Abstract
Background
During long-term anticoagulation in atrial fibrillation, temporary interruptions (TIs) of therapy are common, but the relationship between patient outcomes and TIs has not been well studied. We sought to determine reasons for TI, the characteristics of patients undergoing TI, and the relationship between anticoagulant and outcomes among patients with TI.
Methods and Results
In the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF), a randomized, double-blind, double-dummy study of rivaroxaban and warfarin in nonvalvular atrial fibrillation, baseline characteristics, management, and outcomes, including stroke, non–central nervous system systemic embolism, death, myocardial infarction, and bleeding, were reported in participants who experienced TI (3–30 days) for any reason. The at-risk period for outcomes associated with TI was from TI start to 30 days after resumption of study drug. In 14 236 participants who received at least 1 dose of study drug, 4692 (33%) experienced TI. Participants with TI were similar to the overall ROCKET AF population in regard to baseline clinical characteristics. Only 6% (n=483) of TI incidences involved bridging therapy. Stroke/systemic embolism rates during the at-risk period were similar in rivaroxaban-treated and warfarin-treated participants (0.30% versus 0.41% per 30 days; hazard ratio [confidence interval]=0.74 [0.36–1.50]; P=0.40). Risk of major bleeding during the at-risk period was also similar in rivaroxaban-treated and warfarin-treated participants (0.99% versus 0.79% per 30 days; hazard ratio [confidence interval]=1.26 [0.80–2.00]; P=0.32).
Conclusions
TI of oral anticoagulation is common and is associated with substantial stroke risks and bleeding risks that were similar among patients treated with rivaroxaban or warfarin. Further investigation is needed to determine the optimal management strategy in patients with atrial fibrillation requiring TI of anticoagulation.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00403767.
Keywords: anticoagulation, atrial fibrillation, stroke
In patients with atrial fibrillation (AF), anticoagulation reduces the risk of stroke and embolic events and improves survival.1–4 Annually, nearly 250 000 AF patients in the United States alone require temporary interruption (TI) of anticoagulation for invasive procedures,5 acute illness, or bleeding events. Bridging therapy may or may not be pursued on the basis of clinical evaluation and physician perception of thrombotic risk versus bleeding risk.5,6 Although several clinical studies have examined the characteristics of patients with TI of anticoagulant therapy and the potential need for bridging therapy,7–10 there are limited data regarding downstream thrombotic and bleeding outcomes in these populations,11 and even less is known about TI of novel oral anticoagulants in periprocedural settings.12
The Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) demonstrated that rivaroxaban was noninferior to warfarin in the prevention of stroke and systemic thromboembolic events in moderate- to high-risk participants with nonvalvular AF.3 During the course of the trial, many participants required TI of anticoagulant medication. The objectives of the present analyses are to characterize those participants who required TI of oral anticoagulation, to examine reasons for TI, to describe patterns of bridging therapy, and to evaluate outcomes associated with rivaroxaban and warfarin.
Methods
Patient Population
The design, methods, and primary results of the ROCKET AF trial have been described previously.13 Briefly, ROCKET AF was a randomized, double-blind, double-dummy trial comparing fixed-dose rivaroxaban (20 mg daily or 15 mg daily in participants with a cre-atinine clearance of 30–49 mL/min) versus dose-adjusted warfarin (maintaining an international normalized ratio in the therapeutic range [2.0–3.0]) in participants with nonvalvular AF and moderate to high risk for stroke. The principal efficacy outcome was the occurrence of any stroke or non–central nervous system (CNS) systemic embolism. The principal safety outcome was the composite of International Society on Thrombosis and Hemostasis major bleeding and nonmajor clinically relevant (NMCR) bleeding.14
The ROCKET AF trial was supported by research grants from Johnson & Johnson Pharmaceutical Research and Development (Raritan, NJ) and Bayer Healthcare AG (Leverkusen, Germany). The Duke Clinical Research Institute (Durham, NC) coordinated the trial and performed the statistical analyses for this article independent of the sponsors. All appropriate national regulatory authorities and ethics committees at participating centers approved the study. An international executive committee designed the study and takes responsibility for the accuracy and completeness of the analyses.
For baseline summaries and occurrence of TI, all participants in the safety on-treatment population, defined as those who were randomized and received at least 1 dose of study drug, were included. All other analyses included the subset of patients with 1 or more TIs.
Outcomes and Definitions
TI of oral anticoagulation was defined as cessation of study drug for ≥ 3 days, due to any cause without transition to an open-label anticoagulant, with resumption of study drug within 30 days. The at-risk period for outcomes associated with TI was defined as the duration of the TI plus 30 days after the resumption of study drug. This period was chosen in efforts to evaluate the outcomes associated with TI and the time period after resumption of study drug. This is also the time frame recommended by a task force consensus document derived from available data.15 In cases in which study drug was resumed and stopped again within 30 days, the first at-risk period extended only to the day before the new interruption. Information on the type of procedure, date of procedure, and details concerning study drug interruption were captured in electronic case report form for the ROCKET AF trial. Invasive procedures were defined as any percutaneous or surgical procedure that would typically require cessation of warfarin. These included coronary angiography, percutaneous coronary intervention, peripheral vascular intervention, surgery, endoscopy, electrophysiology procedures, endoscopy/colonoscopy, and tissue biopsies as well as major dental procedures.
Stroke was defined as a sudden, focal neurological deficit resulting from a presumed cerebrovascular cause that is not reversible within 24 hours and not due to a readily identifiable cause, such as a tumor or seizure. Non-CNS systemic embolism was defined as abrupt vascular insufficiency associated with clinical or radiological evidence of arterial occlusion in the absence of other likely mechanisms (eg, trauma, atherosclerosis, or instrumentation). Myocardial infarction was defined by clinical symptoms consistent with myocardial infarction and cardiac biomarker elevation (troponin I or T, creatine kinase–muscle and brain subunit) greater than the upper limit of normal, the development of new pathological Q waves in ≥2 contiguous ECG leads, or confirmed by autopsy.
Major bleeding was defined as clinically overt bleeding associated with any of the following: fatal outcome, involving a critical site (ie, intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, or retroperitoneal), or clinically overt bleeding associated with a fall in hemoglobin concentration of ≥2 g/dL or leading to transfusion of ≥2 U of packed red blood cells or whole blood.14 NMCR bleeding was defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact with a physician (visit or telephone call), temporary (ie, by delaying the next study drug administration) cessation of study drug, pain, or impairment of daily activities.14 All other overt bleeding episodes not meeting the criteria for major or NMCR bleeding are classified as minor bleeding. All primary and secondary outcome events were adjudicated by a blinded, independent multispecialty clinical end point committee as part of the ROCKET AF trial.
In the ROCKET AF trial protocol, investigators were given the option to utilize bridging therapy in appropriate participants at intermediate to high risk for thromboembolic events (considered as those with >2 risk factors for stroke) that experienced TI. Both use of bridging therapy and selection of medications were left to the investigators’ discretion. Information on the use of bridging therapy was collected through the ROCKET AF trial electronic case report form by matching the dates of medication use forms (specifically for heparins and other parental or subcutaneous anticoagulants) to TI dates.
Statistical Analyses
Baseline characteristics are summarized according to TI status, randomized treatment, and use of bridging therapy. Continuous variables are shown as median (25th, 75th percentile), and categorical variables are shown as frequency (percentage). Among participants with at least 1 TI, characteristics of participants who received bridging therapy at least once were compared with those of participants who did not with the use of Wilcoxon rank sum tests or Pearson 2 tests as appropriate. Because early study drug discontinuations resulted in a high level of early censoring, rates of temporary interruption at different times were estimated by cumulative incidence functions that treat early permanent drug discontinuation and death as competing risks to TI. Cumulative incidence curves were compared with the Gray test.16
Outcomes occurring during TI risk periods are summarized by randomized treatment and by bridging therapy as number of events and event rates per 30 days. Association of randomized treatment with risk of outcome was assessed with Cox proportional hazards models in which each TI was an observation and that used robust sandwich variance estimators to account for correlation of multiple TIs within participants. Each participant could meet each end point only once; when a participant met an end point, subsequent TIs for that participant were not included in that model. Risk relationships are expressed as hazard ratios with 95% confidence intervals. Relationships between bridging therapy and risk of outcomes were not formally tested because of the very small number of events within the small subgroup of bridged TIs and complexities caused by the occurrence of both bridged and unbridged TIs in the same patients.
Sensitivity Analyses
Given concern for possible confounding for TIs associated with an adverse bleeding event, resulting in higher reported resultant bleeding risks, sensitivity analyses were performed to evaluate risks of bleeding in the subset of TI participants, excluding those TIs caused by an adverse bleeding event. Additional sensitivity analyses were also performed to evaluate risk relationships in the surgical/invasive procedure TI population alone. All analyses were performed with the use of SAS software version 9.2 (SAS Institute Inc, Cary, NC). A 2-sided P value <0.05 was considered statistically significant.
Results
Temporary Interruption Population
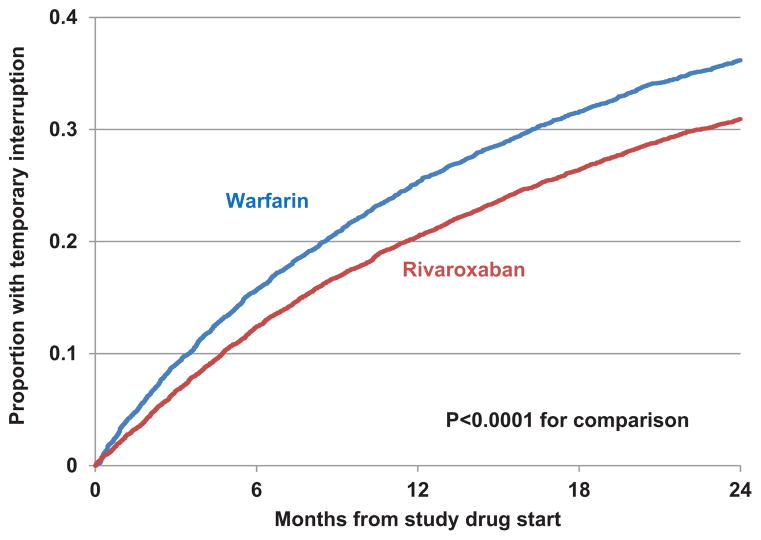
A total of 4692 participants in ROCKET AF (33% of 14 236 who were randomized and received study drug) experienced 7555 episodes of TI, of which 3393 occurred in participants treated with rivaroxaban and 4162 in participants treated with warfarin. Warfarin-treated participants experienced higher rates of TI over a 24-month follow-up period compared with rivaroxoban-treated participants (Figure 1; P<0.0001). The median duration of TI was 5 (4, 9) days. Among those with TI, 63% had 1 TI, 24% had 2 episodes, and 13% had ≥3 episodes of TI.
Figure 1.
Cumulative incidence plot of time to first temporary interruption by treatment group, showing the longitudinal analysis for time to first temporary interruption for warfarin- and rivaroxaban-treated patients. Warfarin-treated patients were significantly more likely to experience temporary interruption during follow-up.
Baseline patient characteristics are reported in Table 1. In general, participants who experienced TI were similar to the overall population of participants, with comparable median age, creatinine clearance, time of exposure to treatment, mean CHADS2 scores, and rates of hypertension, diabetes mellitus, congestive heart failure, prior stroke, prior myocardial infarction, and prior vitamin K antagonist use. Participants with TI receiving rivaroxaban were similar to those receiving warfarin for each of these characteristics as well.
Table 1.
Baseline Characteristics of Temporary Interruption Participants
| Characteristic | All Patients in Safety/On-Treatment Population (n=14 236) | Patients With Temporary Interruption
|
||
|---|---|---|---|---|
| All (n=4692) | Rivaroxaban (n=2165) | Warfarin (n=2527) | ||
| Age, y, median (25th, 75th percentiles) | 73 (65, 78) | 73 (66, 78) | 73 (66, 78) | 73 (66, 78) |
| Female, n (%) | 5645 (39.7) | 1714 (36.5) | 765 (35.3) | 949 (37.6) |
| BMI, kg/m2, median (25th, 75th percentiles) | 28.2 (25.1, 32.0) | 28.4 (25.4, 32.3) | 28.6 (25.4, 32.4) | 28.3 (25.4, 32.1) |
| Blood pressure, systolic, mm Hg, median (25th, 75th percentiles) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) |
| Blood pressure, diastolic, mm Hg, median (25th, 75th percentiles) | 80 (70, 85) | 80 (70, 85) | 80 (70, 85) | 80 (70, 85) |
| Treatment exposure period, day, median (25th, 75th percentiles) | 590 (399, 807) | 674 (473, 864) | 677 (477, 868) | 673 (467, 857) |
| Type of atrial fibrillation, n (%) | ||||
| Persistent | 11 525 (81.0) | 3753 (80.0) | 1740 (80.4) | 2013 (79.7) |
| Paroxysmal | 2511 (17.6) | 879 (18.7) | 397 (18.3) | 482 (19.1) |
| Newly diagnosed or new onset | 200 (1.4) | 60 (1.3) | 28 (1.3) | 32 (1.3) |
| Prior chronic aspirin, n (%) | 5194 (36.5) | 1727 (36.8) | 814 (37.6) | 913 (36.2) |
| Prior vitamin K antagonist, n (%) | 8889 (62.4) | 3030 (64.6) | 1388 (64.1) | 1642 (65.0) |
| CHADS2 score, mean (SD) | 3.47 (0.94) | 3.41 (0.95) | 3.40 (0.95) | 3.42 (0.96) |
| 1 | 3 (<0.1) | 2 (<0.1) | 0 (0.0) | 2 (0.1) |
| 2 | 1855 (13.0) | 740 (15.8) | 344 (15.9) | 396 (15.7) |
| 3 | 6203 (43.6) | 2029 (43.2) | 942 (43.5) | 1087 (43.0) |
| 4 | 4085 (28.7) | 1280 (27.3) | 595 (27.5) | 685 (27.1) |
| 5 | 1809 (12.7) | 549 (11.7) | 243 (11.2) | 306 (12.1) |
| 6 | 281 (2.0) | 92 (2.0) | 41 (1.9) | 51 (2.0) |
| History of stroke, TIA, embolism, n (%) | 7794 (54.7) | 2358 (50.2) | 1077 (49.7) | 1281 (50.7) |
| History of CHF, n (%) | 8894 (62.5) | 2926 (62.4) | 1344 (62.1) | 1582 (62.6) |
| History of hypertension, n (%) | 12 887 (90.5) | 4273 (91.1) | 1959 (90.5) | 2314 (91.6) |
| History of diabetes mellitus, n (%) | 5683 (39.9) | 1955 (41.7) | 926 (42.8) | 1029 (40.7) |
| History of myocardial infarction, n (%) | 2460 (17.3) | 874 (18.6) | 375 (17.3) | 499 (19.7) |
| Peripheral vascular disease, n (%) | 836 (5.9) | 303 (6.5) | 131 (6.1) | 172 (6.8) |
| COPD, n (%) | 1493 (10.5) | 573 (12.2) | 250 (11.5) | 323 (12.8) |
| Creatinine clearance (Cockcroft-Gault equation), mL/min, median (25th, 75th percentiles) | 67 (52, 87) | 68 (53, 88) | 68 (54, 89) | 67 (52, 86) |
| Temporary interruptions, median (25th, 75th percentiles) | 0 (0, 1) | 1 (1,2) | 1 (1,2) | 1 (1,2) |
| 0 | 9544 (67.0) | |||
| 1 | 2958 (20.8) | 2958 (63.0) | 1399 (64.6) | 1559 (61.7) |
| 2 | 1118 (7.9) | 1118 (23.8) | 517 (23.9) | 601 (23.8) |
| 3 | 347 (2.4) | 347 (7.4) | 150 (6.9) | 197 (7.8) |
| 4 | 153 (1.1) | 153 (3.3) | 54 (2.5) | 99 (3.9) |
| 5 | 54 (0.4) | 54 (1.2) | 19 (0.9) | 35 (1.4) |
| 6–18 | 62 (0.4) | 62 (1.3) | 26 (1.2) | 36 (1.4) |
BMI indicates body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; and TIA, transient ischemic attack.
TI Characteristics
The reasons for TI in the ROCKET AF trial are shown in Table 2. Forty percent experienced TI for a surgical or invasive procedure, 25% of participants had TI for an adverse event unrelated to bleeding, and 13% had TI for an adverse bleeding event. The remaining (12%) were attributed to subject error (majority), site error, and logistic difficulties. These results were similar when only the first episode of TI was considered.
Table 2.
Characteristics of TIs
| Characteristic | All TIs (n=7555) | TIs Among Rivaroxaban-Treated Patients (n=3393) | TIs Among Warfarin-Treated Patients (n=4162) |
|---|---|---|---|
| Duration of interruption, d, median (25th, 75th percentiles) | 5 (4, 9) | 6 (4, 10) | 5 (4, 9) |
| Duration of risk period for events, d, median (25th, 75th percentiles) | 35 (33, 38) | 35 (33, 39) | 35 (33, 38) |
| Reason for interruption,* n (%) | |||
| Surgical/invasive procedure | 2997 (39.7) | 1309 (38.6) | 1688 (40.6) |
| Adverse event: nonbleeding | 1874 (24.8) | 816 (24.0) | 1058 (25.4) |
| Subject error | 1366 (18.1) | 624 (18.4) | 742 (17.8) |
| Adverse event: bleeding | 995 (13.2) | 540 (15.9) | 455 (10.9) |
| Logistic difficulty | 449 (5.9) | 163 (4.8) | 286 (6.9) |
| Site error | 48 (0.6) | 22 (0.6) | 26 (0.6) |
| Reason unknown | 23 (0.3) | 3 (0.1) | 20 (0.5) |
| TIs with bridging therapy | n=483 (6.4) | n=277 (8.2) | n=206 (4.9) |
| Type of bridging therapy, n (%) | |||
| Fondaparinux | 7 (1.4) | 6 (2.2) | 1 (0.5) |
| LMWH | 476 (98.6) | 271 (97.8) | 205 (99.5) |
| Duration of bridging therapy, d, median (25th, 75th percentiles) | 6 (4, 9) | 6 (4, 10) | 6 (4, 8) |
LMWH indicates low-molecular-weight heparin; and TI, temporary interruption.
More than 1 reason could be provided for each TI.
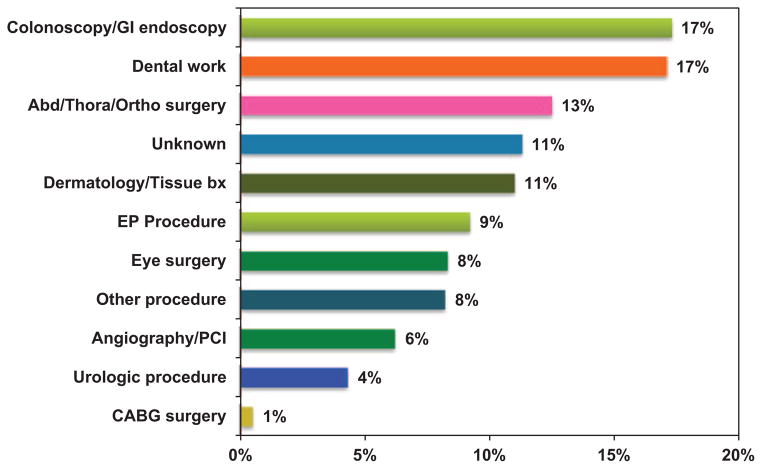
Among TIs for a surgical or invasive procedure, the interruption was prompted by a variety of invasive procedures (Figure 2). The most common causes of TI were colonoscopy or gastrointestinal endoscopic procedures (17%) and dental work (17%). Various abdominal, thoracic, and orthopedic surgeries (13%), electrophysiology procedures (9%), and dermatologic procedures (11%) also contributed significantly, whereas angiography, percutaneous coronary intervention, peripheral intervention (4%), and eye surgery (8%) contributed to a lesser degree. Eleven percent of TIs were due to surgical/invasive procedures in which details of the procedure are unknown. There were no meaningful differences in the frequency of different types of invasive procedures that prompted TI between rivaroxaban- and warfarin-treated patients (Figure I in the online-only Data Supplement).
Figure 2.
Distribution of surgical/invasive procedures requiring temporary interruption, showing the frequency of different types of surgical procedures that were listed as reasons for temporary interruptions in the Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Abd indicates abdominal; Bx, biopsy; CABG, coronary artery bypass graft; EP, electrophysiology; GI, gastrointestinal; Ortho, orthopedic; PCI, percutaneous coronary intervention; and Thora, thoracic.
Abd = Abdominal, Thora = Thoracic, Ortho = Orthopedic, Bx = Biopsy, EP = Electrophysiology, PCI = Percutaneous coronary intervention, CABG = Coronary artery bypass graft
Bridging Patterns and Patient Characteristics
Only 9% (n=431) of patients with TI during the ROCKET AF trial received bridging therapy during at least 1 TI. Most patients with TI who received bridging therapy were treated with low-molecular-weight heparin (98.6%), whereas the remaining patients were treated with fondaparinux (1.4%). The median duration of bridging therapy was 6 (4, 9) days (Table 2). Patient characteristics according to the presence or absence of bridging therapy are summarized in Table 3. Bridged and unbridged participants had similar rates of hypertension, diabetes mellitus, prior stroke, and congestive heart failure. Participants who received bridging therapy were older, were less commonly on prior aspirin therapy, more commonly had prior exposure to vitamin K antagonists, and had slightly higher mean CHADS2 risk scores (3.52 versus 3.40; P=0.009). These results were similar when only the first episode of TI was considered.
Table 3.
Population Characteristics of Participants Who Underwent Bridging
| Characteristic | Bridging Therapy at Least Once
|
||
|---|---|---|---|
| Yes (n=431) | No (n=4261) | P Value | |
| Age, y, median (25th, 75th percentiles) | 74 (68, 78) | 73 (65, 78) | 0.019 |
| Female, n (%) | 140 (32.5) | 1574 (36.9) | 0.067 |
| BMI, kg/m2, median (25th, 75th percentiles) | 28.8 (26.0, 32.1) | 28.4 (25.4, 32.3) | 0.22 |
| Blood pressure, systolic, mm Hg, median (25th, 75th percentiles) | 130 (120, 140) | 130 (120, 140) | 0.81 |
| Blood pressure, diastolic, mm Hg, median (25th, 75th percentiles) | 80 (70, 85) | 80 (70, 85) | 0.91 |
| Type of atrial fibrillation, n (%) | 0.44 | ||
| Persistent | 338 (78.4) | 3415 (80.1) | |
| Paroxysmal | 85 (19.7) | 794 (18.6) | |
| Newly diagnosed or new onset | 8 (1.9) | 52 (1.2) | |
| Prior chronic aspirin, n (%) | 124 (28.8) | 1603 (37.6) | 0.0003 |
| Prior vitamin K antagonist, n (%) | 340 (78.9) | 2690 (63.1) | <0.0001 |
| CHADS2 score, mean (SD) | 3.52 (0.93) | 3.40 (0.96) | 0.0094 |
| 1 | 0 (0.0) | 2 (<0.1) | |
| 2 | 41 (9.5) | 699 (16.4) | |
| 3 | 203 (47.1) | 1826 (42.9) | |
| 4 | 117 (27.1) | 1163 (27.3) | |
| 5 | 60 (13.9) | 489 (11.5) | |
| 6 | 10 (2.3) | 82 (1.9) | |
| History of stroke, TIA, embolism, n (%) | 226 (52.4) | 2132 (50.0) | 0.34 |
| History of CHF, n (%) | 261 (60.6) | 2665 (62.5) | 0.42 |
| History of hypertension, n (%) | 390 (90.5) | 3883 (91.1) | 0.66 |
| History of diabetes mellitus, n (%) | 207 (48.0) | 1748 (41.0) | 0.0049 |
| History of myocardial infarction, n (%) | 73 (16.9) | 801 (18.8) | 0.34 |
| Peripheral vascular disease, n (%) | 44 (10.2) | 259 (6.1) | 0.0009 |
| COPD, n (%) | 51 (11.8) | 522 (12.3) | 0.80 |
| Creatinine (Cockcroft-Gault equation), mL/min, median (25th, 75th percentiles) | 67 (53, 84) | 68 (53, 88) | 0.74 |
| Temporary interruptions, median (25th, 75th percentiles) | 1 (1, 2) | 1 (1, 2) | <0.0001 |
| 1 | 224 (52.0) | 2734 (64.2) | |
| 2 | 125 (29.0) | 993 (23.3) | |
| 3 | 59 (13.7) | 288 (6.8) | |
| 4 | 15 (3.5) | 138 (3.2) | |
| 5 | 4 (0.9) | 50 (1.2) | |
| 6–18 | 4 (0.9) | 58 (1.4) | |
BMI indicates body mass index; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; and TIA, transient ischemic attack.
Outcomes in TI
By Treatment Arm
Outcomes according to randomized treatment assignment are shown in Table 4. The risk of stroke or non-CNS systemic embolism during any at-risk period of TI was not significantly different in rivaroxaban-treated patients versus warfarin-treated patients. Risks of myocardial infarction and death were also not different between treatment groups. Similarly, risks of major bleeding and the composite NMCR bleeding and major bleeding were not different in those receiving rivaroxaban versus warfarin.
Table 4.
Outcomes in the TI Population
| Events | Rivaroxaban (n=2165 Patients, 3393 TIs)
|
Warfarin (n=2527 Patients, 4162 TIs)
|
HR (CI) for Rivaroxaban vs Warfarin | P Value | ||
|---|---|---|---|---|---|---|
| No. of Events | Event Rate per 30 d, % | No. of Events | Event Rate per 30 d % | |||
| Stroke/systemic embolism | 12 | 0.30 | 20 | 0.41 | 0.74 (0.36, 1.50) | 0.40 |
| Death | 7 | 0.17 | 9 | 0.18 | 0.93 (0.34, 2.56) | 0.89 |
| MI | 11 | 0.28 | 18 | 0.37 | 0.75 (0.36, 1.59) | 0.46 |
| Stroke/systemic embolism/MI/death | 26 | 0.66 | 46 | 0.95 | 0.70 (0.43, 1.13) | 0.14 |
| Major/NMCR bleeding | 95 | 3.33 | 111 | 3.00 | 1.12 (0.85, 1.47) | 0.43 |
| Major bleeding | 37 | 0.99 | 37 | 0.79 | 1.26 (0.80, 2.00) | 0.32 |
Stroke/systemic embolism/MI/death as a composite does not include multiple events in the same patient. CI indicates confidence interval; HR, hazard ratio; MI, myocardial infarction; NMCR, nonmajor clinically relevant; and TI, temporary interruption.
In the Subset of TI Patients Undergoing a Surgical/ Invasive Procedure
More than 40% of TI patients (2130) experienced TI for a surgical or invasive procedure, and their outcomes are shown in Table 5. The risk of stroke or non-CNS systemic embolism during any at-risk period of TI for a surgical or invasive procedure was not significantly different in rivaroxaban-treated patients versus warfarin-treated patients. Risks of myocardial infarction and death were also not different between treatment groups. Risks of major bleeding and the composite NMCR bleeding and major bleeding were not different in those receiving rivaroxaban versus warfarin in this subset of TIs. Results for a sensitivity analysis evaluating all TIs excluding those specifically for a bleeding event showed no differences between treatment arms, consistent with the aforementioned results (Table I in the online-only Data Supplement).
Table 5.
Outcomes During TIs for Only Surgical/Invasive Procedures Without Adverse Event Bleeding
| Events | Rivaroxaban (n=968 Patients, 1297 TIs)
|
Warfarin (n=1162 Patients, 1683 TIs)
|
HR (CI) for Rivaroxaban vs Warfarin | P Value | ||
|---|---|---|---|---|---|---|
| No. of Events | Event Rate per 30 d, % | No. of Events | Event Rate per 30 d, % | |||
| Stroke/systemic embolism | 4 | 0.27 | 8 | 0.42 | 0.65 (0.20, 2.13) | 0.48 |
| Death | 1 | 0.07 | 3 | 0.16 | 0.44 (0.05, 4.25) | 0.48 |
| MI | 4 | 0.27 | 3 | 0.16 | 1.70 (0.39, 7.44) | 0.48 |
| Stroke/systemic embolism/MI/death | 8 | 0.55 | 14 | 0.73 | 0.75 (0.31, 1.77) | 0.51 |
| Major/NMCR bleeding | 34 | 3.03 | 42 | 2.69 | 1.13 (0.72, 1.78) | 0.59 |
| Major bleeding | 14 | 0.99 | 18 | 0.97 | 1.02 (0.50, 2.06) | 0.96 |
Stroke/systemic embolism/MI/death as a composite does not include multiple events in the same patient. CI indicates confidence interval; HR, hazard ratio; MI, myocardial infarction, NMCR, nonmajor clinically relevant; and TI, temporary interruption.
By Timing of Study Drug Discontinuation
The occurrence of bleeding outcomes in relation to cessation of study drug before an invasive procedure is shown in Table 6. For the majority of TIs due to invasive procedures (90%; 2299/2547), study drug was stopped ≥3 days before the procedure. The vast majority of bleeding events also occurred in this group (88%; 61/69), although the distribution and frequency were similar to those in the other groups.
Table 6.
Risk of Major and NMCR Bleeding by Timing of Preprocedure Study Drug Discontinuation
| Time of Last Study Drug Dose Relative to Procedure | Rivaroxaban (n=1131 TIs)
|
Warfarin (n=1416 TIs)
|
||||
|---|---|---|---|---|---|---|
| n | Major Bleeds | Major/ NMCR Bleeds | n | Major Bleeds | Major/ NMCR Bleeds | |
| Same day | 20 | 0 | 0 | 24 | 0 | 0 |
| 1 d prior | 23 | 1 | 1 | 19 | 0 | 1 |
| 2 d prior | 105 | 0 | 5 | 57 | 0 | 1 |
| ≥3 d prior | 983 | 13 | 24 | 1316 | 16 | 37 |
This table contains all TIs for which “surgical/invasive procedure” is the only reason for interruption and for which the procedure date is known (n=2547). NMCR indicates nonmajor clinically relevant; and TI, temporary interruption.
By Use of Bridging Therapy
Outcomes according to use of bridging therapy are depicted in Table 7. Stroke/systemic embolism rates during TIs with bridging compared with those without bridging were not different. Rates of major bleeding were similar between bridged and nonbridged TIs, whereas rates of major/NMCR bleeding appeared numerically higher in patients receiving bridging therapy (4.83% versus 3.02%).
Table 7.
Outcomes During TIs With and Without Bridging Therapy
| Events | Bridging Therapy (n=483 TIs)
|
No Bridging Therapy (n=7072 TIs)
|
||
|---|---|---|---|---|
| No. of Events | Event Rate per 30 d, % | No. of Events | Event Rate per 30 d, % | |
| Stroke/systemic embolism | 1 | 0.17 | 31 | 0.37 |
| Death | 2 | 0.33 | 14 | 0.17 |
| MI | 4 | 0.69 | 25 | 0.30 |
| Stroke/systemic embolism/MI/death | 5 | 0.86 | 67 | 0.82 |
| Major/NMCR bleeding | 22 | 4.83 | 184 | 3.02 |
| Major bleeding | 5 | 0.91 | 69 | 0.88 |
Stroke/systemic embolism/MI/death as a composite does not include multiple events in the same patient. MI indicates myocardial infarction; NMCR, nonmajor clinically relevant; and TI, temporary interruption.
Discussion
This analysis of the ROCKET AF trial population represents one of the largest TI cohorts ever studied, with nearly 4700 participants experiencing >7000 TI and 431 participants receiving bridging therapy. The majority of TI episodes in ROCKET AF were due to invasive procedures (40%); however, a considerable proportion of TIs were also secondary to bleeding-related events and patient factors. Our report on this broad population is the first of its kind for novel oral anticoagulant therapy. Patterns of TI were due to a variety of minor and major invasive medical procedures, which are similar to those in prior observational studies.9,11 Duration of TI was often short (5 days) in our population, and bridging therapy with enoxaparin or fondaparinux was used in only 6% of TIs. TI was associated with a 30-day stroke/systemic embolism rate of 0.36% and major bleeding rate of 0.88%, which is notable compared with the overall rates in the ROCKET trial of 2.2%/y and 3.5%/y, respectively.3 However, the risks of stroke/systemic embolism and clinically relevant bleeding associated with TI were similar in rivaroxaban- and warfarin-treated participants.
Characteristics of TI
Participants treated with warfarin had higher rates of TI compared with those on rivaroxaban in our analyses. Although clinicians might surmise that the higher rate of TI in warfarin patients would be due to a greater rate of adverse events or procedures, risks of adverse events and surgical/invasive procedures were similar among participants in both treatment groups experiencing TI. Participants and investigators were blinded and a double dummy procedure was in place, and therefore bias from knowledge of working with warfarin was mostly eliminated. There may have been incidences of pseudo-unblinding that were not documented, possibly by performing coagulation measures as part of a routine screening before surgical/invasive procedures or during adverse events. This may account for part of the discrepancy seen between warfarin and rivaroxaban, which is admittedly difficult to explain in this context.
Stroke With TI
The 30-day rate of stroke or non-CNS embolism in our study population was 0.36%, which is notable compared with the overall stroke/systemic embolism rate in the ROCKET AF trial of 2.2%/y.3 The observed embolic event rates are high if prorated over a 1-year period but are consistent with findings in other populations in which there is TI of anticoagulant therapy.8 Possible factors that explain such higher-than-expected rates of embolic events include a prothrombotic perioperative milieu among patients having TI for surgery and a prothrombotic milieu associated with TI due to bleeding. In addition, patients enrolled in the ROCKET AF trial were considered high risk, and the embolic event rate during TI likely also reflects their intrinsic susceptibility to such outcomes. Our finding neither supports nor refutes a “rebound effect” with TI of either anticoagulant, especially given the short period (median=5 days) of anticoagulant interruption, and the clinical evidence for this phenomenon remains tenuous.15 Although differences were seen in stroke rates in the end-of-study analyses of the ROCKET AF trial, we believe that this was due to the unbalanced (worse in rivaroxaban patients) lack of continuous therapeutic anticoagulation at the end of the study period; this has been described elsewhere.17 However, the results compare favorably with a recently published large meta-analysis of patients on vitamin K antagonists receiving bridging or no bridging therapy in the periprocedural setting.8 That study described an acute thromboembolism event rate of ≈ 1.0% and a rate of periprocedural stroke/systemic embolism events of ≈ 1%.8 In addition, the risk of stroke/systemic embolism events compares favorably to the only other published database of periprocedural outcomes with the novel oral anticoagulant dabigatran in a lower-risk population as part of the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial, with a 30-day acute thromboembolism event rate of 1%, with ≈ 17% of patients receiving heparin bridging therapy.12 In our study, there was no detectable difference in the risk of stroke and systemic embolism for participants treated with rivaroxaban versus warfarin undergoing TI, consistent with the overall trial results.3
Bleeding With TI
Bleeding rates in those experiencing TI can be significant.9,18,19 The aforementioned large meta-analysis described a 30-day major bleeding rate of ≈ 4% in warfarin-treated patients in periprocedural settings, with bridging therapy conferring a >3-fold increase risk of major bleeding.8 In our study, participants who underwent TI for invasive procedures had rates of major bleeding and NMCR bleeding of ≈ 1% and 3% per 30 days, respectively, which were similar between treatment groups. This finding was consistent with data from the main trial, which showed that rates of major and NMCR bleeding were similar (14.9% versus 14.5% per year; P=0.44) between treatment groups.3 This is also consistent with periprocedural data from the RE-LY trial, which showed major bleeding and minor bleeding rates of ≈ 4% and ≈ 8%, respectively. Thus, our data in a high-risk population with AF on chronic anticoagulation in periprocedural settings compare favorably with previous evidence for postprocedural major bleeding rates with vitamin K antagonists and dabigatran.12
Patterns of Bridging Therapy
The use of bridging therapy was infrequent, at 6% of all TIs in the on-treatment safety population despite the moderate-to high-risk nature of this group (mean CHADS2 score=3.4). This observation was similar to data from Garcia et al,11 showing that rates of bridging were low (8.3%) in an observational cohort of chronically anticoagulated participants with diverse indications. Current guidelines indicate that the thromboembolic risk of TI should be estimated and weighed against the estimated risk of perioperative bleeding, and then bridging therapy should be utilized predominantly in the individuals at highest risk of thromboembolism.5 A possible explanation for the pattern of therapy seen in our analyses may be due to the blinding of participants and investigators in the ROCKET AF trial. Often clinicians are hesitant to introduce a second anticoagulant therapy to a patient experiencing TI if they are unsure of the current status of anticoagulation (in this case, because they are blinded to therapy). This may have led to the very low rates of bridging therapy utilization observed in this study. Participants were predominantly bridged with low-molecular-weight heparin in this analysis, consistent with current guideline recommendations.5
The rates of major bleeding were ≈ 1% among TIs with bridging therapy, whereas only 1 stroke or systemic embolism occurred in our small group. This is consistent with recent meta-analyses data showing low rates of thromboembolism (<1%) and bleeding (≈4%) in patients on chronic vitamin K antagonist therapy undergoing elective procedures with and without heparin bridging.8 Significance testing was not performed because of the small absolute number of events in the relatively small set of TIs that were bridged and because of the confounding present between bridged and unbridged TIs in the same participants. However, the data raise the question of whether the benefit of lower thrombotic events might be mitigated by an increased risk of bleeding associated with bridging anticoagulation. Current data available to answer this question are mixed,9,10,18 and there is no clear consensus to the risks and potential benefits of bridging therapy. Although published guidelines exist in regard to the management of novel oral anticoagulants in the periprocedural setting,15 they are based primarily on expert opinion, with little evidence to drive clinical management. Further work on this topic, including whether there is a role for heparin bridging therapy in patients on rivaroxaban in periprocedural settings, is needed.
Limitations
Our study has several limitations. First, it is a retrospective analysis of clinical trial data, and thus there is likely selection bias in regard to patient patterns of TI and bridging therapy. However, a particular strength of the analyses is that all participants and investigators were blinded to treatment assignment. Thus, the majority of comparisons made in this study were between the randomized treatment groups from the original trial, which should strengthen its validity by mitigating intentional biases. We acknowledge that utilization of bridging therapy was not randomized, and therefore any comparisons made between bridged and unbridged TIs may be subject to multiple biases. Thus, we chose to limit these comparisons. Although our study represents one of the largest populations of TI participants studied, with a large number of temporary interruptions, the number of events compared between groups is low. As a post hoc analysis, there was insufficient power to compare the TI subpopulation with the overall ROCKET AF population and examine the effect of TIs on clinical outcomes. In addition, because these are nonrandomized comparisons and because of the low number of events, we did not pursue multivariable statistical modeling. In addition, we did not have full and detailed data for the characteristics of these temporary interruptions, including the urgency (eg, elective versus emergent) of the surgical or invasive procedures leading to TI, as well as the relationship of study drug restart to the date of potential surgical or invasive procedures. Finally, for 11% of TIs due to surgical/invasive procedures, we did not have details on type, indication, or duration of that procedure. Lacking these data limited the depth of analyses that could be performed for the outcomes of TI.
Conclusions
In the ROCKET AF study, a large international population of participants with nonvalvular AF, TI of oral anticoagulation occurred in ≈ 10% of patients per year, with only a minority of patients (<10%) receiving bridging therapy. During the at-risk period associated with TIs, we observed a 30-day stroke/systemic embolism rate of 0.4% and a major bleeding rate of 0.9%. The risks of thrombotic and bleeding complications were comparable between rivaroxaban- and warfarin-treated participants experiencing TI. TI of oral anticoagulation should be avoided to minimize adverse outcomes. Given the lack of randomized data in this area, further research is necessary to determine the safest way to manage both warfarin and novel anticoagulant medications, such as rivaroxaban, during TIs.
CLINICAL PERSPECTIVE.
The Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) demonstrated that rivaroxaban was noninferior to warfarin in the prevention of stroke and systemic thromboembolic events in moderate- to high-risk participants with nonvalvular atrial fibrillation. During the course of the trial, many participants required temporary interruption of anticoagulant medication for surgery, invasive procedures, or adverse events. Our analysis provides one of the largest experiences of patients undergoing temporary interruption of oral anticoagulation for procedural and nonprocedural reasons and is one of the first to evaluate this clinical scenario in patients treated with a novel oral anticoagulant. In this clinical trial setting, the use of bridging anticoagulation was very low despite the high thrombotic risk of the population. There was no difference in thrombotic or bleeding events during or after temporary interruption in patients treated with rivaroxaban compared with patients treated with warfarin. However, given the substantial thrombotic and bleeding risks associated with temporary interruption of oral anticoagulation, it should be avoided if at all possible.
Footnotes
Disclosures
Dr Sherwood reports consulting fees from Boehringer Ingelheim. Dr Douketis reports past participation in Advisory Boards for the following companies: Sanofi-Aventis, Astra-Zeneca, Boehringer-Ingelheim, Pfizer; he has performed as a consultant to the following companies: AGEN Biomedical, Ortho-Janssen Pharmaceuticals, Boehringer-Ingelheim Pharmaceuticals. Dr Patel has received honoraria from Johnson & Johnson and Bayer HealthCare for serving on the executive committee of the ROCKET AF trial; consulting fees from Ortho McNeil Janssen and Bayer HealthCare; and advisory board fees from Genzyme. Dr Piccini has received grants for clinical research from Johnson & Johnson and Boston Scientific and consulting and/or advisory board fees from Medtronic, Forest Laboratories, Sanofi Aventis, and Johnson & Johnson. Dr Hankey has received honoraria from Johnson & Johnson, Bayer, and Sanofi-Aventis and has received fees for serving on trial adjudication committees and an advisory board for Boehringer Ingelheim. Dr Singer is supported in part by the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital (Boston, MA); has received consulting fees from Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, Pfizer, and Sanofi; and serves as a member of the executive committee of the ROCKET AF trial of rivaroxaban versus warfarin in patients with atrial fibrillation sponsored by Johnson & Johnson and Bayer HealthCare. Dr Nessel is an employee of Johnson & Johnson Pharmaceutical Research & Development. Dr Spyropoulos is a consultant for Boehringer-Ingelheim, Jansen, Bayer, The Medicines Company, Sanofi, and Daiichi Sankyo. Dr Mahaffey reports grant support (significant) from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Momenta Pharmaceuticals, Novartis, Portola, Pozen, Regado Biotechnologies, Sanofi-Aventis, Schering-Plow (now Merck), and The Medicines Company; consulting fees (significant) from AstraZeneca and Johnson & Johnson, (modest) Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Ortho/McNeill, Pfizer, Polymedix, Sanofi-Aventis, and Schering-Plow (now Merck). Dr Fox has received grants and honoraria from Bayer, Lilly, Boehringer Ingelheim, Sanofi-Aventis, and GlaxoSmithKline. Dr Califf reports consulting fees and research funding from Johnson & Johnson; all other industry interactions are listed at http://ww.dcri.org. Dr Becker has received research support from Bayer and Johnson & Johnson. Dr Nessel is an employee of Johnson & Johnson. The other authors report no conflicts.
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, Dunn AS, Kunz R American College of Chest Physicians. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141 (2 suppl):e326S–e350S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korte W, Cattaneo M, Chassot PG, Eichinger S, von Heymann C, Hofmann N, Rickli H, Spannagl M, Ziegler B, Verheugt F, Huber K. Peri-operative management of antiplatelet therapy in patients with coronary artery disease: joint position paper by members of the Working Group on Perioperative Haemostasis of the Society on Thrombosis and Haemostasis Research (GTH), the Working Group on Perioperative Coagulation of the Austrian Society for Anesthesiology, Resuscitation and Intensive Care (ÖGARI) and the Working Group Thrombosis of the European Society for Cardiology (ESC) Thromb Haemost. 2011;105:743–749. doi: 10.1160/TH10-04-0217. [DOI] [PubMed] [Google Scholar]
- 7.Jafri SM. Periprocedural thromboprophylaxis in patients receiving chronic anticoagulation therapy. Am Heart J. 2004;147:3–15. doi: 10.1016/j.ahj.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126:1630–1639. doi: 10.1161/CIRCULATIONAHA.112.105221. [DOI] [PubMed] [Google Scholar]
- 9.Spyropoulos AC, Turpie AG, Dunn AS, Spandorfer J, Douketis J, Jacobson A, Frost FJ REGIMEN Investigators. Clinical outcomes with unfractionated heparin or low-molecular-weight heparin as bridging therapy in patients on long-term oral anticoagulants: the REGIMEN registry. J Thromb Haemost. 2006;4:1246–1252. doi: 10.1111/j.1538-7836.2006.01908.x. [DOI] [PubMed] [Google Scholar]
- 10.Douketis JD, Johnson JA, Turpie AG. Low-molecular-weight heparin as bridging anticoagulation during interruption of warfarin: assessment of a standardized periprocedural anticoagulation regimen. Arch Intern Med. 2004;164:1319–1326. doi: 10.1001/archinte.164.12.1319. [DOI] [PubMed] [Google Scholar]
- 11.Garcia DA, Regan S, Henault LE, Upadhyay A, Baker J, Othman M, Hylek EM. Risk of thromboembolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168:63–69. doi: 10.1001/archinternmed.2007.23. [DOI] [PubMed] [Google Scholar]
- 12.Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, Themeles E, Heidbuchel H, Heidbuchle H, Avezum A, Reilly P, Connolly SJ, Yusuf S, Ezekowitz M RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation. 2012;126:343–348. doi: 10.1161/CIRCULATIONAHA.111.090464. [DOI] [PubMed] [Google Scholar]
- 13.Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–347. e341. doi: 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 15.Spyropoulos AC, Douketis JD, Gerotziafas G, Kaatz S, Ortel TL, Schulman S Subcommittee on Control of Anticoagulation of the SSC of the ISTH. Periprocedural antithrombotic and bridging therapy: recommendations for standardized reporting in patients with arterial indications for chronic oral anticoagulant therapy. J Thromb Haemost. 2012;10:692–694. doi: 10.1111/j.1538-7836.2012.04630.x. [DOI] [PubMed] [Google Scholar]
- 16.Bagdonavicius V, Bikelis A, Kazakevicius V. Statistical analysis of linear degradation and failure time data with multiple failure modes. Lifetime Data Anal. 2004;10:65–81. doi: 10.1023/b:lida.0000019256.59372.63. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Hellkamp AS, Lokhnygina Y, Piccini JP, Zhang Z, Mohanty S, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Becker RC, Nessel CC, Berkowitz SD, Califf RM, Fox KA, Mahaffey KW. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) J Am Coll Cardiol. 2013;61:651–658. doi: 10.1016/j.jacc.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 18.Wysokinski WE, McBane RD, Daniels PR, Litin SC, Hodge DO, Dowling NF, Heit JA. Periprocedural anticoagulation management of patients with nonvalvular atrial fibrillation. Mayo Clin Proc. 2008;83:639–645. doi: 10.4065/83.6.639. [DOI] [PubMed] [Google Scholar]
- 19.Malato A, Saccullo G, Lo Coco L, Caramazza D, Abbene I, Pizzo G, Casuccio A, Siragusa S. Patients requiring interruption of long-term oral anticoagulant therapy: the use of fixed sub-therapeutic doses of low-molecular-weight heparin. J Thromb Haemost. 2010;8:107–113. doi: 10.1111/j.1538-7836.2009.03649.x. [DOI] [PubMed] [Google Scholar]