Abstract
The ATG genes are highly conserved in eukaryotes including yeasts, plants, and mammals. However, these genes appear to be only partially present in most protists. Recent studies demonstrated that, in the apicomplexan parasites Plasmodium (malaria parasites) and Toxoplasma, ATG8 localizes to the apicoplast, a unique nonphotosynthetic plastid with 4 limiting membranes. In contrast to this established localization, it remains unclear whether these parasites can induce canonical macroautophagy and if ATG8 localizes to autophagosomes. Furthermore, the molecular function of ATG8 in its novel workplace, the apicoplast, is totally unknown. Here, we review recent studies on ATG8 in Plasmodium and Toxoplasma, summarize both consensus and controversial findings, and discuss its potential role in these parasites.
Keywords: apicoplast, ATG8, autophagy, plasmodium, toxoplasma
ATGs are only Partially Conserved in Protists
Macroautophagy (hereafter referred to as autophagy) is a major intracellular degradation system that, in eukaryotes, is mediated by a special organelle, the autophagosome.1,2 Upon autophagy induction, small pieces of the cytoplasm are enclosed by phagophores, which mature into autophagosomes. Then, completed autophagosomes fuse with the lysosome, and the cytoplasm-derived materials inside the autophagosomes are degraded by lysosomal hydrolases. These degrading structures, called autolysosomes, undergo autolysosomal reformation to terminate autophagy.3 Thus, the autophagosome is a transient structure. Autophagy is important for many physiological and pathological processes: for example, production of amino acids during starvation, development, quality control of intracellular proteins and organelles, and degradation of intracellular bacteria.4-6
Autophagosome formation requires autophagy-related (Atg) proteins, which were originally identified in the yeast Saccharomyces cerevisiae.2,7 These Atg proteins function as distinct complexes in a hierarchical order.8 The Atg1-Atg13-Atg17-Atg31-Atg29 complex and Atg9-containing vesicles are required for initiation steps. Next, the phosphatidylinositol (PtdIns) 3-kinase complex (Vps30/Atg6-Atg14-Vps15-Vps34) produces PtdIns3P, which recruits the Atg2-Atg18 complex. Finally, the Atg12–Atg5-Atg16 complex and the Atg8– phosphatidylethanolamine (PE) conjugate are required for elongation of the phagophore membrane or completion of closure of the autophagosome (Fig. 1A). These Atg proteins are highly conserved in eukaryotes.9
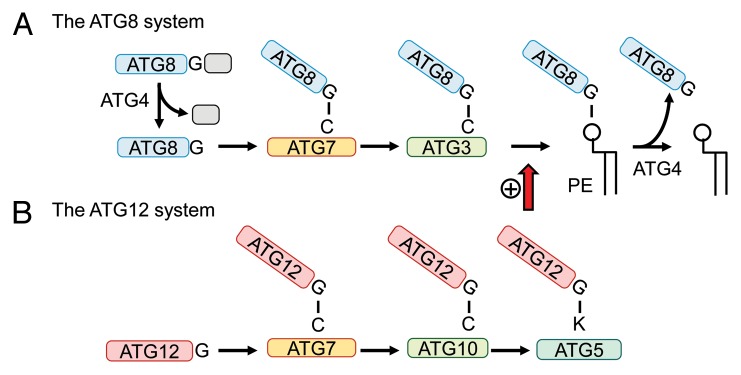
Figure 1. General model of the ATG8 and ATG12 conjugation systems. (A) The ATG8 conjugation system. Immediately after synthesis, the C-terminal extension of ATG8 is cleaved by ATG4 to expose a glycine residue, which is important for the subsequent reaction. After this processing, ATG8 is activated by the E1-like enzyme ATG7 in an ATP-dependent manner and forms a thioester bond between the C-terminal glycine of ATG8 and the active site cysteine of ATG7. Then, ATG8 is transferred to the E2-like enzyme ATG3. ATG8 is finally conjugated with phosphatidylethanolamine (PE). On autophagosomes or autolysosomes, ATG8–PE is deconjugated by ATG4. This system is highly conserved in apicomplexan parasites, but ATG8 in Plasmodium and Toxoplasma lacks the C-terminal extension sequence (shown in gray) and the glycine residue is already exposed without ATG4-mediated processing. (B) The ATG12 conjugation system. ATG12 has a C-terminal glycine and is activated by ATG7 as in the ATG8 system. Then, ATG12 is transferred to ATG10, another E2-like enzyme specific for ATG12, and finally conjugated to a lysine residue of ATG5 via an isopeptide bond. Although the ATG12 system is highly conserved in almost all eukaryotes, ATG10-like proteins have not been clearly identified in apicomplexan parasites. Potential ATG12 (but lacking the C-terminal glycine) and ATG5 proteins can be found in these organisms.
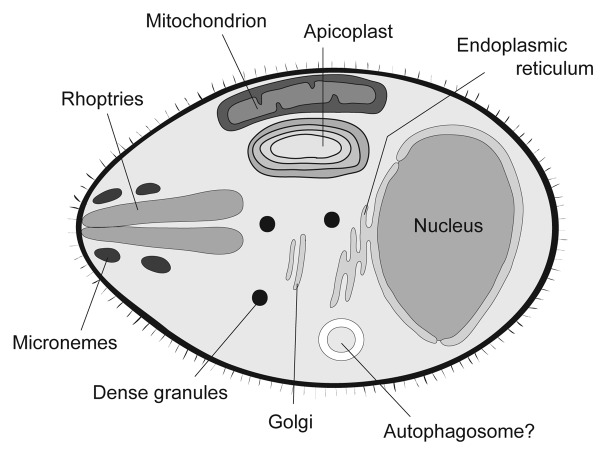
However, these ATG genes appear to be only partially conserved in protists.10 This finding indicates either that these protists can still generate autophagosomes using a partial set of ATG proteins or that ATG proteins have some roles other than autophagy in these protists. Recently, special attention has been paid to apicomplexan parasites. Apicomplexa is a large phylum of parasitic protists, including human pathogens such as Plasmodium and Toxoplasma, as well as many other parasites, which are nonpathogenic to humans, but affect a wide variety of animals. Most of these parasites have unique life cycles. Plasmodium parasites, which cause malaria, grow in the host hepatocytes and then in erythrocytes, and mosquitos act as a vector. When the vector mosquito takes a blood meal, sporozoites enter the human blood stream and subsequently enter hepatocytes to multiply. Merozoites realeased from hepatocytes infect red blood cells, producing the clinical manifestation of the malaria (please see http://www.cdc.gov/malaria/about/biology/ for more details).10 Toxoplasma gondii, the causative agent of toxoplasmosis, undergoes sexual reproduction in cats and asexual reproduction in intermediate hosts (warm-blooded animals) including humans (http://www.cdc.gov/parasites/toxoplasmosis/biology.html). Apicomplexa are characterized by the phylum-defining apical complex (comprising rhoptries, micronemes, and dense granules as well as the conoid and polar ring) involved in host-cell invasion. Many also possess a plastid organelle called the apicoplast, which the parasites acquired through secondary endosymbiosis (Fig. 2).11,12 Blood-stage Plasmodium parasites have a food vacuole, an acidic organelle, where hemoglobin is actively degraded. However, typical lysosomes appear to be missing in Toxoplasma and Plasmodium (at the liver stage). In the human malaria parasite Plasmodium falciparum, rodent malaria parasite Plasmodium berghei, and Toxoplasma gondii, ATG proteins belonging to the Atg8 conjugation system (i.e., ATG3, ATG4, ATG7, and ATG8) are completely conserved. Besides the ATG8 system, ATG1, ATG2, ATG5, ATG6, ATG12, ATG14, ATG17, ATG18, and VPS34 may be conserved in P. falciparum,10,13,14 and ATG1, ATG5, ATG6, ATG9, ATG18, VPS34, and ATG15 in T. gondii.15 Other candidates with weak homology are also found.10 However, as ATG8 is the most widely used marker protein for the autophagosome in many organisms and ATG8 is undoubtedly conserved in Plasmodium and Toxoplasma, most studies have thus far focused on this protein.
Figure 2. Intracellular organelles in the Plasmodium falciparum merozoite. The rhoptries, micronemes, and dense granules are secretory organelles at the apical pole, which are exocytosed upon host cell invasion. The apicoplast is a nonphotosynthetic plastid, which was acquired by secondary endosymbiosis. See the text for more details about the apicoplast.
Consensus Findings
ATG8 associates with membranes likely as a PE-conjugated form
ATG8 is a ubiquitin-like molecule. Immediately after synthesis, a C-terminal extension sequence of ATG8 (and most of its homologs in other organisms) is cleaved by ATG4, exposing a glycine residue at the C terminus.16,17 This glycine residue is conjugated to PE by the action of ATG7 (ubiquitin E1-like enzyme) and ATG3 (ubiquitin E2-like enzyme) (Fig. 1A). The ATG12–ATG5 conjugate behaves as if it is an E3-like enzyme for ATG8 conjugation (Fig. 1B). Therefore, in most organisms, ATG8 exists in 2 forms: cytosolic unconjugated ATG8 (ending with glycine) and membrane-bound ATG8–PE. On immunoblotting, mammalian ATG8 homologs (LC3s and GABARAPs) can be easily separated into the 2 forms (ATG8–PE migrates faster than ATG8) by conventional SDS-PAGE.17,18 In contrast, yeast Atg8 and Atg8–PE migrate to almost the same position by conventional SDS-PAGE and can be separated only by SDS-PAGE using gels containing urea.19 P. falciparum (Pf) ATG813,14 and P. berghei (Pb) ATG820 are detected as a single band at ~13–14 kDa by conventional SDS-PAGE (one report shows 2 PfATG8 bands at over 15 kDa21) (Table 1). Most PfATG8 and PbATG8 are recovered in the membrane fraction, which can be solubilized by detergent but not by high concentrations of NaCl or urea.13,14,20 By urea-containing SDS-PAGE, mCherry-PfATG8 can be separated into 2 bands; the appearance of the lower bands depends on the C-terminal glycine.25 Although there have been no data showing urea-containing SDS-PAGE of endogenous ATG8 proteins, these data suggest that most endogenous PfATG8 and PbATG8, and a part of fluorescent protein-tagged PfATG8, tightly associate with membranes, most probably in the PE-conjugated form.
Table 1. Intracellular localization and biochemical features of ATG8, and functions of related ATG proteins in Plasmodium and Toxoplasma.
| Sp. | Localization | Evidence | Immunoblotting | Membrane-bound | Function | Ref. | Epub Date | |
|---|---|---|---|---|---|---|---|---|
| Plasmodium | Pf | Apicoplast | IF: endogenous PfATG8 colocalizes with ACP in RBC (wortmannin-resistant, CQ-resistant) IEM: endogenous PfATG8 on apicoplasts |
Single band (endogenous PfATG8) |
Yes Triton-sensitive Urea-resistant |
13 | 08/18/2012 | |
| Pb | Unknown puncta | IF: endogenous PbATG8 | 22 | 09/18/2012 | ||||
| Pb | Apicoplast | IF: endogenous PbATG8 and GFP-PbATG8 colocalize with ACP in liver cells (dependent on C-terminal Gly) | 23 | 02/08/2013 | ||||
| Pf | (Function of PfATG7) Essential for growth |
24 | 07/05/2013 | |||||
| Pf | Apicoplast Autophagosome Food vacuole |
IF: mCherry-PfATG8 and endogenous PfATG8 colocalize with aLipDH-GFP in RBC, and partially colocalize with PfRab7 and lysotracker signals during starvation IEM: PfATG8 on double membrane structures and inside the food vacuole in starved parasites |
Two bands (mCherry-PfATG8, Urea SDS-PAGE) | 25 | 09/13/2013 | |||
| Pf | Apicoplast Maurer's cleft? |
IF: endogenous PfATG8colocalize with ACP and additional vesicles near PfRex1 (induced by starvation and CQ) in RBC | Two bands (endogenous PfATG8) | 21 | 11/28/2013 | |||
| Pf | Apicoplast Small vesicles? |
IF: GFP-PfATG8 and endogenous PfATG8 colocalize with ACP (wortmannin-resistant, CQ-resistant) | Single band (endogenous PfATG8) |
Yes | 14 | 11/28/2013 | ||
|
Pb Pf |
Apicoplast (with tubules and vesicles) | IF: endogenous PbATG8 colocalize with ACP in liver cells IEM: endogenous PbATG8 on the outermost apicoplast membrane |
Single band (endogenous PbATG8) |
Yes Triton-sensitive Urea-resistant |
20 | 12/18/2013 | ||
| Toxoplasma | Tg | Autophagosome Cytosol |
IF: GFP-TgATG8 puncta are induced by starvation (sensitive to wortmannin and 3-MA) IEM: GFP-TgATG8 on double membrane structures |
Two bands (endogenous TgATG8, GFP-TgATG8, Urea SDS-PAGE) | Yes Deoxycholate-sensitive NaCl-resistant Urea-resistant |
(Function of TgATG3) Essential for growth Important for mitochondrial homeostasis |
15 | 12/07/2011 |
| Tg, Pf | Unknown puncta Cytosol |
IF: TgATG8, PfATG8 | 26 | 07/24/2012 | ||||
| Tg | Apicoplast Cytosol |
IF: GFP-TgATG8 induced by monensin colocalize with ATRX1 (dependent on C-terminal Gly, inhibited by 3-MA) | Important for mitochondria? | 27 | 08/01/2012 | |||
| Tg | Apicoplast Cytosol |
IF: endogenous TgATG8 and GFP-TgATG8 colocalize with ATrx1 (dependent on C-terminal Gly) | Two bands (GFP-TgATG8, Urea SDS-PAGE) | (Function of TgATG4) Important for growth and homeostasis of mitochondria and apicoplasts (apicoplasts are lost also in ATG3KO) |
28 | 06/12/2013 |
Pf, P. falciparum; Pb, P. berghei; Tg, T. gondii; IF, immunofluorescence microscopy; IEM, immunoelectron microscopy; ACP, acyl carrier protein; RBC, red blood cell; CQ, chloroquine; Gly, glycine; 3-MA, 3-methyladenine.
Similarly, endogenous T. gondii (Tg) ATG8 is detected as a single band at 14 kDa by conventional SDS-PAGE without urea.15 Both endogenous TgATG8 and GFP-TgATG8 can be separated into 2 bands by urea-containing SDS-PAGE.15,28 As 3H-labeled ethanolamine can be incorporated into the lower GFP-ATG8 band and mutation of the C-terminal glycine abolishes formation of the lower band, the lower band should represent the PE-conjugated form.15 The PE-conjugated form associates with membranes and is solubilized by detergent but not by salt or urea.15,28 All these studies show that ATG8 proteins in Plasmodium and Toxoplasma share biochemical features with ATG8 homologs in other organisms.
ATG8 is on the apicoplast
To date, at least 6 studies on PfATG8/PbATG813,14,20,21,23,25 and 2 studies on TgATG827,28 have reached the unexpected but consistent conclusion that ATG8 localizes, at least partially, on the unique organelle, the apicoplast (Table 1). The apicoplast is a nonphotosynthetic plastid present in apicomplexan parasites and is thought to have been acquired by secondary endosymbiosis of red algae.11,12 This plastid is essential for these species and is involved in the synthesis of fatty acids, isoprenoids, and heme. The apicoplast has 4 membranes and is often found to be in close contact with the mitochondrion.
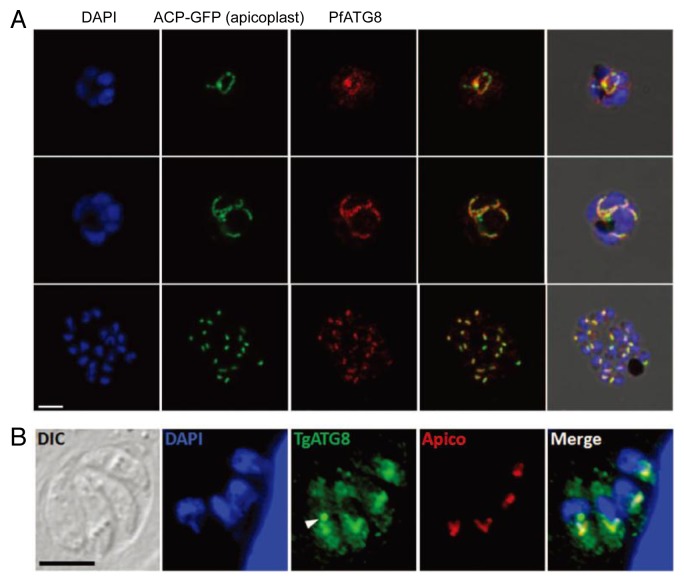
Immunofluorescence microscopy shows that endogenous PfATG8,13,14,20,21,25 GFP/mCherry-PfATG8,14,25 endogenous PbATG8,23 green fluorescent protein (GFP)-PbATG8,23 endogenous TgATG8,28 and GFP-TgATG827,28 constitutively colocalize with apicoplast markers such as acyl carrier protein and apicoplast lipoamide dehydrogenase-GFP in Plasmodium and ATRX1 in Toxoplasma (Fig. 3). Electron microscopy also detects endogenous PfATG8 and PbATG8 signals on apicoplasts, most likely on the outermost membrane of the 4 apicoplast membranes.13,20
Figure 3. PfATG8 and TgATG8 localize to apicoplasts. Localization of PfATG8 in early schizonts (the multinucleated replicating form inside a host cell; upper), late schizonts (middle), and merozoites (daughter cells within the host formed by division of the schizont; lower) (A) and TgATG8 in tachyzoites (B) are shown together with apicoplast (Apico) markers. The arrowhead indicates a putative autophagosome. Scale bars: 5 μm. The images in (A and B) were published in references 14,28, respectively, with permission of the publisher.
Localization of ATG8 on the apicoplast appears to be stable and is detected throughout the liver and blood stages. This observation argues against the possibility that the colocalization of ATG8 represents autophagic degradation of apicoplasts. In fact, the electron microscopy studies do not show any autophagic membranes surrounding apicoplasts.13,20
ATG8 mutants in which the C-terminal glycine is replaced with alanine can no longer colocalize with apicoplasts, suggesting that the ubiquitin-like conjugation is required for localization of ATG8 to the apicoplast.23,27,28 Together with the above-mentioned biochemical data, Plasmodium and Toxoplasma ATG8 proteins are most likely conjugated with PE in the apicoplast membrane.
Controversies
Is PtdIns 3-kinase required for the apicoplast localization of ATG8?
It is not known how the association of ATG8 with the apicoplast is regulated in Plasmodium and Toxoplasma. In canonical starvation-induced autophagy, recruitment of ATG8/LC3 is clearly dependent on PtdIns 3-kinase activity.8,29 In Plasmodium, the apicoplast membrane contains a class III PtdIns 3-kinase, VPS34, and its product PtdIns3P.30 Thus, the effects of PtdIns 3-kinase inhibitors on apicomplexan ATG8 and TgATG8 have been tested. In Plasmodium, the apicoplast localization of PfATG813,14 is not affected by wortmannin, an inhibitor of PtdIns 3-kinase. In contrast, in Toxoplasma, the accumulation of starvation- or monensin-induced GFP-TgATG8 puncta is inhibited by wortmannin and 3-methyladenine, both of which are PtdIns 3-kinase inhibitors.15,27 The discrepancies observed indicate that the requirement of PtdIns 3-kinase may be different between Plasmodium and Toxoplasma. Alternatively, although both wortmannin and 3-methyladenine seem to inhibit accumulation of starvation-induced GFP-TgATG8 puncta,15,27 these chemicals may have different effects under nonstarved conditions, as observed in mammalian cells.31 Involvement of other ATG homologs also needs to be investigated in a more comprehensive manner.
Do Plasmodium and Toxoplasma have the canonical autophagy system?
Although an obvious question is whether apicomplexan parasites have canonical autophagy and whether ATG8 proteins localize in autophagic membranes, the answers have been quite controversial. Several fluorescence microscopy studies identified PfATG8,13 PbATG8,20,23 and TgATG827 solely on the apicoplast or its related structures. Relatively mild starvation treatment does not increase the number of PfATG8 puncta.14 These reports suggest either that these parasites do not produce autophagosomes or that ATG8 does not translocate to the autophagosomes, if they exist. In contrast, other studies showed starvation-induced ATG8-positive structures in P. falciparum21,26 and T. gondii.15,26 Furthermore, an increase in the number of PfATG8- and PfRAB7-double positive structures is also observed under starvation conditions.25 Thus, localization of ATG8 can be changed by starvation. However, these latest studies were done under severe starvation conditions (e.g., without amino acids) and it is unknown whether these changes can also be induced under physiological starvation conditions. As a whole, these reports suggest that while apicoplast localization of ATG8 is a certainty, whether or not these parasites produce a classical autophagosome structure remains to be confirmed.
To better address these questions, we need to reconfirm the definition of (macro)autophagy, in particular that a region of the cytoplasm is enclosed by the double-membrane autophagosome and delivered to the lysosome/vacuole to be degraded. Thus, at least 2 distinct structures must be involved to classify a process as (macro)autophagy: the autophagosome and lysosome/vacuole. Kitamura et al.13 showed by electron microscopy that there is no autophagosome-like double-membrane structure in blood-stage P. falciparum at least under growing conditions.13 Some vacuoles can be observed in untreated P. berghei23 or in T. gondii treated with monensin, an antimicrobial drug.27 However, these “vacuoles” cannot be recognized as autophagosomes because cytoplasmic material (i.e., cytosolic ribosomes or organelles) are not clearly detected inside these vacuoles. Furthermore, these vacuoles are devoid of ATG8.23,27
In this regard, autophagosome-like structures have been observed in some reports in these apicomplexan parasites. In T. gondii, TgATG8 shows a diffuse cytosolic pattern in tachyzoites (the motile infectious forms of the parasites) immediately after release from host cells.15 However, when these extracellular parasites ae subjected to amino acid starvation, TgATG8 forms several punctate structures.15 Following 8 h starvation, approximately 80% of the parasites demonstrate these TgATG8 puncta (mostly more than 1 per cell), in contrast to only ~15% of the cells before starvation.15 Electron microscopy identified several structures of ~300–900 nm in diameter, some of which clearly possess double membranes.15 These structures contain cytosolic materials, sometimes including mitochondria, which are reminiscent of autophagosomes or mitophagosomes. However, the same group later reported that many TgATG8 signals are detected on apicoplasts.28 Thus, it would be important to carefully distinguish these TgATG8 signals on apicoplasts and potential autophagosomes, in general. Double- or multiple-membrane structures are also observed in starved Toxoplasma in another study, but immunoelectron microscopy was not performed and the identities of these structures are unknown.32 In contrast to these reports, Lavine et al.27 reported that even though monensin treatment induces TgATG8 puncta, they still colocalize with apicoplasts.27 Thus, monensin and starvation treatment may result in different ATG8 localization patterns in T. gondii. Also in P. falciparum, the immunoelectron microscopy study shows colocalization of PfATG8 and PfRAB7 on small double-membrane structures, particularly under starvation conditions.25 Although all these structures are reminiscent of autophagosomes or autolysosomes, it should be remembered that apicoplasts also have multiple membranes and may look similar to autophagosomes. Additional methods such as correlative light and electron microscopy, which allow us to observe identical structures by both immunofluorescence microscopy and conventional microscopy, would provide more information to allow us to distinguish between, and understand the precise nature of, these 2 ATG8-positive structures.
Lysosomes or acidic organelles are essential to achieve autophagy, but it is also unclear whether apicomplexan parasites possess lysosome-like organelles besides the food vacuole. Plasmodium may actually use the food vacuole for autophagy because PfATG8 and PfRAB7 can be detected inside this compartment.25 In T. gondii, the presence of lysosome-like organelles was recently reported.33,34 If these parasites can induce autophagy with their partial sets of ATG proteins, how is this achieved? For example, both Plasmodium and Toxoplasma seem to lack most subunits of the ATG1 initiation complex. ATG1 is not a simple signaling molecule, but it has an important mechanistic role in autophagosome formation in yeast.35,36 However, a recent study showed the surprising result that the chicken ATG1 homologs ULK1 and ULK2 are not essential for starvation-induced autophagy.37 Thus, a complete set of ATGs may not be necessary in all organisms. Alternatively, as components of the ATG1 complex already differ between yeast and mammals,38 apicomplexan parasites might have evolved a different set of ATG1 complex subunits (ATG1 itself seems to be conserved), which may not be easily identified based on sequence homology.
Are there other ATG8-positive structures/populations?
Besides the localization of ATG8 on the apicoplast and potential autophagosomes, several studies also report that some population of ATG8 may exist at different places, which can be induced under certain conditions. Gaviria et al.21 showed that the number of PfATG8 punctate structures is increased by starvation or chloroquine treatment.21 As acyl carrier protein colocalizes with only a certain population of these structures, they may represent entities other than the apicoplast. These inducible structures are detected in close proximity to PfREX1 signals, suggesting that they may be related to Maurer’s clefts,21 membranous structures involved in protein sorting and export.39 However, other studies failed to observe such chloroquine-induced ATG8 punctate structures.13,14 As Gaviria et al.21 use antibodies against TgATG8 to detect PfATG8, the colocalization between PfATG8 and Maurer’s cleft should be confirmed by using other anti-PfATG8 antibodies or fluorescent protein-tagged PfATG8.
In contrast to Plasmodium ATG8, a significant population of GFP-TgATG8 is detected in the cytosol, but this may be due to overexpression and/or tagging of GFP.15,27,28 In general, the PE-conjugation efficiency of the Atg8 family proteins is reduced when they are tagged with large molecules such as GFP. In fact, the amount of the PE-conjugated form is reduced for GFP-TgATG8 compared with endogenous ATG8, and unconjugated GFP-TgATG8 is detected in the soluble cytosolic fraction.15 Thus, it remains uncertain whether Toxoplasma has a larger amount of the cytosolic ATG8 pool compared with Plasmodium.
Should the C-terminal end of newly synthesized ATG8 be a glycine?
In most organisms, ATG8 has an amino acid extension downstream of the C-terminal glycine, which needs to be cleaved off by ATG4 prior to the conjugation reaction (Fig. 1). This cleavage occurs immediately after synthesis of the ATG8 protein, not upon autophagy induction. However, ATG8 proteins of Plasmodium and Toxoplasma are unique because their primary sequences already end with a glycine residue and do not have any C-terminal extension even though these parasites have ATG4, which can cleave off an artificially tagged C-terminal peptide, at least in T. gondii.28 In these parasites, ATG4 is likely important for the deconjugation step; TgATG8–PE can be cleaved by TgATG4.28 The fact that TgATG4 is essential for growth and organelle homeostasis suggests that recycling of conjugated TgATG8 is important.28 It remains unknown why Plasmodium and Toxoplasma have ATG8 with the C-terminal glycine exposed. However, this unique character of ATG8, ending with glycine, is not observed in all apicomplexan parasites; a C-terminal extension sequence is found, for example, in Cryptosporidium species.15
What is the role of the ATG8 system in apicomplexa and other parasites?
Interfering with proteins of the ATG8 conjugation system such as PbATG8,10 PfATG7,24 TgATG3,15 and TgATG428 results in defects in growth, suggesting that the ATG8 system is essential for survival.24 Importantly, depletion of TgATG3 and TgATG4 causes mitochondrial abnormalities and apicoplast loss.15,28 These results are consistent with the consensus observation that ATG8 is mainly present on the apicoplast, which is an essential organelle. Thus, the mitochondrial phenotype may be secondary to the apicoplast impairment. However, how ATG8 exerts an essential role for the apicoplast is unknown. As TgATG3 and TgATG4 are required for maintenance of the apicoplast,15,28 TgATG8 could be involved in membrane biogenesis of this organelle. These studies also suggest that the ATG8 system can be a good therapeutic target for human diseases caused by these parasites.
It is unknown whether the potential role of ATG8 in autophagy contributes to the defects in growth and organelle homeostasis seen in ATG-deficient parasites. Given that autophagy is not essential for survival in most organisms under nutrient-rich conditions, these phenotypes are likely caused by a loss of function of ATG8 on the apicoplast. However, it is likely that the ATG8 system has some non-apicoplast functions in protists because the complete ATG8 system can be found in other apicomplexan parasites that lack the apicoplast, such as genus Cryptosporidium and related parasites.10,12 It would be interesting to know the function of ATG8 in these apicoplast-less parasites.
So far 3 studies have tested if apicomplexan ATG8 can complement the autophagy-defective phenotype in yeast ATG8-deleted cells. The results were not simple; PfATG8 can complement the mutant,25 but PbATG8 cannot.20,23 These discrepancies may be due to a slight difference in structure between PfATG8 and PbATG8, but the precise reason is unknown. Although these complementation assays are sometimes useful, they may not provide further detailed information for the molecular function of these apicomplexan proteins as their primary sequences are usually not very conserved.
Future Prospective
In contrast to the clear agreement for the apicoplast localization of Plasmodium and Toxoplasma ATG8 proteins, currently available evidence suggesting the existence of canonical autophagy in apicomplexan parasites is limited. To convincingly show the presence of autophagy, it is essential to determine autophagic flux in these parasites. Typically flux can be demonstrated by showing that degradation of ATG8 depends on acidic compartments by quantitative biochemical or morphological methods.40,41 Alternatively, demonstration of fusion between the ATG8 puncta and acidic compartments such as food vacuoles or lysosome-like organelles by live imaging will provide another line of strong evidence.
Another remaining question is whether the ATG12–ATG5 conjugation system exists in apicomplexan parasites. In general, the ATG8–PE conjugation reaction requires the ATG12–ATG5 conjugate at least in vivo (Fig. 1).7 As ATG8 exists primarily in the ATG8–PE conjugated form, it is speculated that these parasites possess the ATG12–ATG5 conjugate. However, PfATG12 ends with tyrosine and lacks the C-terminal glycine that is required for conjugation with putative PfATG5. In addition, PfATG10 homologs have not been clearly identified in the genome of Plasmodium and Toxoplasma. Thus, more extensive searching and experiments will be required to elucidate whether the complete ATG12 system is conserved and ATG8–PE conjugation requires ATG12–ATG5 in apicomplexan parasites.
Finally, is there any common function of ATG8–PE shared by autophagy and apicoplast biology? One attractive idea is that, as mentioned above, ATG8–PE may be involved in biogenesis of both autophagosome and apicoplast membranes. However, the nature of the outer autophagosomal and apicoplast membranes appears to be very different. Typical endoplasmic reticulum (ER) proteins have been identified on the outer 2 membranes of the apicoplast,42,43 whereas ER proteins are essentially absent on the outer autophagosomal membrane even though autophagosomes may be generated in close proximity to the ER.1 Recently, LC3, a mammalian homolog of ATG8, was found in membranes of several non-autophagic structures such as phagosomes.44 LC3 association in this pathway, called LC3-associated phagocytosis, could accelerate the fusion between phagosomes and lysosomes. Notably, association of LC3 with phagosomes is independent of the ULK1 initiation complex even in mammalian cells.44
Besides their role in phagocytosis, “unconventional” functions of ATG proteins have also been proposed. These include unconventional secretion, tuning of ER-associated degradation on the EDEMosome, axon guidance, cell division, and cell death.45,46 The finding that ATG8 associates with the apicoplast membrane could reveal new unexplored roles of the ATG proteins, which are required for these parasites but for a process distinct from autophagy. More studies are required to reveal the molecular basis of these diverse functions of the ATG8 family proteins. Studies using parasites would provide unique and important insights into this challenging topic.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by JSPS KAKENHI Grants-in-Aid for Scientific Research on Innovative Areas (Grant Number 25111005) and The Tokyo Biochemical Research Foundation (TBRF). We thank Ms Nozomi Sato for preparing a figure illustration.
Glossary
Abbreviations:
- ATG
autophagy-related
- GFP
green fluorescent protein
- Pb
P. berghei
- PE
phosphatidylethanolamine
- PtdIns
phosphatidylinositol
- Pf
P. falciparum
- Tg
T. gondii
- VPS
vacuolar protein sorting
References
- 1.Tooze SA, Yoshimori T. The origin of the autophagosomal membrane. Nat Cell Biol. 2010;12:831–5. doi: 10.1038/ncb0910-831. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–32. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Yu L. Autophagic lysosome reformation. Exp Cell Res. 2013;319:142–6. doi: 10.1016/j.yexcr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell. 2008;15:344–57. doi: 10.1016/j.devcel.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–35. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol. 2009;10:458–67. doi: 10.1038/nrm2708. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–18. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 9.Meijer WH, van der Klei IJ, Veenhuis M, Kiel JAKW. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy. 2007;3:106–16. doi: 10.4161/auto.3595. [DOI] [PubMed] [Google Scholar]
- 10.Duszenko M, Ginger ML, Brennand A, Gualdrón-López M, Colombo MI, Coombs GH, Coppens I, Jayabalasingham B, Langsley G, de Castro SL, et al. Autophagy in protists. Autophagy. 2011;7:127–58. doi: 10.4161/auto.7.2.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalanon M, McFadden GI. Malaria, Plasmodium falciparum and its apicoplast. Biochem Soc Trans. 2010;38:775–82. doi: 10.1042/BST0380775. [DOI] [PubMed] [Google Scholar]
- 12.Sato S. The apicomplexan plastid and its evolution. Cell Mol Life Sci. 2011;68:1285–96. doi: 10.1007/s00018-011-0646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura K, Kishi-Itakura C, Tsuboi T, Sato S, Kita K, Ohta N, Mizushima N. Autophagy-related Atg8 localizes to the apicoplast of the human malaria parasite Plasmodium falciparum. PLoS One. 2012;7:e42977. doi: 10.1371/journal.pone.0042977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cervantes S, Bunnik EM, Saraf A, Conner CM, Escalante A, Sardiu ME, Ponts N, Prudhomme J, Florens L, Le Roch KG. The multifunctional autophagy pathway in the human malaria parasite, Plasmodium falciparum. Autophagy. 2014;10:80–92. doi: 10.4161/auto.26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besteiro S, Brooks CF, Striepen B, Dubremetz JF. Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites. PLoS Pathog. 2011;7:e1002416. doi: 10.1371/journal.ppat.1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, Ohsumi M, Takao T, Noda T, Ohsumi Y. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151:263–76. doi: 10.1083/jcb.151.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–12. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 18.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–92. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 20.Jayabalasingham B, Voss C, Ehrenman K, Romano JD, Smith ME, Fidock DA, Bosch J, Coppens I. Characterization of the ATG8-conjugation system in 2 Plasmodium species with special focus on the liver stage: possible linkage between the apicoplastic and autophagic systems? Autophagy. 2014;10:269–84. doi: 10.4161/auto.27166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaviria D, Paguio MF, Turnbull LB, Tan A, Siriwardana A, Ghosh D, Ferdig MT, Sinai AP, Roepe PD. A process similar to autophagy is associated with cytocidal chloroquine resistance in Plasmodium falciparum. PLoS One. 2013;8:e79059. doi: 10.1371/journal.pone.0079059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hain AU, Weltzer RR, Hammond H, Jayabalasingham B, Dinglasan RR, Graham DR, Colquhoun DR, Coppens I, Bosch J. Structural characterization and inhibition of the Plasmodium Atg8-Atg3 interaction. J Struct Biol. 2012;180:551–62. doi: 10.1016/j.jsb.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eickel N, Kaiser G, Prado M, Burda PC, Roelli M, Stanway RR, Heussler VT. Features of autophagic cell death in Plasmodium liver-stage parasites. Autophagy. 2013;9:568–80. doi: 10.4161/auto.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker DM, Mahfooz N, Kemme KA, Patel VC, Spangler M, Drew ME. Plasmodium falciparum erythrocytic stage parasites require the putative autophagy protein PfAtg7 for normal growth. PLoS One. 2013;8:e67047. doi: 10.1371/journal.pone.0067047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlins AM, Ben-Rached F, Williams RA, Proto WR, Coppens I, Ruch U, Gilberger TW, Coombs GH, Mottram JC, Müller S, et al. Plasmodium falciparum ATG8 implicated in both autophagy and apicoplast formation. Autophagy. 2013;9:1540–52. doi: 10.4161/auto.25832. [DOI] [PubMed] [Google Scholar]
- 26.Sinai AP, Roepe PD. Autophagy in Apicomplexa: a life sustaining death mechanism? Trends Parasitol. 2012;28:358–64. doi: 10.1016/j.pt.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavine MD, Arrizabalaga G. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS One. 2012;7:e42107. doi: 10.1371/journal.pone.0042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong-Hap MA, Mouammine A, Daher W, Berry L, Lebrun M, Dubremetz JF, Besteiro S. Regulation of ATG8 membrane association by ATG4 in the parasitic protist Toxoplasma gondii. Autophagy. 2013;9:1334–48. doi: 10.4161/auto.25189. [DOI] [PubMed] [Google Scholar]
- 29.Itakura E, Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–76. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tawk L, Chicanne G, Dubremetz JF, Richard V, Payrastre B, Vial HJ, Roy C, Wengelnik K. Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast. Eukaryot Cell. 2010;9:1519–30. doi: 10.1128/EC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–61. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh D, Walton JL, Roepe PD, Sinai AP. Autophagy is a cell death mechanism in Toxoplasma gondii. Cell Microbiol. 2012;14:589–607. doi: 10.1111/j.1462-5822.2011.01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, de Haas F, de Souza W, et al. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol Microbiol. 2010;76:1358–75. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parussini F, Coppens I, Shah PP, Diamond SL, Carruthers VB. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Mol Microbiol. 2010;76:1340–57. doi: 10.1111/j.1365-2958.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheong H, Nair U, Geng J, Klionsky DJ. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol Biol Cell. 2008;19:668–81. doi: 10.1091/mbc.E07-08-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawamata T, Kamada Y, Kabeya Y, Sekito T, Ohsumi Y. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol Biol Cell. 2008;19:2039–50. doi: 10.1091/mbc.E07-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alers S, Löffler AS, Paasch F, Dieterle AM, Keppeler H, Lauber K, Campbell DG, Fehrenbacher B, Schaller M, Wesselborg S, et al. Atg13 and FIP200 act independently of Ulk1 and Ulk2 in autophagy induction. Autophagy. 2011;7:1423–33. doi: 10.4161/auto.7.12.18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–9. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Sam-Yellowe TY. The role of the Maurer’s clefts in protein transport in Plasmodium falciparum. Trends Parasitol. 2009;25:277–84. doi: 10.1016/j.pt.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalanon M, Tonkin CJ, McFadden GI. Characterization of two putative protein translocation components in the apicoplast of Plasmodium falciparum. Eukaryot Cell. 2009;8:1146–54. doi: 10.1128/EC.00061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spork S, Hiss JA, Mandel K, Sommer M, Kooij TW, Chu T, Schneider G, Maier UG, Przyborski JM. An unusual ERAD-like complex is targeted to the apicoplast of Plasmodium falciparum. Eukaryot Cell. 2009;8:1134–45. doi: 10.1128/EC.00083-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends Cell Biol. 2012;22:374–80. doi: 10.1016/j.tcb.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bestebroer J, V’kovski P, Mauthe M, Reggiori F. Hidden behind autophagy: the unconventional roles of ATG proteins. Traffic. 2013;14:1029–41. doi: 10.1111/tra.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramani S, Malhotra V. Non-autophagic roles of autophagy-related proteins. EMBO Rep. 2013;14:143–51. doi: 10.1038/embor.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]