Abstract
Autophagy and microRNA (miRNA) are important regulators during cancer cell tumorigenesis. Impaired autophagy and high expression of the oncogenic microRNA MIR224 are prevalent in hepatocellular carcinoma (HCC); however, the relationship between the 2 phenomena remains elusive. In this study, we are the first to reveal that autophagy selectively regulates MIR224 expression through an autophagosome-mediated degradation system. Based on this finding, we further demonstrated that in hepatitis B virus (HBV)-related HCC, aberrant autophagy (low autophagic activity) results in accumulation of MIR224 and decreased expression of the target gene Smad4, which leads to increased cell migration and tumor formation. Preferential recruitment of MIR224 into the autophagosome was clearly demonstrated by a) miRNA in situ hybridization under confocal microscopy, and b) immunogold labeling of MIR224 under electron microscopy compared with a ubiquitously expressed microRNA MIRlet7e/let-7. Furthermore, we found that off-label use of amiodarone, an antiarrhythmic agent, effectively suppressed HCC tumorigenesis through autophagy-mediated MIR224 degradation both in vitro and in vivo. In summary, we identified amiodarone as a new autophagy inducer, which may provide an alternative approach in HCC therapy through a novel tumor suppression mechanism.
Keywords: HCC, miR-224, autophagy, HBV, miRNA
Ding et al. and Inami et al. studied Atg5- and Atg7-knockout mice, which develop liver tumors, and detected impaired autophagy in the clinical HCC specimens. These data support the notion that autophagy plays a suppressive role in liver tumorigenesis. Autophagic progression involves either nonselective or selective degradation pathways. The former is activated by metabolic stresses, such as starvation, to degrade unnecessary cytoplasmic organelles and proteins. However, the latter targets and degrades selective protein aggregates, infective pathogens, lipid droplets, and dysfunctional organelles. Accumulating evidence has revealed that selective autophagic degradation regulates various signaling pathways related to tumorigenesis, including apoptotic and WNT signaling pathways.
MiRNAs are small non-coding RNAs, which are involved in diverse diseases including cancers. MIR224 plays an oncogenic role in liver, colorectal, breast, and renal cancers, and overexpression of MIR224 is most frequently detected among dysregulated miRNAs in HCC.
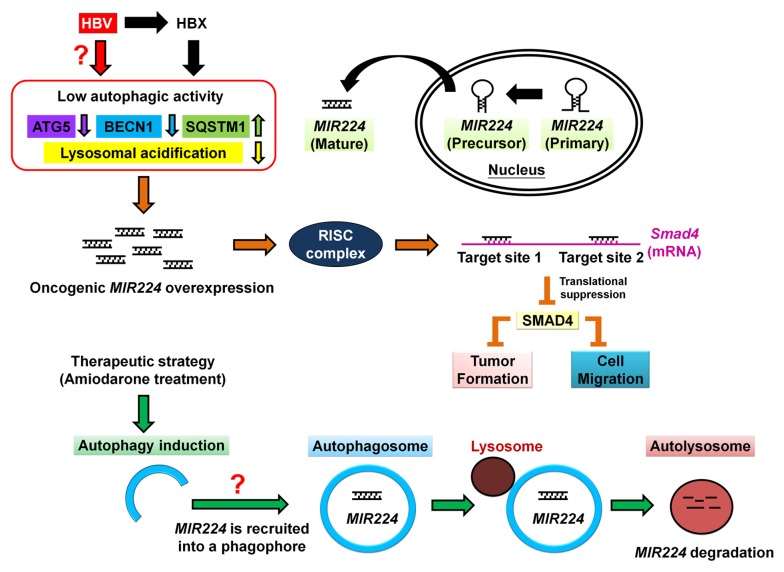
In this study, we found an association between the autophagy machinery and MIR224 degradation, and showed that impaired autophagy together with high MIR224 expression is significantly associated with HBV-associated HCC in 46 HCC patients (r = −0.412, P = 0.0043). Decreased autophagic activity accompanied with increased Mir224 expression detected in the liver tumors of HBV transgenic mice (harboring the liver-specific promoter-driven Hbx gene) further supports our hypothesis that low autophagic activity and high MIR224 expression contribute to tumorigenesis in HBV-associated HCC. Furthermore, the missing link between HBV-associated HCC and dysfunction of autophagy was identified in a study by Liu et al. that showed the HBVX protein impairs lysosomal acidification and then abolishes the autophagic degradation pathway in HBV-associated HCC. The above data provide evidence of a molecular mechanism in which HBV possibly triggers HCC tumorigenesis through the abolition of autophagic flux.
Most previous studies on the relationship between autophagy and miRNA have primarily investigated miRNA-targeted genes that are related to autophagy progression (Mir130a to Atg2b, Mir376b to Atg4, Mir30a to Becn1, and Mir204 to Map1lc3). We adopted a different approach to screen autophagy-regulated miRNAs and identified Mir224 as the most autophagy downregulated miRNA by microRNA microarray (unpublished data). Various autophagic stimuli (starvation, rapamycin, and amiodarone) could induce autophagy and significantly suppress MIR224 expression, suggesting that autophagy regulation of MIR224 through its degradation mechanism is a general event in the cell (Fig. 1). In summary, we identified a noncanonical degradation machinery, which negatively regulates MIR224 expression.
Figure 1. Schematic presentation of hypothetical model of autophagy regulation of MIR224 in HBV-related tumorigenesis. In HBV-associated tumorigenesis, impaired autophagy (low expression of ATG5 and BECN1, possibly caused by the HBVX gene) causes oncogenic MIR224 accumulation and then promotes tumorigenesis by silencing expression of its target gene Smad4. Use of amiodarone as an off-label agent can induce autophagy for degradation of oncogenic MIR224 expression in HCC therapy.
We then investigated how autophagy negatively regulates MIR224 and found that autophagy preferentially suppresses MIR224 expression at the post-transcriptional level through the autophagic degradation pathway, in contrast to the ubiquitously expressed miRNA MIRlet7e. It is possible that MIR224, after binding with an unidentified autophagic adaptor protein (SQSTM1/p62, NBR1, CALCOCO2/NDP52, VCP, or OPTN/optineurin), is recruited to the phagophore for degradation. We are the first to demonstrate the preferential recruitment of MIR224 into the phagophore by in situ hybridization under confocal microscopy and under electron microscopy with immunogold labeling of MIR224. The methodology utilized in this study may lead to the identification of other autophagy degraded miRNAs in the cell.
Amiodarone is an FDA-approved agent used to treat patients with various types of cardiac dysrhythmias. Balgi et al. reported that amiodarone is the most stable agent for inducing autophagy among 3,584 small molecules that were screened. We are the first to use amiodarone alone in vivo as an off-label use drug to suppress HCC tumorigenesis, and our findings demonstrate that it indeed induces the autophagic degradation machinery, which preferentially recruits and regulates the level of MIR224. The dosage used to effectively suppress tumor formation in rats was 30 mg/kg, which is equivalent to 4.8 mg/kg in a human adult, based on Reagan-Shaw’s conversion formula, which is much lower than the dosage used to treat antiarrhythmic disease (1,000 mg). Studies by Guiu et al. and Boilin et al. showed that the application of amiodarone as a supportive adjuvant of an anti-HCC drug increases the survival rate and prolongs the life span of HCC patients who receive transcatheter arterial chemoembolization. In addition, no toxicity was observed in the patients who received amiodarone. Taken together, our findings and previously published results strongly suggest that amiodarone is a novel autophagy inducer with the potential for tumor suppression in HCC therapy. Further research on the clinical treatment of HCC patients who show lower autophagic activity is warranted.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.