Abstract
Macroautophagy is a vesicular catabolic trafficking pathway that is thought to protect cells from diverse stressors and to promote longevity. Recent studies have revealed that transcription factors play important roles in the regulation of autophagy. In this study, we have identified GA binding protein (GABP) as a transcriptional regulator of the combinatorial expression of BECN1-PIK3C3 complex genes involved in autophagosome initiation. We performed bioinformatics analyses that demonstrated highly conserved putative GABP sites in genes that encode BECN1/Beclin 1, several BECN1 interacting proteins, and downstream autophagy proteins including the ATG12–ATG5-ATG16L1 complex. We demonstrate that GABP binds to the promoter regions of BECN1-PIK3C3 complex genes and activates their transcriptional activities. Knockdown of GABP reduced BECN1-PIK3C3 complex transcripts, BECN1-PIK3C3 complex protein levels and autophagy in cultured cells. Conversely, overexpression of GABP increased autophagy. Nutrient starvation increased GABP-dependent transcriptional activity of BECN1-PIK3C3 complex gene promoters and increased the recruitment of GABP to the BECN1 promoter. Our data reveal a novel function of GABP in the regulation of autophagy via transcriptional activation of the BECN1-PIK3C3 complex.
Keywords: autophagy, transcription, GA binding protein, BECN1, PIK3C3
Introduction
Macroautophagy, hereafter designated autophagy, is a vesicular trafficking pathway for autolysosomal degradation that is thought to protect cells from nutrient starvation,1 infection,2 toxic protein aggregates,3 injured/defective mitochondria,4 and other cellular stressors. Autophagy deficiencies have been implicated in aging5 and neurodegeneration.6,7 A comprehensive understanding of the regulatory mechanisms that govern autophagy efficacy may lead to novel therapeutic strategies to augment protective autophagy in aging and disease.
Autophagosomes, or autophagic vacuoles (AVs), are double-membrane vacuoles that sequester and deliver cellular organelles, cytoplasm, and membranes to the lysosome for degradation. The induction, formation, and trafficking of AVs is regulated by several macromolecular protein complexes. In AV formation, the BECN1-PIK3C3-PIK3R4 core complex catalyzes phosphorylation of membrane phosphatidylinositol to phosphatidylinositol-3-phosphate.8 Phosphatidylinositol-3-phosphate then recruits downstream autophagy regulatory proteins8-10 including the ATG12–ATG5-ATG16L1 complex which facilitates the incorporation of lipidated LC3 (LC3-II) in the autophagosome membrane. Several autophagy regulatory proteins, including ATG14 and UVRAG, bind to the BECN1-PIK3C3-PIK3R4 core complex to activate lipid phosphorylation. ATG1411 increases AV induction, whereas UVRAG12 promotes the maturation of AVs and endocytic vesicles. BECN1 deficiency reduces autophagy,13 promotes tumorigenesis,13 and likely contributes to proteinopathies including amyloid-β pathology in Alzheimer disease.14-16 Augmenting BECN1 levels ameliorates SNCA/α-synuclein17 and HTT/huntingtin18,19 neuropathologies. Furthermore, disinhibiting endogenous BECN1 protects cells from mutant huntingtin toxicity and infectious pathogens.20 A more comprehensive understanding of how BECN1-PIK3C3-PIK3R4 complex protein levels and activity are regulated to impact autophagy will likely have implications for new therapeutic strategies in neurodegeneration, cancer, and infectious diseases.
Gene transcription, an important factor that regulates protein levels, plays a role in autophagy regulation.21 Transcription of autophagy regulatory genes, including BECN1, decreases with aging.5,18 BECN1 transcript levels are also reduced in patients with Alzheimer disease.14 Reduced BECN1 transcription may thus predispose to age-related diseases through autophagy deficiency. Recent studies have identified TFEB,22 ZKSCAN3,23 E2F1,24 and histone methylation via methyltransferase EHMT2/G9a25 as important transcriptional regulators of autophagy. Notably, these factors tend to regulate expression of genes predominantly involved in late steps of autophagy regulation. Transcriptional regulation of autophagy initiation complexes, including the BECN1-PIK3C3 complex, is poorly understood. NFKB26 has been implicated in transcriptional regulation of the BECN1 gene and E2F1 has been suggested to bind to the BECN1 promoter.27 FOXO3 and CLOCK-ARNTL/BMAL1 regulate ATG14 transcription.28 TFEB plays a role in transcriptional activation of UVRAG.22 However, regulatory mechanisms that activate the combinatorial transcription of autophagy initiation complexes have not been reported.
The ETS-domain transcription factor (ETS-TF) family is well known for combinatorial regulation of gene expression.29 Among the family of ETS-TFs, GA binding protein (GABP, also known as NRF2 or nuclear respiratory factor 2) functions as either heterodimers or heterotetramers of GABPA and GABPB subunits. GABPA contains the DNA binding domain and is required for the recruitment of GABPB to DNA, whereas GABPB contains the transactivation domains and directs the transportation of GABP complexes from the cytoplasm to the nucleus.30 GABP was first identified to regulate the expression of several nuclear-encoded mitochondrial genes31,32 and the prolactin gene.33,34 Recent studies have shown that GABP activates the expression of genes involved in the differentiation of myeloid cells35-40 and skeletal muscle.41-46 Furthermore, GABP interacts with E2F to promote cell cycle progression through the S-phase via the transcription of DNA synthesis genes, indicating its regulatory role in cell proliferation and differentiation.47 A role for GABP and other ETS-TFs in autophagy regulation has not been reported.
In this study, we examined a novel role for GABP in the combinatorial transcriptional regulation of genes encoding BECN1-PIK3C3 complexes. We found that putative ETS-TF/GABP motifs are ubiquitously present in genes encoding protein subunits of BECN1-PIK3C3 complexes. Our data show that GABP binds to the promoters of these genes and activates their transcription. Furthermore, we found that GABP knockdown reduces the levels of BECN1-PIK3C3 complex proteins and inhibits basal autophagy whereas GABP overexpression increases autophagy. Finally, we found that nutrient starvation increases GABP-dependent transcriptional activity of genes encoding BECN1-PIK3C3 complexes and increases GABP binding to the endogenous BECN1 promoter. Our study identifies GABP as a novel transcriptional regulator of autophagy and as a potential therapeutic target to augment autophagy.
Results
Genes encoding BECN1-PIK3C3 complexes contain functional ETS-TF core sequences in their promoter regions
We performed bioinformatics analyses to determine whether autophagy genes contain consensus core ETS-TF sequences (5′-SGGAAG-3′) in their 5′ regulatory regions. We found putative ETS-TF sequences in the promoter regions of key genes encoding protein subunits of BECN1-PIK3C3 complexes, including BECN1, PIK3C3, UVRAG, ATG14, and PIK3R4 (Fig. 1; Figs. S1–S6). We also noted consensus core ETS-TF sequences in ATG5, ATG12, and ATG16L1 (Fig. S1; Figs. S7–S9). Sequence alignments from mammalian species demonstrated that these ETS-TF consensus sequences are highly conserved (Figs. S2–S9), suggesting they may have functional importance for the transcriptional regulation of these autophagy genes. Our sequence alignments also corroborated previously reported autophagy gene promoter regulatory elements including NFKB (5′-GGGRNTTTCC-3′) in intron 1 of BECN1,26 TFEB (5′-CANNTG-3′ or E-box) in the promoter region of UVRAG,22 and FOXO (5′-TGAAAACA-3′) and E-box in the promoter region of ATG14 (Fig. 1; Figs. S2, S4, and S5).28 Our statistical analysis of putative autophagy gene ETS-TF binding sites via the TRANSFAC database rendered GABP, an ETS-TF that has high affinity for the core ETS-TF binding consensus sequence,32,48 as the only ubiquitous putative ETS-TF likely to bind to genes encoding BECN1-PIK3C3 complex proteins (Table S1). These results suggested that GABP is a strong candidate for the combinatorial regulation of BECN1-PIK3C3 complex gene transcription.

Figure 1.BECN1, PIK3C3, UVRAG, and ATG14 5′ regulatory regions contain putative ETS-TF sites. Schematic diagrams of 5′ regulatory regions of human BECN1 (A), PIK3C3 (B), UVRAG (C), and ATG14 (D) demonstrate known and putative TF sites revealed by our bioinformatics analyses (also see Figs. S2–S5). Dark blue rectangles represent noncoding exons and light blue rectangles represent coding exons. Major transcription start sites are identified with arrows and translational start codons (ATG) are marked. Conserved transcription factor binding sites are presented as follows: ETS (orange stars), Sp1 and Sp-family (Sp-fam; purple ovals), CREB (gray diamond), NFKB (red oval), E-boxes (pink ovals), NFYA (green oval) and FOXO (dark blue oval). Red lines below the promoter schematics delineate the ETS-TF containing sites cloned into our luciferase reporter constructs.
Putative GABP elements activate BECN1-PIK3C3 complex gene promoters
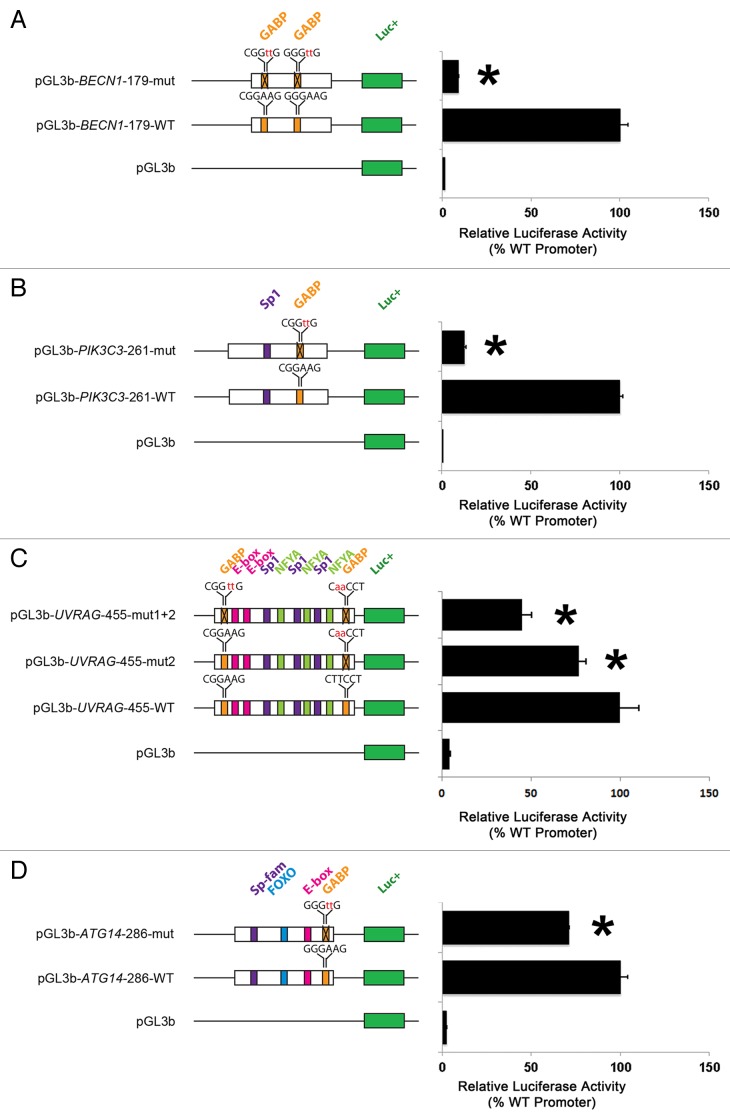
To address the functional significance of putative GABP elements in BECN1-PIK3C3 complex genes, we cloned highly conserved sequences from the promoter regions of BECN1, PIK3C3, UVRAG, and ATG14 into luciferase reporter constructs. We compared relative luciferase activities of each WT promoter-luciferase construct with constructs containing mutations in the GABP sites (Fig. 2). Mutations of the 2 GABP sites in the BECN1 promoter fragment (Fig. 2A) and of a single GABP site in the PIK3C3 promoter fragment (Fig. 2B) dramatically reduced luciferase activities by ~90% compared with the corresponding WT constructs, indicating that GABP elements are functional and responsible for most of the transcriptional activities in these 2 promoter fragments. Mutation of the 3′ GABP site in the UVRAG promoter decreased luciferase activity by ~25% compared with the WT promoter fragment, whereas mutation of both 5′ and 3′ GABP sites reduced luciferase activity by ~55% (Fig. 2C). Mutation of a single GABP site in the ATG14 promoter reduced luciferase activity by ~30% compared with the WT promoter (Fig. 2D). In summary, our data suggest that putative GABP elements activate the transcriptional activities of BECN1-PIK3C3 complex gene promoters.
Figure 2. Putative GABP sites activate transcriptional activities of BECN1-PIK3C3 complex gene promoters. (A–D) Promoter sequences containing GABP recognition sites were cloned into pGL3b luciferase reporter constructs. GABP sites are identified and mutated bases are highlighted in red lowercase. Mutations of GABP sites were associated with marked reductions in transcriptional activity of the BECN1 (A) and PIK3C3 (B) promoter constructs and moderate reductions in transcriptional activity of the UVRAG (C) and ATG14 (D) promoter constructs. *P < 0.05, compared with transcriptional activities of corresponding wild-type promoter constructs, ANOVAs with post-hoc Dunnett tests, n = 3 for each construct.
GABP binds to the promoter regions of autophagy regulatory genes and activates their transcription
To determine whether GABP interacts with the promoters of autophagy regulatory genes, we performed chromatin immunoprecipitation (ChIP) assays in cultured cells using an antibody to GABPA, the DNA binding subunit of GABP. These experiments confirmed that GABPA binds to the promoter regions of BECN1, PIK3C3, PIK3R4, ATG14, and UVRAG (Fig. 3A; Fig. S10A–S10C). ChIP experiments also demonstrated interaction of GABPA with the promoter regions of ATG5, ATG12, and ATG16L1 (Fig. S10D), suggesting that GABP also potentially regulates transcription of genes encoding the ATG12–ATG5-ATG16L1 complex. GABPA did not show significant binding to the promoter region of RAG2 (Fig. 3A; Fig. S10A), a previously reported negative control gene40 that is expressed in HeLa cells (Fig. 3B) and SH-SY5Y cells (Fig. S10E). Furthermore, we confirmed that GABPA binds to the Becn1 promoter in B103 rat neuroblastoma cells and cultured rat cortical neurons (Fig. S10B and S10C). To determine whether GABP regulates the expression of genes encoding BECN1-PIK3C3 complexes, we used siRNA oligonucleotides to knock down GABPA and then examined autophagy gene transcripts by RT-PCR. Seventy percent knockdown of GABPA mRNA levels was associated with significant reductions in BECN1, PIK3C3, UVRAG, ATG14, and PIK3R4 mRNA levels (Fig. 3B). Taken together, these data demonstrate that GABP binds to the promoter regions of autophagy regulatory genes and functions as a novel transcriptional activator of autophagy gene expression.

Figure 3. GABP binds to the promoter regions of BECN1-PIK3C3 complex genes and activates transcription. (A) ChIP assays performed in SH-SY5Y human neuroblastoma cells demonstrate immunoprecipitation of promoter DNA from BECN1-PIK3C3 complex genes with a GABPA antibody. The GABPA promoter was used as a positive control and the RAG2 promoter was employed as a negative control. Nonspecific rabbit IgG was used as an antibody control. H2O denotes the negative PCR controls. (B) GABPA knockdown with siRNA oligonucleotides significantly reduced BECN1-PIK3C3 complex mRNA transcript levels as determined by real-time RT-PCR. RAG2 mRNA levels were investigated as a negative control. *P < 0.05, compared with mRNA levels with scrambled control (siControl) oligonucleotide transfection, unpaired t tests, n = 4.
GABPA knockdown reduces autophagy
We performed western blots to determine whether siRNA-mediated knockdown of GABPA protein attenuates BECN1-PIK3C3 complex protein levels and autophagy. GABPA knockdown to approximately ~75% of control levels was associated with significant reductions in BECN1, PIK3C3, and UVRAG (Fig. 4A). Furthermore, the same cultures showed significantly decreased autophagosomal LC3-II levels and significantly increased levels of the autophagy substrate SQSTM1 (Fig. 4B), indicating reduced autophagy flux with GABPA knockdown. SQSTM1 transcript levels were unaffected by GABPA knockdown (Fig. S11A and S11B). Furthermore, GABPA knockdown reduced cytoplasmic autophagosomes and autolysosomes as detected with the mRFP-GFP-LC3 tandem reporter assay (Fig. 4C–E). Cell cycle arrest in G1 phase via double thymidine blockade did not recapitulate the inhibition of autophagy observed with GABPA knockdown (Fig. S12), suggesting that the reduction in autophagy observed with GABPA knockdown is not an artifact of cell cycle arrest. Taken together, our data show that GABP plays an important role in maintaining BECN1-PIK3C3 complex protein levels and driving basal autophagy.
Figure 4.siRNA-mediated knockdown of GABPA inhibits autophagy in HeLa cells. (A) GABPA knockdown with siRNA oligonucleotides was associated with reductions in BECN1, PIK3C3 and UVRAG levels in HeLa cells (immunoblots with 3 replicates per treatment group depicted on left; densitometric quantification of 6 replicates graphically depicted on right). *P < 0.05 compared with protein levels with scrambled control (siControl) oligonucleotide transfection, unpaired t tests. (B) Western blots demonstrated significantly increased SQSTM1 levels and decreased autophagosomal LC3-II levels following GABPA knockdown in HeLa cells (immunoblots with 2 replicates per treatment group depicted on left; densitometric quantification of 8 replicates graphically depicted on right). *P < 0.05 compared with protein levels with scrambled control (siControl) oligonucleotide transfection, unpaired t tests. (C) Deconvoluted fluorescence microscopic images of siControl and siGABPA-transfected HeLa cells transiently expressing the mRFP-GFP-LC3 tandem reporter. AVs, quantified as GFP and RFP colocalizing signals, and autolysosomes (ALs), quantified as RFP-positive signals, were both reduced with GABPA knockdown (D). * and **P < 0.05 compared with pcDNA vector control-transfected cells for colocalizing GFP-RFP puncta (AVs) and solitary RFP fluorescent puncta (ALs), respectively; unpaired t tests. (E) Western blot confirming GABPA knockdown and reduced LC3 and mRFP-GFP-LC3 shifts with siGABPA transfection.
GABP overexpression increases autophagy
To determine whether ectopic GABP increases autophagy, we transfected GABP expression constructs into HeLa cells and examined cellular markers of autophagy. GABPA overexpression and coexpression of GABPA and GABPB1 increased levels of the autophagosomal marker LC3-II and decreased levels of the autophagy substrate SQSTM1 (Fig. 5A–C). GABP-induced increases in autophagosomal LC3-II persisted following inhibition of AV maturation with bafilomycin A1, consistent with increased autophagy induction (Fig. 5D). GFP expression did not recapitulate the increase in autophagy observed with GABP expression (Fig. S13). GABP expression also increased cellular levels of PIK3C3, BECN1, and UVRAG (Fig. 5E). Furthermore, GABP expression increased transcriptional activity of the wild-type BECN1 luciferase reporter construct but had no effect on the transcriptional activity of the GABP mutant BECN1 reporter construct (Fig. 5F).
Figure 5. GABP overexpression upregulates autophagy induction, autophagic flux, and expression of BECN1-PIK3C3 complexes. (A) A western blot demonstrates GABPA and GABPB1 overexpression in HeLa cells. (B and C) GABP overexpression was associated with increased autophagosomal LC3-II and reduced levels of the autophagy substrate SQSTM1. Overexpression of BECN1 was employed as a positive control to increase SQSTM1 degradation. *P < 0.05 compared with pcDNA vector control, ANOVA with post-hoc Dunnett test, n = 12. (D) GABP induced increases in LC3-II persisted during treatment with 10 nM bafilomycin A1, an inhibitor of autophagosome-lysosome fusion, suggesting that GABP overexpression increases autophagy induction. (E) GABP overexpression was associated with increases in the levels of PIK3C3, BECN1, and UVRAG. *P < 0.05 compared with pcDNA vector control. ANOVA with post-hoc Dunnett test, n = 4. (F) GABP overexpression increased the transcriptional activity of the BECN1 luciferase reporter construct but had no impact on transcriptional activity following mutations of the GABP binding sites in the BECN1 promoter. *P < 0.05 GABP(A+B1) compared with pcDNA vector control transfection, unpaired t tests, n = 3.
Overexpression of GABPA or GABPB1 also increased GFP-LC3 labeled AVs as detected by fluorescence microscopy (Fig. 6A and B) and by western blot analysis of GFP-LC3 (Fig. 6C). Increased GFP-LC3 labeled AVs were also observed with GABPA or GABPB1 overexpression in stable GFP-LC3-expressing HeLa cells (Fig. S14). Furthermore, GABP-induced increases in autophagosomes and autolysosomes were detected in tandem reporter experiments by fluorescence microscopy (Fig. 6D and E) and by western blot detection of ectopic mRFP-GFP-LC3 (Fig. 6F). Transmission electron micrographs of HeLa cells cotransfected with GABPA and GABPB1 expression constructs confirmed the increase in cytoplasmic autolysosomes seen with fluorescence microscopy reporter experiments (Fig. 7A and B). Interestingly, we detected a decrease in mature endosomes in GABP-overexpressing HeLa cells (Fig. 7C) and a decrease in RAB5-positive early endosomes in HeLa cells with GABPA siRNA knockdown (Fig. S15). These results suggest that GABP-induced alterations in BECN1-PIK3C3 levels impact autophagy more specifically than endosomal trafficking. In summary, these data demonstrate that GABP augments the expression of genes encoding BECN1-PIK3C3 complexes and autophagy.
Figure 6. GABP overexpression increases GFP-LC3-labeled autophagosomes. (A) Representative images of HeLa cells cotransfected with GFP-LC3 and either pcDNA vector, GABPA, GABPB1, or BECN1 overexpression vectors are shown. Cells overexpressing GABPA, GABPB1, or BECN1 demonstrate increased punctate GFP-LC3-labeled autophagosomes (quantified in B). *P < 0.05 compared with pcDNA vector control, ANOVA with post-hoc Dunnett test. (C) A GFP-LC3 immunoblot performed on HeLa lysates confirmed increased levels of autophagosomal GFP-LC3-II in cultures cotransfected with GABPA, GABPB1, or BECN1. (D) Representative images of tandem-reporter mRFP-GFP-LC3-transfected HeLa cells cotransfected with GABPA or GABPB1 overexpression vectors demonstrate increased punctate autophagosomes (AVs) and autolysosomes (ALs) compared with pcDNA vector-transfected cells (quantified in E). * and **P < 0.05 compared with pcDNA vector control-transfected cells for colocalizing GFP-RFP puncta (AVs) and solitary RFP fluorescent puncta (ALs), respectively; ANOVAs with post-hoc Dunnett tests. (F) An LC3 immunoblot demonstrates increased levels of tandem reporter mRFP-GFP-LC3-II in GABP-transfected cells consistent with increased autolysosomes.

Figure 7. GABP overexpression increases autolysosomes but not mature endosomes. (A) Representative transmission electron microscopy images show that overexpression of GABPA and GABPB1 in HeLa cells evoked increases in cytoplasmic autolysosomes (ALs). Black arrows identify autolysosomes, white arrows identify late endosomes and white arrowheads identify multivesicular bodies. (B) Cumulative data demonstrate an increase in autolysosomes with GABP overexpression. *P < 0.05 compared with pcDNA vector control, unpaired t tests. (C) Cumulative data demonstrate no increase, but rather a decrease, in mature endosomal structures with GABP overexpression. *P < 0.05 compared with pcDNA vector control, unpaired t tests.
Nutrient starvation increases GABP-dependent autophagy gene promoter activity
To determine whether GABP upregulates autophagy gene transcription during cell stress, we subjected B103 cells expressing wild-type or mutant luciferase reporter constructs (Fig. 2) to nutrient starvation. We observed robust increases in the transcriptional activities of BECN1, PIK3C3, UVRAG, and ATG14 wild-type reporter constructs during incubation in HBSS (Fig. 8A). GABP binding site mutations markedly blunted the baseline transcriptional activities of BECN1 and PIK3C3 reporter constructs and rendered them essentially unresponsive to nutrient starvation. GABP binding site mutations resulted in more modest attenuations of baseline transcriptional activity of UVRAG and ATG14 reporter constructs. GABP binding site mutations blunted the response of the UVRAG promoter, but not the ATG14 promoter, to nutrient starvation. Furthermore, we observed an increase in GABPA recruitment to the endogenous BECN1 promoter with nutrient starvation via ChIP assays (Fig. 8B). These data suggest that, in addition to the maintenance of baseline BECN1-PIK3C3 complex gene transcriptional activity, GABP plays a role in upregulating transcriptional activity of BECN1-PIK3C3 complex genes during nutrient starvation.
Figure 8. Nutrient starvation increases GABP-dependent transcriptional activities of BECN1-PIK3C3 complex gene promoters. (A) Luciferase assays demonstrate robust increases in transcriptional activity of BECN1, PI3KC3, ATG14, and UVRAG promoters during nutrient starvation (HBSS). HBSS-induced increases in BECN1, PI3KC3, and UVRAG transcriptional activities were markedly attenuated in promoter constructs with mutated GABP binding sites. The HBSS-induced increase in transcriptional activity of the ATG14 promoter, which also contains FOXO and E-box sequences, was not eliminated by mutation of the GABP binding site. *P < 0.05, comparisons of normal media and HBSS conditions for each promoter; ns, not significant; unpaired t tests with Bonferroni corrections for multiple comparisons. (B) Semiquantitative BECN1 promoter ChIP assay using a GABPA antibody demonstrates an increase in GABPA binding to the endogenous BECN1 promoter during nutrient starvation. BECN1 promoter DNA was not immunoprecipitated with a nonspecific IgG. Negative control RAG2 promoter DNA was not immunoprecipitated by the GABPA antibody under control or nutrient starvation conditions. *P < 0.05, comparison of normal media and HBSS conditions for BECN1 promoter ChIP with the GABPA antibody, unpaired t test.
Discussion
Autophagy is a complex vesicular trafficking pathway for autolysosomal degradation. Autophagy dysregulation, including impaired autophagy initiation6,7,49 and impaired maturation,50,51 evokes detrimental consequences for cells. Accordingly, autophagy is tightly regulated at multiple levels, including autophagosome formation, autophagosome trafficking, and upstream signaling pathways that respond to nutrients, growth factors, and stressors.21 The induction, formation, and trafficking of AVs is regulated by several macromolecular regulatory protein complexes, including the BECN1-PIK3C3 complex. In this study, we found that the ETS-TF GABP augments the combinatorial expression of autophagy genes encoding the BECN1-PIK3C3-PIK3R4 core complex, genes encoding the BECN1 binding proteins ATG14 and UVRAG, and autophagy. Furthermore, the impairment of autophagy with GABPA knockdown suggests that GABP plays an important role in the basal maintenance of autophagic machinery and capacity. Finally, we observed GABP-dependent increases in transcriptional activity of autophagy gene reporter constructs and increased GABP recruitment to the endogenous BECN1 promoter during nutrient starvation, suggesting a novel role for GABP in the regulation of BECN1-PIK3C3 complex gene expression during cellular stress.
Autophagy gene transcription is an important factor in the regulation of autophagy and autophagy capacity; however, autophagy transcriptional regulatory mechanisms are incompletely understood.21 Recent studies have identified E2F1,24 FOXOs,28,52 TFEB,22 and ZKSCAN323 as TFs that regulate the expression of autophagy genes. However, TFs that regulate the combinatorial expression of autophagy initiation complex genes are unknown. In our study, we found that the ETS-TF consensus binding element, which is present in approximately 7% of human gene promoters,53 is ubiquitously found in the 5′ regulatory regions of BECN1-PIK3C3 complex genes, including BECN1, PIK3C3, UVRAG, ATG14, and PIK3R4, and regulates the expression of these genes. Additionally, we found that GABP binds to the promoter regions of genes encoding the downstream ATG12–ATG5-ATG16L1 complex. Furthermore, our analysis of GABP ChIP-seq data from the ENCODE project in the UCSC genome browser54 revealed that GABP binds to 22 of 58 (38%) of analyzed autophagy-related genes (Table S2), indicating that GABP may serve as master regulatory transcription factor of autophagy.
Several TFs have been previously implicated in the transcriptional regulation of genes encoding subunits of the BECN1-PIK3C3 complex, including NFKB26 in the BECN1 promoter, TFEB in the UVRAG promoter22 and FOXO3 and CLOCK-ARNTL/BMAL1 in the ATG14 promoter.28 The presence of an E-box element55 in the ATG14 promoter raises the possibility of regulation by TFEB. Additionally, our bioinformatics analyses implicate SP1 and NFYA, which are transcriptional coactivators,53,56 with possible roles in the regulation of UVRAG transcription. However, none of these transcription factors demonstrate the combinatorial overlap in BECN1-PIK3C3 complex gene regulation we have documented for GABP. The combinatorial regulation of autophagy gene expression by GABP likely augments its impact on autophagy.
Although our TRANSFAC analyses rendered GABP as the only ubiquitous likely ETS-TF for the regulation of BECN1-PIK3C3 complex genes, the possibility that other ETS-TFs may also regulate expression of these autophagy genes remains to be explored. Furthermore, the possibility that GABP itself might have diverse effects on autophagy gene transcription is intriguing. GABP is a unique multimeric member of the ETS-TF family consisting of 2 subunits, the DNA binding subunit GABPA and the transactivation subunit GABPB.29,30 In humans, the GABPA subunit is encoded by GABPA on chromosome 21 and the GABPB subunit is encoded either by GABPB1 on chromosome 15 or by GABPB2 on chromosome 1.57 Structural heterogeneity of the GABPB subunit secondary to alternative splicing has an impact on GABP heterodimer formation and its interactions with transcriptional cofactors.36,57,58 Thus, GABP and other ETS-TFs may have a broad repertoire of regulatory effects on the transcriptional output of autophagy genes.
GABP regulates the expression of genes involved in the differentiation of hematopoietic cells,35-40 skeletal muscle,41-46 and adenohypophysis.33,34 Furthermore, GABP regulates the expression of several nuclear-encoded mitochondrial genes31,32 and plays a role in mitochondrial dynamics during embryogenesis.59 Autophagic proteolysis also plays important roles in cell remodeling.60 GABP therefore might also facilitate cell differentiation by increasing the expression of autophagy initiation complexes. Furthermore, GABP, which promotes cell cycle entry into S-phase via interaction with E2F and upregulation of DNA synthesis genes,47 might contribute to autophagy activation during G1 and S-phases61 and inhibition during the G2/M phases.61,62 The possibility that GABP regulates autophagy during these contexts will require further investigation.
Autophagy is generally thought to protect cells from diverse stressors, including toxic protein aggregates, injured mitochondria, and infectious organisms. Furthermore, autophagy deficiency renders cells more vulnerable to stress and is implicated in nervous system aging and neurodegeneration. For example, autophagy deficiencies in Atg5 and Atg7 knockout mice are associated with precipitous neurodegeneration.6,7,49 Deficiencies of BECN1 and PIK3C3 are associated with nervous system aging and Alzheimer disease.14,18,63 Cleavage of BECN1 by caspases may contribute to BECN1 deficiency in AD.64 Augmentation of autophagy capacity by BECN1 overexpression has been proposed as a therapeutic strategy and has shown promising results for the clearance of amyloid-β pathology,65 α-synuclein,17 and huntingtin.18 The possibility that GABP and other transcription factors may be leveraged to therapeutically augment Beclin-dependent autophagy underlies the importance of understanding the transcriptional regulation of autophagy.
In conclusion, our study is the first to identify a role of the ETS-TF GABP in regulating the combinatorial expression of autophagy initiation genes. We showed that GABP activates the transcription of genes encoding BECN1-PIK3C3 complexes at baseline and during nutrient starvation. Furthermore, we demonstrated that GABP deficiency reduces autophagy whereas GABP overexpression increases autophagy. Our work identifies a novel role for GABP in autophagy gene transcription and highlights ETS-TF and GABP-mediated gene regulation as a potential target to therapeutically augment autophagy.
Materials and Methods
All animal procedures were designed in accordance with the Guidelines for Animal Use of the Stanford University and were approved by the Institutional Animal Care and Use Committee (Protocol 26911).
Bioinformatics and phylogenetic analysis
Sequences for human BECN1, PIK3C3, UVRAG, ATG14, PIK3R4, ATG5, ATG12, and ATG16L1 were obtained from the Ensembl database (www.ensembl.org). A search for the core consensus binding sequence for ETS-TF, “5′-SGGAAG-3′,” was performed 2 kb upstream of transcriptional start site (TSS) and at least 1 kb into intron 1 of each gene. When the ETS-TF consensus sequence (5′-SGGAAG-3′) was found in the promoters of human autophagy genes, similar searches were performed in rat and mouse genes. When corresponding ETS-TF consensus sequences were found in all three species, human promoter sequences were queried in the whole genome shotgun (WGS) database to retrieve sequences from other mammalian species. Next, 5′ regulatory regions of mammalian autophagy genes were aligned using Clustal W2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html) and adjusted manually to maximize parsimony. Finally, putative ETS-TF binding sites were analyzed via matrix alignment using the TRANSFAC database to reveal likely candidate ETS-TFs for each promoter.66
Cell culture, transfection, and drug treatments
HeLa cells, B103 rat neuroblastoma cells, and SH-SY5Y (ATCC, CRL2266) human neuroblastoma cells were grown to 70% to 80% confluence in DMEM (Corning, 10-017-CV) with 10% Fetal Bovine Serum (Lonza, 14-502F) and 1% Pen-Strep (Corning, 30-002-Cl)] and supplemented with 20 mM L-glutamine (SH-SY5Y; Gibco, 25030-081) and 10 mM HEPES (SH-SY5Y; Gibco, 15630–080). B103 cells were supplemented with 5% Horse Serum (Corning, 35-030-CV). Cells were maintained at 37 °C in 5% CO2. E16 rat cortical neuron cultures were maintained at 37 °C with 5% ambient CO2 in Neurobasal medium (Invitrogen, 21103-049) supplemented with B27 (Invitrogen, 17504-044) and 1% Glutamax-I (Invitrogen, 35050-061). Cell lines were transfected with overexpression constructs using Lipofectamine LTX (Invitrogen, 15338100) or with siRNA oligonucleotides using RNAiMAX (Life Technology, 13778075) according to the manufacturer’s instructions. The following expression constructs were employed: GABP (pCAGGS-GABPA and pCAGGS-GABPB1), pEGFP-LC3,67 mRFP-GFP-LC3 (tandem reporter),68 pcDNA4-BECN1-FLAG69 and pRK7-GFP. Stably transfected HeLa-GFP-LC3 were generated under G418 (250 μg/ml, Gibco, 10131-035) selection. Cells in which GABP knockdown was induced with 100 nM OnTarget SmartPool siGABPA (Dharmacon, Fisher Scientific, L-011662-00-0020) were compared with cells transfected with scrambled siRNA control oligonucleotide pool (Dharmacon, Fisher Scientific, D-001810-10-20). To induce nutrient starvation, cells were incubated in Hank's balanced salt solution (HBSS, Gibco, 24020–117) for 3 h. To inhibit AV fusion with lysosomes, cultures were treated with 10 nM bafilomycin A1 (Sigma, B1793) in DMSO (Sigma, 276855) for 5 h. To induce cell cycle arrest, normal media was replaced with media containing 2.5 mM thymidine (Fisher Scientific, TCI, T0233) according to the protocol of Whitfield and colleagues.70
Luciferase reporter assays
Proximal promoter regions from BECN1, PIK3C3, UVRAG, and ATG14 were cloned from human SH-SY5Y DNA using Pfu Ultra High Fidelity DNA polymerase (Agilent Technologies, 600385-51) and the following primers (enzyme digestion sites underlined and mutated sites in lowercase, Fig. 1 and Fig. 2, and Figs. S1–S4): BECN1-179-WT [promoter region of human BECN1, a 179-bp fragment from −47 to +132 (transcript ID: ENST00000590099) relative to transcription start site (TSS, +1])]: 5′-AATTCTCGAG GTCACGTCCG GTCTCG-3′ (F) and 5′-TCGTAAGCTTCCTCAGCCCC CGATGCTC-3′ (R); PIK3C3-261-WT [promoter region of human PIK3C3, a 261-bp fragment from −180 to +81 (transcript ID: ENST00000262039) relative to TSS]: 5′-AATTCTCGAGGACAGGAAAA CTGGAATGTA AC-3′ (F) and 5′-TCGAAAGCTTCGTCTGCAAA GGTACCACCT AC-3′ (R); UVRAG-455-WT [promoter region of human UVRAG, a 455-bp fragment from −390 to +65 (transcript ID: ENST00000356136) relative to TSS]: 5′-AATTCTCGAGCAACCAGTGA GGGCGGAAGT G-3′ (F) and 5′-TCGAAAGCTTGCTAAGGAAG AGCCATATTA CCG-3′ (R); and ATG14-286-WT [promoter region of human ATG14, a 286-bp fragment from −154 to +132 (transcript ID: ENST00000247178) relative to TSS]: 5′-AATTCTCGAG CTGCTTCGGG ACCTGTTGCC-3′ (F) and 5′-TCGAAAGCTT ATCGTCCACG GAGTCCACCA G-3′ (R). Mutations of putative GABP binding sites48 were made either in the cloning primers or by synthesizing mutant promoter regions (Integrated DNA Technologies, Inc., Fig. 2). PCR products were digested with XhoII (New England Biolabs, R0146S) and HindIII (New England Biolabs, R0104S), and ligated into the pGL3basic vector by a T4 ligase (New England Biolabs, M0203S). All wild-type (WT) and mutant inserts were confirmed with DNA sequencing. pGL3b generated constructs were cotransfected with pRL-TK into B103 rat neuroblastoma cells. Cells were lysed 24 h after transfection with passive lysis buffer (Promega, E1910). Luciferase activity was measured using a Dual-luciferase reporter assay (Promega, E1910) on a Lumat LB 9507 luminometer (Berthold Technology, Germany). For each sample, the firefly luciferase activity was normalized to the Renilla luciferase activity. Each experiment contained 3 replicates per luciferase reporter construct. Experiments were repeated for reproducibility.
Chromatin immunoprecipitation (ChIP)
Cells were fixed with 4% formaldehyde, diluted from 37% formaldehyde solution (LabChem Inc., Thermo Fisher, LC14650-2), scraped and collected. Protein-DNA complexes were extracted, sonicated, immunoprecipitated and eluted with a Chromatin Immunoprecipitation Assay Kit (Millipore, 17-295) according to the manufacturer’s instructions using a GABPA antibody (H-180X, Santa Cruz Biotechnology, sc-22810). A nonspecific rabbit IgG (Millipore, PP64) was employed as a negative immunoprecipitation control. Immunoprecipitated genomic DNA was recovered and used in polymerase chain reactions (PCR) with GoTaq DNA polymerase (Promega, M3005) and the following primers flanking GABP sites in the corresponding promoters: GABPA promoter (employed as a positive control40: 5′-ACGCCTACCC GCCATCGCAA TG-3′ (F) and 5′-GAGCTTGAAC TAGGGGAAAG GC-3′ (R); RAG2 promoter (employed as a negative control40: 5′-CATTGTTGCT AGTAGTGAAA GAG-3′ (F) and 5′-GTGGCAAGCA GAAGGCTGAC TG-3′ (R); BECN1 promoter: 5′-GTGAGCCTGT GGACCAGGAG-3′ (F) and 5′-AGCCGTGAGG GTTCCCAGAC-3′(R); PIK3C3 promoter: 5′-TGGAATGTAA CCAGATTAGC CG-3′ (F) and 5′-GGGAACTTAG GTACAGGAAA AAC-3′ (R); UVRAG promoter: 5′-GGGAAAAGAC TGCACAAGGG-3′ (F) and 5′-CGGGAGAGTC ACCTGATTAG G-3′ (R); ATG14 promoter: 5′-CGGTTGAAAA CAAAATCCCA CG-3′ (F) and 5′-CGCATCGTCC ACGGAGTCCA C-3′ (R); PIK3R4 promoter: 5′-GCCCAAAGTA AGACCACGC-3′ (F) and 5′-AGAGAAGTGC AGACCGCC-3′ (R); ATG5 promoter: 5′-ACGTGGGGTC TCGTGACGT-3′ (F) and 5′-CGCCTGACAC ACTGTCCTG-3′ (R); ATG12 promoter: 5′-GATTTGAATG ACTAGCCG-3′ (F) and 5′-CATCTTGCTT GGAGACACTC G-3′ (R), and ATG16L1 promoter: 5′-ACGCTTCCGG CTACAGGCTG-3′ (F) and 5′-TCACCTCCAC ACACTGGCAG TC-3′ (R). PCR products were analyzed by agarose gel electrophoresis. Real-time PCR using Power SYBR Green reagent (Life Technology, 4367659) was also employed to analyze BECN1 promoter DNA immunoprecipitation.
RNA extraction and RT-PCR analysis
Total RNA was extracted from cells using TRIzol (Life Technologies, 15596-018) according to the manufacturer’s instructions. Reverse transcription was performed using QuantiTect Reverse Transcription Kit (Qiagen, 205310). Real-time PCR was performed using SYBR Green reagent (POWER SYBR GREEN, Life Technologies, 4367659) and primer sets as follows: GAPDH: 5′-TCACCAGGGC TGCTTTTAAC TC-3′ (F) and 5′-ATGGGTGGAA TCATATTGGA AC-3′ (R); GABPA: 5′-GCCTTGGGAT ACCCTATGAT C-3′ (F) and 5′- TGAGTGTGGT GAGGTCTATA TC-3′ (R); BECN1: 5′-GATTCATCCC CCCAGCCAGG-3′ (F) and 5′-CCCAGTGACC TTCAGTCTTC G-3′ (R); PIK3C3: 5′-AACTGGAATG AATGGCTGAA AC-3′ (F) and 5′-GCGAAACCGT TGTTCCTCCT AC-3′ (R); ATG14: 5′-GGGAGAGGTT TATCGACAAG AAGG-3′ (F) and 5-TATTTTCCAT CTCAACTGAT CTG-3′ (R); UVRAG: 5′-GAACATAAGG GTTATTCAAA TGC-3′ (F) and 5′-GTTACCTGAG TCTGTTTAAT TGC-3′ (R); PIK3R4: 5′-GGACATGAGG TTCCAGTTGC-3′ (F) and 5′-TTGTTGCCCT GAACAGCTGC-3′ (R); CCND1: 5′-GAACACTTCC TCTCCAAAAT G-3′ (F) and 5′-GGAAATGAAC TTCACATCTG TG-3′ (R); RAG2: 5′-GGTGTTCTCT TTGGAGGACG-3′ (F) and 5′-TCAGCTACAC TATTCCATTT TTC-3′ (R). Experiments were performed with a real-time PCR machine (Applied Biosystems StepOne PLUS, Life Technologies, Grand Island, NY, USA). For each sample, ∆CT value was obtained by normalizing each gene of interest against GAPDH in each assay. The relative ∆∆CT value was obtained by subtracting the ∆CT values for each sample from the ∆CT values of GAPDH in each corresponding assay. All ∆∆CT values were converted to fold change. Data was compiled from 4 independent cultures for each treatment. Three technical RT-PCR replicates were performed for each cDNA sample. Finally, for the analysis of SQSTM1 transcript levels, semiquantitative PCR was performed on 1 µl cDNA samples using the following primers: F: 5′-TGAGGAAGAT CGCCTTGGAG-3′ and R: 5′-ACAGATGGGT CCAGTCATCA TC-3′.
Western blot
Total protein was extracted 24 to 48 h following transfection using HEPES buffer [20 mM HEPES (Gibco, 15630-080), 150 mM NaCl, 1 mM EDTA and 1% Triton X-100 (Sigma, T8787)] or modified RIPA buffer [50 mM Tris, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.5% deoxycholate (Sigma, D6750), 0.1% SDS (Amresco, 0837)] supplemented with a protease inhibitor cocktail (Sigma, P8340). Protein quantification was performed via the Bradford method (Bio-Rad, 500-0006) using a BioTek Epoch 96-well microplate reader (BioTek, Winooski, VT, USA). Protein samples were loaded into 4–15% Tris-Glycine gels (Bio-Rad, 456-1085 and 456-1086) and transferred onto PVDF membranes (Immobilon-P, Millipore, IPVH00010). Immunoblotting was performed using primary antibodies for BECN1 (Cell Signaling Technology, 3495S), GABPA (Santa Cruz Biotechnology, sc-22810), PIK3C3 (Cell Signaling Technology, 4263S), UVRAG (Medical and Biological Laboratory Co, Ltd, M160-3), MAP1LC3 (NanoTools, 026-100), SQSTM1/p62 (Cell Signaling Technology, 5114S), GABPB1 (Abcam, ab90861), GFP (Millipore, MAB2510), FLAG (Sigma, F3165), GAPDH (Abcam, ab9485) and ACTB/β-actin (Cell Signaling Technology, 4970). Blots were then labeled with secondary antibodies: HRP-conjugated anti-mouse IgG (GE Healthcare, NXA-931) or HRP-conjugated anti-rabbit IgG (GE Healthcare, NA934V). Immunoreactive bands were detected by exposure of X-ray film (Denville, E3018) following incubation of blots with ECL Prime chemiluminesence reagents (GE Healthcare, RPN2236). Densitometry was performed using NIH ImageJ software.
Immunofluorescence staining and fluorescence microscopy
Cell cultures were grown in 4-well Lab-Tek coverglass chambers (growth area 1.8 cm2, Thermo Scientific Nunc, 155382). Cultures were fixed with 4% paraformaldehyde in phosphate-buffered saline (Corning Cellgro, 21-031-CV), rinsed and permeabilized with 0.1% Triton-X in PBS. Cultures were then blocked with 5% bovine serum albumin (Fisher Scientific, BP1600-100), incubated with RAB5 antibody (1:200; Santa Cruz Biotechnology, sc-28570) and stained with Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies, A11008).
Stained cultures were imaged with a Leica DMI6000 wide-field fluorescence microscope (Leica Microsystems, Buffalo Grove, IL). Z-stacks from 15 random medium power fields (200×) containing 4 to 6 transfected cells were acquired from the central portion of the coverglass chambers. Z-stacks were processed with Leica 3-D deconvolution software and colocalizing events were detected with the colocalization module (Leica Advanced Fluorescence Application Suite). The central image from each deconvoluted stack was then used to quantify fluorescent puncta in a plane that included the center of the nucleus. All transfected cells were analyzed in all images, rendering data from 180 to 320 cells per experiment. Images were analyzed with NIH ImageJ using the Analyze Particles function after fluorescence signals were thresholded at the mean background cytoplasm signal + 4× the standard deviation of the background cytoplasm signal.
Transmission electron microscopy
Cell cultures were fixed in 3% glutaraldehyde/4% paraformaldehyde (Electron Microscopy Sciences, 16020 and 15710) in 0.1 M sodium cacodylate buffer (pH7.4) (Electron Microscopy Sciences, 11650) and post fixed in 1% osmium tetroxide (Electron Microscopy Sciences, 19110). Cultures were then scraped with a rubber soldier, pelleted, and re-suspended in 10% gelatin in PBS. Cells were then pelleted again and stained in cold 1% uranyl acetate (Electron Microscopy Sciences, 22400) overnight. Samples were then dehydrated in a series of ethanol washes and acetonitrile, infiltrated with EMbed-812 resin (Electron Microscopy Sciences, 14120) and then placed into molds filled with fresh resin and polymerized overnight. Sections cut at 75 nm on a Leica Ultracut S (Leica, Wetzlar, Germany) were picked up on formvar/carbon coated grids (100 mesh copper; Electron Microscopy Sciences, FCF100-Cu-50) and contrast stained in 3.5% uranyl acetate in 50% acetone followed by staining in 0.2% lead citrate (Electron Microscopy Sciences, 17900) for 3 min. Grids were observed in a JEM-1400 transmission electron microscope (JEOL USA, Peabody, MA) at 120kV. Photos were taken at 1500× and 4000× using a Gatan Orius digital camera. AVs, autolysosomes (ALs), multivesicular bodies and late endosomes were quantified from 50 consecutive cells in each treatment group.71 Quantifications were performed blind to the treatment group. These transmission electron microscopy experiments were performed with the assistance of the Cell Sciences Imaging Facility at Stanford University.
Statistical analyses
All data are expressed as mean ± standard errors (SEM). Data from multiple treatment groups were compared with ANOVAs. Data from experimental groups were compared with control groups using post-hoc 2-tailed Dunnett tests. Data from dual treatment groups were compared with unpaired Student t tests. P < 0.05 was accepted as significantly different. Statistical analyses were performed with the SYSTAT version 13 software suite.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the American Federation for Aging Research (RAG12401, New Investigator in Alzheimer Disease Award, EDP) and by the National Institutes of Health (NS085324, EDP). The transmission electron microscope used in these studies was provided with support from Shared Instrumentation Grant 1S10RR02678001 from the National Institutes of Health. We thank Dr Tony Wyss-Coray for providing B103 rat neuroblastoma cells, Dr Alex Lee for providing pGL3basic and pRL-TK vectors, Dr Alan R Rosmarin for providing the GABPA and GABPB1 expression constructs, Dr Tamotsu Yoshimori for providing pEGFP-LC3 and mRFP-GFP-LC3 tandem reporter constructs and Dr Qing Zhong for providing pcDNA4-FLAG-BECN1 construct.
Glossary
Abbreviations:
- ACTB
actin, beta isoform
- AL
autolysosome
- ATG5
autophagy-related 5
- ATG12
autophagy-related 12
- ATG14
autophagy-related 14
- ATG16L1
autophagy-related 16-like 1
- AV
autophagic vacuole
- BECN1
Beclin 1, autophagy related
- ChIP
chromatin immunoprecipitation
- ETS-TF
ETS-domain-transcription factor
- GABP/NRF2
GA binding protein transcription factor composed of GABPA and GABPB
- GABPA
GA binding protein transcription factor, alpha subunit 60 kDa
- GABPB1
GA binding protein transcription factor, beta subunit 1
- GFP
green fluorescent protein
- MAP1LC3 (LC3)
microtubule-associated protein 1 light chain 3
- mRFP
monomeric red fluorescent protein
- Sp-fam
Sp-family of transcription factors
- SQSTM1/p62
sequestosome 1
- PCR
polymerase chain reaction
- PIK3C3/VPS34
phosphatidylinositol 3-kinase, catalytic subunit type 3
- PIK3R4/VPS15
phosphoinositide-3-kinase, regulatory subunit 4
- UVRAG
UV radiation-resistance associated
- WT
wild type
References
- 1.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumpter R, Jr., Levine B. Autophagy and innate immunity: triggering, targeting and tuning. Semin Cell Dev Biol. 2010;21:699–711. doi: 10.1016/j.semcdb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2012;8:108–17. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]
- 4.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, et al. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:14164–9. doi: 10.1073/pnas.1009485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 8.Tassa A, Roux MP, Attaix D, Bechet DM. Class III phosphoinositide 3-kinase--Beclin1 complex mediates the amino acid-dependent regulation of autophagy in C2C12 myotubes. Biochem J. 2003;376:577–86. doi: 10.1042/BJ20030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–5. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy. 2005;1:46–52. doi: 10.4161/auto.1.1.1542. [DOI] [PubMed] [Google Scholar]
- 11.Matsunaga K, Morita E, Saitoh T, Akira S, Ktistakis NT, Izumi T, Noda T, Yoshimori T. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J Cell Biol. 2010;190:511–21. doi: 10.1083/jcb.200911141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–99. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 13.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–20. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–9. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaeger PA, Pickford F, Sun CH, Lucin KM, Masliah E, Wyss-Coray T. Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One. 2010;5:e11102. doi: 10.1371/journal.pone.0011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucin KM, O’Brien CE, Bieri G, Czirr E, Mosher KI, Abbey RJ, Mastroeni DF, Rogers J, Spencer B, Masliah E, et al. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer’s disease. Neuron. 2013;79:873–86. doi: 10.1016/j.neuron.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–88. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T, MacDonald M, Yankner B, Yuan J. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281:14474–85. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 19.Wu JC, Qi L, Wang Y, Kegel KB, Yoder J, Difiglia M, Qin ZH, Lin F. The regulation of N-terminal Huntingtin (Htt552) accumulation by Beclin1. Acta Pharmacol Sin. 2012;33:743–51. doi: 10.1038/aps.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–6. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–33. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan S, Goodwin JG, Chauhan S, Manyam G, Wang J, Kamat AM, Boyd DD. ZKSCAN3 is a master transcriptional repressor of autophagy. Mol Cell. 2013;50:16–28. doi: 10.1016/j.molcel.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polager S, Ofir M, Ginsberg D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene. 2008;27:4860–4. doi: 10.1038/onc.2008.117. [DOI] [PubMed] [Google Scholar]
- 25.Artal-Martinez de Narvajas A, Gomez TS, Zhang JS, Mann AO, Taoda Y, Gorman JA, Herreros-Villanueva M, Gress TM, Ellenrieder V, Bujanda L, et al. Epigenetic regulation of autophagy by the methyltransferase G9a. Mol Cell Biol. 2013;33:3983–93. doi: 10.1128/MCB.00813-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copetti T, Bertoli C, Dalla E, Demarchi F, Schneider C. p65/RelA modulates BECN1 transcription and autophagy. Mol Cell Biol. 2009;29:2594–608. doi: 10.1128/MCB.01396-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinmann AS, Bartley SM, Zhang T, Zhang MQ, Farnham PJ. Use of chromatin immunoprecipitation to clone novel E2F target promoters. Mol Cell Biol. 2001;21:6820–32. doi: 10.1128/MCB.21.20.6820-6832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong X, Tao R, DePinho RA, Dong XC. The autophagy-related gene 14 (Atg14) is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism. J Biol Chem. 2012;287:39107–14. doi: 10.1074/jbc.M112.412569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–37. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 30.Sawa C, Goto M, Suzuki F, Watanabe H, Sawada J, Handa H. Functional domains of transcription factor hGABP beta1/E4TF1-53 required for nuclear localization and transcription activation. Nucleic Acids Res. 1996;24:4954–61. doi: 10.1093/nar/24.24.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blesa JR, Hernández-Yago J. Distinct functional contributions of 2 GABP-NRF-2 recognition sites within the context of the human TOMM70 promoter. Biochem Cell Biol. 2006;84:813–22. doi: 10.1139/o06-064. [DOI] [PubMed] [Google Scholar]
- 32.Virbasius JV, Virbasius CA, Scarpulla RC. Identity of GABP with NRF-2, a multisubunit activator of cytochrome oxidase expression, reveals a cellular role for an ETS domain activator of viral promoters. Genes Dev. 1993;7:380–92. doi: 10.1101/gad.7.3.380. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang L, Jacob KK, Stanley FM. GABP mediates insulin-increased prolactin gene transcription. J Biol Chem. 1996;271:10425–8. doi: 10.1074/jbc.271.18.10425. [DOI] [PubMed] [Google Scholar]
- 34.Schweppe RE, Gutierrez-Hartmann A. Pituitary Ets-1 and GABP bind to the growth factor regulatory sites of the rat prolactin promoter. Nucleic Acids Res. 2001;29:1251–60. doi: 10.1093/nar/29.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang ZF, Drumea K, Cormier J, Wang J, Zhu X, Rosmarin AG. GABP transcription factor is required for myeloid differentiation, in part, through its control of Gfi-1 expression. Blood. 2011;118:2243–53. doi: 10.1182/blood-2010-07-298802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuchprayoon I, Simkevich CP, Luo M, Friedman AD, Rosmarin AG. GABP cooperates with c-Myb and C/EBP to activate the neutrophil elastase promoter. Blood. 1997;89:4546–54. [PubMed] [Google Scholar]
- 37.Bush TS, St Coeur M, Resendes KK, Rosmarin AG. GA-binding protein (GABP) and Sp1 are required, along with retinoid receptors, to mediate retinoic acid responsiveness of CD18 (beta 2 leukocyte integrin): a novel mechanism of transcriptional regulation in myeloid cells. Blood. 2003;101:311–7. doi: 10.1182/blood.V101.1.311. [DOI] [PubMed] [Google Scholar]
- 38.Rosmarin AG, Caprio DG, Kirsch DG, Handa H, Simkevich CP. GABP and PU.1 compete for binding, yet cooperate to increase CD18 (beta 2 leukocyte integrin) transcription. J Biol Chem. 1995;270:23627–33. doi: 10.1074/jbc.270.40.23627. [DOI] [PubMed] [Google Scholar]
- 39.Rosmarin AG, Yang Z, Resendes KK. Transcriptional regulation in myelopoiesis: Hematopoietic fate choice, myeloid differentiation, and leukemogenesis. Exp Hematol. 2005;33:131–43. doi: 10.1016/j.exphem.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 40.Yu S, Cui K, Jothi R, Zhao DM, Jing X, Zhao K, Xue HH. GABP controls a critical transcription regulatory module that is essential for maintenance and differentiation of hematopoietic stem/progenitor cells. Blood. 2011;117:2166–78. doi: 10.1182/blood-2010-09-306563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fromm L, Burden SJ. Synapse-specific and neuregulin-induced transcription require an ets site that binds GABPalpha/GABPbeta. Genes Dev. 1998;12:3074–83. doi: 10.1101/gad.12.19.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Leary DA, Noakes PG, Lavidis NA, Kola I, Hertzog PJ, Ristevski S. Targeting of the ETS factor GABPalpha disrupts neuromuscular junction synaptic function. Mol Cell Biol. 2007;27:3470–80. doi: 10.1128/MCB.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khurana TS, Rosmarin AG, Shang J, Krag TO, Das S, Gammeltoft S. Activation of utrophin promoter by heregulin via the ets-related transcription factor complex GA-binding protein alpha/beta. Mol Biol Cell. 1999;10:2075–86. doi: 10.1091/mbc.10.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan RY, Boudreau-Larivière C, Angus LM, Mankal FA, Jasmin BJ. An intronic enhancer containing an N-box motif is required for synapse- and tissue-specific expression of the acetylcholinesterase gene in skeletal muscle fibers. Proc Natl Acad Sci U S A. 1999;96:4627–32. doi: 10.1073/pnas.96.8.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duclert A, Savatier N, Schaeffer L, Changeux JP. Identification of an element crucial for the sub-synaptic expression of the acetylcholine receptor epsilon-subunit gene. J Biol Chem. 1996;271:17433–8. doi: 10.1074/jbc.271.29.17433. [DOI] [PubMed] [Google Scholar]
- 46.Koike S, Schaeffer L, Changeux JP. Identification of a DNA element determining synaptic expression of the mouse acetylcholine receptor delta-subunit gene. Proc Natl Acad Sci U S A. 1995;92:10624–8. doi: 10.1073/pnas.92.23.10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang ZF, Mott S, Rosmarin AG. The Ets transcription factor GABP is required for cell-cycle progression. Nat Cell Biol. 2007;9:339–46. doi: 10.1038/ncb1548. [DOI] [PubMed] [Google Scholar]
- 48.Genuario RR, Perry RP. The GA-binding protein can serve as both an activator and repressor of ribosomal protein gene transcription. J Biol Chem. 1996;271:4388–95. doi: 10.1074/jbc.271.8.4388. [DOI] [PubMed] [Google Scholar]
- 49.Komatsu M, Wang QJ, Holstein GR, Friedrich VL, Jr., Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci U S A. 2007;104:14489–94. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu CT. Autophagic stress in neuronal injury and disease. J Neuropathol Exp Neurol. 2006;65:423–32. doi: 10.1097/01.jnen.0000229233.75253.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28:6926–37. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–83. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 53.FitzGerald PC, Shlyakhtenko A, Mir AA, Vinson C. Clustering of DNA sequences in human promoters. Genome Res. 2004;14:1562–74. doi: 10.1101/gr.1953904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, Batzoglou S, Bernstein BE, Bickel P, Brown JB, Cayting P, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 2012;22:1813–31. doi: 10.1101/gr.136184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ellenberger T, Fass D, Arnaud M, Harrison SC. Crystal structure of transcription factor E47: E-box recognition by a basic region helix-loop-helix dimer. Genes Dev. 1994;8:970–80. doi: 10.1101/gad.8.8.970. [DOI] [PubMed] [Google Scholar]
- 56.Maity SN, de Crombrugghe B. Role of the CCAAT-binding protein CBF/NF-Y in transcription. Trends Biochem Sci. 1998;23:174–8. doi: 10.1016/S0968-0004(98)01201-8. [DOI] [PubMed] [Google Scholar]
- 57.Watanabe H, Sawada J, Yano K, Yamaguchi K, Goto M, Handa H. cDNA cloning of transcription factor E4TF1 subunits with Ets and notch motifs. Mol Cell Biol. 1993;13:1385–91. doi: 10.1128/mcb.13.3.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu S, Jing X, Colgan JD, Zhao DM, Xue HH. Targeting tetramer-forming GABPβ isoforms impairs self-renewal of hematopoietic and leukemic stem cells. Cell Stem Cell. 2012;11:207–19. doi: 10.1016/j.stem.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ristevski S, O’Leary DA, Thornell AP, Owen MJ, Kola I, Hertzog PJ. The ETS transcription factor GABPalpha is essential for early embryogenesis. Mol Cell Biol. 2004;24:5844–9. doi: 10.1128/MCB.24.13.5844-5849.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–30. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tasdemir E, Maiuri MC, Tajeddine N, Vitale I, Criollo A, Vicencio JM, Hickman JA, Geneste O, Kroemer G. Cell cycle-dependent induction of autophagy, mitophagy and reticulophagy. Cell Cycle. 2007;6:2263–7. doi: 10.4161/cc.6.18.4681. [DOI] [PubMed] [Google Scholar]
- 62.Eskelinen EL, Prescott AR, Cooper J, Brachmann SM, Wang L, Tang X, Backer JM, Lucocq JM. Inhibition of autophagy in mitotic animal cells. Traffic. 2002;3:878–93. doi: 10.1034/j.1600-0854.2002.31204.x. [DOI] [PubMed] [Google Scholar]
- 63.Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C, Dall’Armi C, Simoes S, Point Du Jour KS, McCabe BD, et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat Commun. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rohn TT, Wirawan E, Brown RJ, Harris JR, Masliah E, Vandenabeele P. Depletion of Beclin-1 due to proteolytic cleavage by caspases in the Alzheimer’s disease brain. Neurobiol Dis. 2011;43:68–78. doi: 10.1016/j.nbd.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian Y, Bustos V, Flajolet M, Greengard P. A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 2011;25:1934–42. doi: 10.1096/fj.10-175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, et al. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res. 2006;34:D108–10. doi: 10.1093/nar/gkj143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–60. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 69.Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2008;105:19211–6. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM, Brown PO, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.