Abstract
Objectives
This study sought to report additional safety results from the ROCKET AF (Rivaroxaban Once-daily oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation).
Background
The ROCKET AF trial demonstrated similar risks of stroke/systemic embolism and major/nonmajor clinically relevant bleeding (principal safety endpoint) with rivaroxaban and warfarin.
Methods
The risk of the principal safety and component bleeding endpoints with rivaroxaban versus warfarin were compared, and factors associated with major bleeding were examined in a multivariable model.
Results
The principal safety endpoint was similar in the rivaroxaban and warfarin groups (14.9 vs. 14.5 events/100 patient-years; hazard ratio: 1.03; 95% confidence interval: 0.96 to 1.11). Major bleeding risk increased with age, but there were no differences between treatments in each age category (<65, 65 to 74, ≥75 years; pinteraction = 0.59). Compared with those without (n = 13,455), patients with a major bleed (n = 781) were more likely to be older, current/prior smokers, have prior gastrointestinal (GI) bleeding, mild anemia, and a lower calculated creatinine clearance and less likely to be female or have a prior stroke/transient ischemic attack. Increasing age, baseline diastolic blood pressure (DBP) ≥90 mm Hg, history of chronic obstructive pulmonary disease or GI bleeding, prior acetylsalicylic acid use, and anemia were independently associated with major bleeding risk; female sex and DBP <90 mm Hg were associated with a decreased risk.
Conclusions
Rivaroxaban and warfarin had similar risk for major/nonmajor clinically relevant bleeding. Age, sex, DBP, prior GI bleeding, prior acetylsalicylic acid use, and anemia were associated with the risk of major bleeding. (An Efficacy and Safety Study of Rivaroxaban With Warfarin for the Prevention of Stroke and Non-Central Nervous System Systemic Embolism in Patients With Non-Valvular Atrial Fibrillation: NCT00403767)
Keywords: anticoagulants, atrial fibrillation, hemorrhage
The ROCKET AF (Rivaroxaban Once-daily oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) demonstrated that rivaroxaban was noninferior to warfarin for the prevention of stroke and systemic embolism (1). Although major and nonmajor clinically relevant bleeding rates were also similar, rivaroxaban led to a lower risk of intracranial hemorrhage and fatal bleeding. In contrast, rivaroxaban caused higher rates of bleeding from gastrointestinal (GI) sites and bleeding that led to a drop in hemoglobin level or required transfusion. The effect of rivaroxaban in stroke/systemic embolism prevention and safety was consistent across a broad range of patient baseline characteristics (1), suggesting that once-daily, fixed-dose rivaroxaban is an alternative to adjusted-dose warfarin in patients with nonvalvular atrial fibrillation (AF) who are at moderate-to-high risk for stroke. We report on additional safety results of rivaroxaban compared with warfarin from the ROCKET AF trial. Furthermore, because several bleeding risk scores have been developed from data on patients receiving warfarin (2–7) and their applicability (8,9)—particularly to newer anticoagulants (10)—is uncertain, we investigated factors associated with major bleeding in AF patients in the ROCKET AF trial.
Methods
The ROCKET AF trial design
The ROCKET AF design has been published (1,11). The primary study objective was to establish the noninferiority of rivaroxaban compared with warfarin for the prevention of stroke or systemic embolism in patients with nonvalvular AF. The trial randomized 14,264 patients with AF who were at moderate-to-high risk for stroke. Elevated risk was indicated by a history of stroke, transient ischemic attack (TIA), or systemic embolism or at least 2 of the following risk factors: heart failure or a left ventricular ejection fraction ≤35%; hypertension; age ≥75 years; or diabetes mellitus. The proportion of patients who had not had a previous ischemic stroke, TIA, or systemic embolism and who had no more than 2 risk factors was limited to 10% of the cohort for each region; the remainder of patients were required to have had either previous thromboembolism or 3 or more risk factors. Key exclusion criteria included: active internal bleeding; a history of or condition associated with increased bleeding risk (e.g., major surgical procedure or trauma ≤30 days; clinically significant GI bleeding ≤6 months; history of intracranial, intraocular, spinal, or atraumatic intra-articular bleeding; chronic hemorrhagic disorder; known intracranial neoplasm, arteriovenous malformation, or aneurysm; platelet count <90,000/μl at the screening visit; sustained uncontrolled hypertension [i.e., systolic blood pressure (SBP) ≥180 mm Hg and/or diastolic blood pressure (DBP) ≥100 mm Hg]; acetylsalicylic acid (ASA) >100 mg daily or in combination with thienopyridines at the time of screening; anticipated need for ≥14 days of treatment with a nonsteroidal anti-inflammatory drug; anemia [hemoglobin <10 g/dl] at the screening visit; calculated creatine clearance <30 ml/min at the screening visit; or significant liver disease (e.g., acute clinical hepatitis, chronic active hepatitis, cirrhosis) or alanine transferase >3× the upper limit of normal).
Patients were randomized to receive fixed-dose rivaroxaban 20 mg daily (or 15 mg daily in patients with a creatinine clearance of 30 to 49 ml/min) or adjusted-dose warfarin (target international normalized ratio [INR]: 2.5; range 2.0 to 3.0). Patients in each group also received a placebo tablet to maintain blinding. A point-of-care device was used to generate encrypted values that were entered into an interactive voice response system to generate either real INR values (for patients in the warfarin group to adjust the dose) or sham values (for patients in the rivaroxaban group receiving placebo warfarin).
All appropriate national regulatory authorities and ethics committees approved the study. All patients provided written informed consent.
Outcome definitions
All primary and secondary outcome events were adjudicated by an independent clinical endpoint committee blinded to treatment assignment.
The principal safety endpoint was defined as major bleeding or nonmajor clinically relevant bleeding. Major bleeding was defined as that which was clinically overt and associated with any of the following: fatal outcome; involvement of a critical anatomic site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal, or intramuscular with compartment syndrome); fall in hemoglobin concentration >2 g/dl; transfusion of >2 U of whole blood; or packed red blood cells (12). Nonmajor clinically relevant bleeding was defined as overt bleeding not meeting criteria for major bleeding but requiring medical intervention, unscheduled contact (visit or telephone) with a physician, temporary interruption of the study drug (i.e., delayed dosing), pain, or impairment of daily activities.
Statistical analysis
We examined the effect of rivaroxaban and warfarin on the risk of major bleeding. Bleeding endpoints are presented as rates/100 patient-years of follow-up. All analyses of rates of bleeding are based on the first event in the safety population during treatment. We considered the issue of competing risk (due to death) and examined the cumulative incidence estimates of major bleeding; however, because the differences between the methods were minimal (data not shown), we used the Kaplan-Meier method. The Cox model was used to derive hazard ratios (HRs) and confidence intervals (CIs) for the rivaroxaban versus warfarin comparison. Baseline characteristics of patients with and without a major bleeding event were summarized as frequencies and percentages for categorical variables and median and quartiles for continuous variables.
The Cox model was also used to compare patients with and without major bleeding in terms of demographic and clinical characteristics; for each characteristic, a univariate Cox model for the risk of major bleeding was derived. The following candidate variables included those pre-specified in the statistical analysis plan of the trial plus those in the HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile International Normalized Ratio, Elderly, Drugs/alcohol) (7) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) (6) bleeding scores: age; sex; body mass index; SBP and DBP; type of AF (persistent, paroxysmal, or newly diagnosed/new onset); history of stroke; TIA or systemic embolism; history of heart failure; diabetes mellitus; myocardial infarction; peripheral arterial disease; chronic obstructive pulmonary disease (COPD); alcohol use; liver disease; prior GI bleeding; labile INR; ASA use (before randomization); prior vitamin K antagonist use; creatinine clearance; and anemia (hemoglobin <13 g/dl in men and <12 g/dl in women). Variables considered in other bleeding scores (2,3,5), such as history of malignancy, prior peptic ulcer disease, prior (non-GI) bleeding, genetic factors, excessive fall risk or neuropsychiatric disease, and prior ethanol or drug abuse, were not recorded in the ROCKET AF trial.
Continuous variables were tested for linearity with restricted cubic splines. Systolic and DBPs were transformed with linear spline. Variables were selected for inclusion in the regression model with a backward selection method with significance level to stay in the model set to 0.05.
All analyses were performed with the use of SAS software (version 9.2, SAS Institute, Inc., Cary, North Carolina). A 2-sided p value <0.05 was considered statistically significant. The authors of the paper had full access to the data and planned the statistical analyses.
Results
Patient characteristics and treatments
As published (1), the median age was 73 years (25th, 75th percentiles: 65, 78), 40% were women, 91% had hypertension, 63% had a history of heart failure, 40% had diabetes, 17% had a prior myocardial infarction, 11% had a history of COPD, and 55% had a history of stroke, systemic embolism, or TIA. The median CHADS2 (Congestive heart failure, hypertension, age >75 years, Diabetes, prior Stroke/TIA/non-central nervous system thromboembolism [doubled]) score was 3 (3,4). Atrial fibrillation was persistent in 81%, 62% had previously used a vitamin K antagonist, and 37% had used ASA before randomization. During the course of the study, 35% of patients in the rivaroxaban group and 36% of those in the warfarin group took ASA concurrently with the assigned study drug. Among patients in the warfarin group, INR values were within the therapeutic range (2 to 3) a mean of 55% of the time (median: 58%; 25th, 75th percentiles range: 43%, 71%) (13). Warfarin-experienced patients had a mean time in the therapeutic range (TTR) of 61%, whereas warfarin-naive patients had a mean TTR of 47%. The mean time with an INR <2 was 29% and >3 was 16% (13).
Bleeding
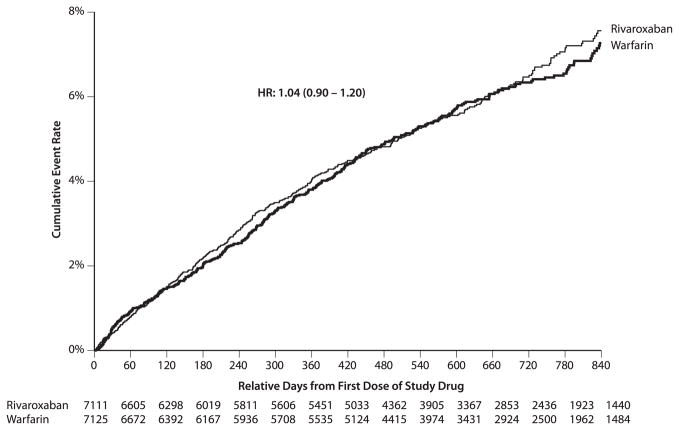
Table 1 presents the risk of the principal safety endpoint, individual endpoint components including transfusions, and minimal bleeding according to randomized treatment allocation. Figure 1 presents Kaplan-Meier curves for major bleeding. Compared with warfarin, rivaroxaban had a similar risk of the principal safety endpoint, including major and nonmajor clinically relevant bleeding. Although rivaroxaban caused a significantly higher risk of hemoglobin decrease ≥2 g/dl and transfusion compared with warfarin, critical bleeding and fatal bleeding were significantly lower in rivaroxaban-treated patients. Intracranial hemorrhage was significantly lower in the rivaroxaban group (0.5 vs. 0.7, HR: 0.67; 95% CI: 0.47 to 0.93). Minimal bleeding (not included in the primary safety endpoint) was similar in the rivaroxaban and warfarin groups (2.4 vs. 2.0, HR: 1.16; 95% CI: 0.97 to 1.39). Epistaxis (6.9% vs. 5.7%; p ≤ 0.001) and hematuria (2.7% vs. 2.2%; p = 0.011) were reported more frequently as an adverse event in the rivaroxaban group.
Table 1.
Event Rates and HRs and 95% CIs for Bleeding Events
| Events (Rate)
|
HR (95% CI) | p Value | ||
|---|---|---|---|---|
| Rivaroxaban (n = 7,111) | Warfarin (n = 7,125) | |||
| Principal safety endpoint | 1,475 (14.91) | 1,449 (14.52) | 1.03 (0.96–1.11) | 0.442 |
|
| ||||
| Major | 395 (3.60) | 386 (3.45) | 1.04 (0.90–1.20) | 0.576 |
| Hemoglobin/hematocrit drop | 305 (2.77) | 254 (2.26) | 1.22 (1.03–1.44) | 0.019 |
| Transfusion | 183 (1.65) | 149 (1.32) | 1.25 (1.01–1.55) | 0.044 |
| Critical organ bleeding | 91 (0.82) | 133 (1.18) | 0.69 (0.53–0.91) | 0.007 |
| Death | 27 (0.24) | 55 (0.48) | 0.50 (0.31–0.79) | 0.003 |
|
| ||||
| Nonmajor clinically relevant | 1,185 (11.80) | 1,151 (11.37) | 1.04 (0.96–1.13) | 0.345 |
|
| ||||
| Minimal | 258 (2.35) | 226 (2.03) | 1.16 (0.97–1.39) | 0.102 |
Event rates/100 patient-years.
CI = confidence interval; HR = hazard ratio.
Figure 1. Major Bleeding by Treatment.
Kaplan-Meier curves for major bleeding for the treatment groups. HR = hazard ratio.
Table 2 presents the risks of major and nonmajor clinically relevant bleeding by site. Among the 1,063 patients with a major or nonmajor clinically relevant bleeding event, warfarin (active or placebo) dose was reduced in 116 (4%) patients. The study drug was temporarily discontinued but then restarted in 1,337 (46.2%) and permanently discontinued in 381 (13.1%). Bleeding led to permanent study drug discontinuation in 322 (4.5%) rivaroxaban and 286 (4%) warfarin patients (absolute difference 0.5; 95% CI: −0.2 to 1.2).
Table 2.
Major Bleeding or Nonmajor Clinically Relevant Bleeding by Site
| Rivaroxaban (n = 7,111) | Warfarin (n = 7,125) | |
|---|---|---|
| Major bleeding or nonmajor clinically relevant bleeding | 1,475 (20.7) | 1,449 (20.3) |
|
| ||
| GI (upper, lower, and rectal)* | 394 (5.5) | 290 (4.1) |
| Intracranial† | 55 (0.77) | 84 (1.18) |
| Intraparenchymal† | 37 (0.52) | 56 (0.79) |
| Nontraumatic† | 33 (0.46) | 54 (0.76) |
| Traumatic | 4 (0.06) | 2 (0.03) |
| Intraventricular‡ | 13 (0.18) | 30 (0.42) |
| Subdural hematoma† | 14 (0.20) | 27 (0.38) |
| Subarachnoid | 7 (0.10) | 14 (0.20) |
| Epidural hematoma | 0 | 1 (0.01) |
| Macroscopic hematuria‡ | 243 (3.4) | 187 (2.6) |
| Bleeding associated with noncardiac surgery | 53 (0.75) | 47 (0.66) |
| Intraocular/retinal | 17 (0.24) | 24 (0.34) |
| Intra-articular | 16 (0.23) | 21 (0.29) |
|
| ||
| Epistaxis | 303 (4.3) | 275 (3.9) |
Values are n (%). Patients can be counted in multiple lines, including for intracranial bleeding.
p < 0.0001.
p < 0.05.
p < 0.01; no adjustments for multiple comparisons were made.
GI = gastrointestinal.
Major bleeding
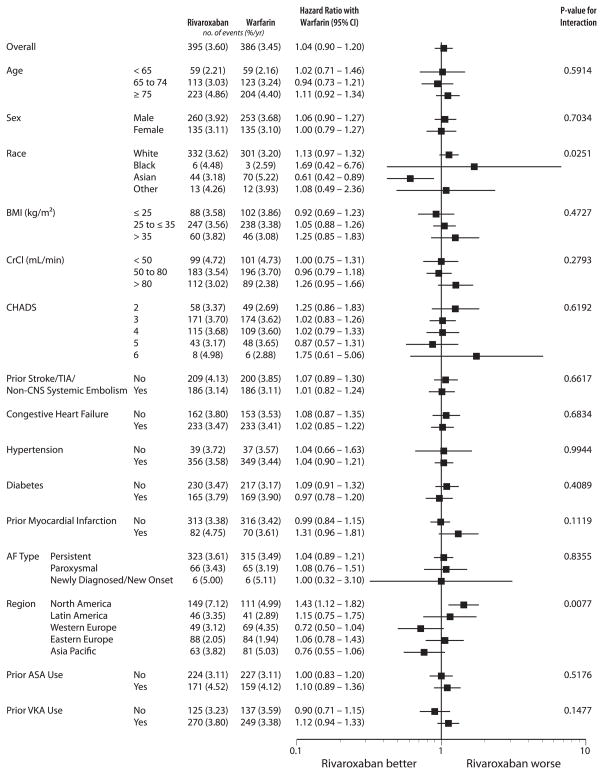
Figure 2 presents the HRs for major bleeding in patients randomized to receive rivaroxaban compared with warfarin in key subgroups according to patient baseline characteristics. The risk of major bleeding increased with increasing age, although there were no apparent differences between rivaroxaban and warfarin in each age category (<65, 65 to 74, ≥75 years; p for interaction = 0.59). The relative risk of major bleeding with rivaroxaban versus warfarin was comparable in those <75 years of age (n = 8,007: 2.7% vs. 2.8%; HR: 0.96; 95% CI: 0.78 to 1.19) and those 75 years or older (n = 6,164: 4.9% vs. 4.4%; HR: 1.11; 95% CI: 0.92 to 1.34) (p for interaction = 0.34). The relative risk of intracranial hemorrhage for rivaroxaban versus warfarin was statistically significantly lower in those under 75 years (0.37% vs. 0.68%; HR: 0.54; 95% CI: 0.33 to 0.89) and numerically lower in those 75 years or older (0.66% vs. 0.83%; HR: 0.80; 95% CI: 0.50 to 1.28) (p value for interaction = 0.27). There was a statistically significant p value for interaction when comparing the HRs for major bleeding across regions, with the North American cohort having the highest overall rates, including a significantly higher frequency in the rivaroxaban-treated patients (7.1% vs. 5.0%; HR: 1.43; 95% CI: 1.12 to 1.82).
Figure 2. Major Bleeding in Key Subgroups.
On-treatment (safety population) major bleeding in key subgroups according to patient baseline characteristics. AF = atrial fibrillation; ASA = acetylsalicylic acid; BMI = body mass index; CHADS = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack (TIA), or non–central nervous system thromboembolism (doubled); CI = confidence interval; CNS = central nervous system; CrCl = creatinine clearance; TIA = transient ischemic attack; VKA = vitamin K antagonists.
Table 3 presents the baseline characteristics of all patients in the ROCKET AF trial according to whether or not they experienced a major bleeding event. Patients with a major bleed (n = 781) were more likely to be older (median 75 vs. 73 years, p < 0.0001), current or prior smokers (40.2% vs. 33.2%, p < 0.0001), have a history of prior GI bleeding (7.8% vs. 3.3%, p < 0.0001), have mild anemia at baseline (25.1% vs. 13.5%, p < 0.0001), use ASA before randomization (42.3% vs. 36.2%, p < 0.0001), and have a lower baseline calculated creatinine clearance (63 vs. 68 ml/min, p < 0.0001); they were less likely to be female (34.3% vs. 40.0%, p = 0.0017) or to have a prior stroke or TIA (46.2% vs. 52.7%, p = 0.0004), compared with those without a major bleed (n = 13,455).
Table 3.
Baseline Characteristics of ROCKET AF Patients According to Major Bleeding Events
| Characteristic | Major Bleed n = 781 | No Major Bleed n = 13,455 | p Value |
|---|---|---|---|
| Age, yrs | 75 (69–80) | 73 (65–78) | <0.0001 |
| <65 yrs | 118 (15.1) | 3,170 (23.6) | |
| ≥65–74 yrs | 236 (30.2) | 4,497 (33.4) | <0.0001 |
| ≥75 yrs | 427 (54.7) | 5,788 (43.0) | |
|
| |||
| Female | 268 (34.3) | 5,377 (40.0) | 0.0017 |
|
| |||
| Race | 0.21 | ||
| White | 633 (81.1) | 11,225 (83.4) | |
| Black | 9 (1.1) | 170 (1.3) | |
| Asian | 114 (14.6) | 1,667 (12.4) | |
| Other | 25 (3.2) | 393 (2.9) | |
|
| |||
| Body mass index, kg/m2 | 28.0 (25.1–32.0) | 28.2 (25.1–32.0) | 0.42 |
|
| |||
| SBP, mm Hg | 130 (120–140) | 130 (120–140) | 0.12 |
|
| |||
| DBP, mm Hg | 80 (70–85) | 80 (70–84) | <0.0001 |
|
| |||
| AF type | |||
| Persistent/permanent | 638 (81.7) | 10,887 (80.9) | |
| Paroxysmal | 131 (16.8) | 2,380 (17.7) | 0.42 |
| Newly diagnosed | 12 (1.5) | 188 (1.4) | |
|
| |||
| Medical history | |||
| Hypertension | 705 (90.3) | 12,182 (90.5) | 0.78 |
| Diabetes mellitus | 334 (42.8) | 5,349 (39.8) | 0.05 |
| Coronary artery disease | 318 (40.7) | 5,142 (38.2) | 0.13 |
|
| |||
| Heart failure | 466 (59.7) | 8,428 (62.7) | 0.35 |
| LV dysfunction (<35% EF)* | 528 (84.9) | 8,431 (83.8) | 0.82 |
|
| |||
| Stroke/TIA | 361 (46.2) | 7,090 (52.7) | 0.0004 |
|
| |||
| Non-CNS systemic embolism | 20 (2.6) | 537 (4.0) | 0.052 |
|
| |||
| CHADS2 score | 3 (3–4) | 3 (3–4) | 0.52 |
|
| |||
| CHADS2 score >3 | 329 (42.1) | 5,846 (43.5) | 0.79 |
|
| |||
| Myocardial infarction | 152 (19.5) | 2,308 (17.2) | 0.033 |
|
| |||
| Peripheral arterial disease | 59 (7.6) | 777 (5.8) | 0.02 |
|
| |||
| COPD* | 111 (14.2) | 1,382 (10.3) | <0.0001 |
|
| |||
| Liver disease | 38 (4.9) | 708 (5.3) | 0.62 |
|
| |||
| Alcohol use* | 277 (35.5) | 4,767 (35.4) | 0.43 |
|
| |||
| Current/prior smoking* | 314 (40.2) | 4,467 (33.2) | 0.0001 |
|
| |||
| Prior GI bleeding | 61 (7.8) | 438 (3.3) | <0.0001 |
|
| |||
| Anemia (hemoglobin <13 g/dl in men, <12 g/dl in women)* | 194 (25.1) | 1,782 (13.5) | <0.0001 |
|
| |||
| Creatinine clearance, ml/min/1.73 m2 | 63 (49–82) | 68 (52–87) | <0.0001 |
|
| |||
| Prior ASA use | 330 (42.3) | 4,864 (36.2) | <0.0001 |
|
| |||
| Prior vitamin K antagonist use | 519 (66.5) | 8,370 (62.2) | 0.018 |
Values are median (25th–75th percentiles) or n (%).
Data not available: left ventricular (LV) dysfunction status: n = 3,557; chronic obstructive pulmonary disease (COPD): n = 6; alcohol use: n = 1; smoking: n = 3; and anemia: n = 270.
AF = atrial fibrillation; ASA = acetylsalicylic acid; CHADS2 = congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, prior stroke, transient ischemic attack (TIA), or noncentral nervous system thromboembolism (doubled); CNS = central nervous system; DBP = diastolic blood pressure; EF = ejection fraction; GI = gastrointestinal; ROCKET AF = Rivaroxaban Once-daily oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; SBP = systolic blood pressure; TIA = transient ischemic attack.
Tables 4 and 5 present the number of major bleeding events, patient-years of follow-up, and the number of bleeding events/100 patient-years according to the contemporary bleeding risk stratification schemas HAS-BLED (7) and ATRIA (6). The predictive value of these schemas deteriorated when applied to the ROCKET AF population when compared with the original derivation cohorts (for HAS-BLED: c-statistic 0.55 [0.53 to 0.57] vs. 0.72 [0.65 to 0.79] (7) and for ATRIA: 0.60 [0.58 to 0.63] vs. 0.74 [0.70 to 0.78]) (6).
Table 4.
HAS-BLED Score in ROCKET AF Trial and Original HAS-BLED Cohort
| Score | ROCKET-AF
|
HAS-BLED Cohort
|
|||
|---|---|---|---|---|---|
| Major Bleedings | Patient-Yrs of Follow-Up | Bleeds/100 Patient-Yrs | Major Bleedings | Bleeds/100 Patient-Yrs | |
| 0 | 1 | 255.2 | 0.71 | 9 | 1.13 |
|
| |||||
| 1 | 45 | 2,686.3 | 2.81 | 13 | 1.02 |
|
| |||||
| 2 | 201 | 9,323.0 | 2.95 | 14 | 1.88 |
|
| |||||
| 3 | 330 | 7,643.7 | 3.63 | 7 | 3.74 |
|
| |||||
| 4 | 170 | 2,087.0 | 4.37 | 4 | 8.70 |
|
| |||||
| 5 | 31 | 139.8 | 5.51 | 1 | 12.50 |
|
| |||||
| 6 | 2 | 2.0 | 7.07 | 0 | 0.00 |
|
| |||||
| Any score | 780 | 22,137.0 | 3.52 | 48 | 1.56 |
|
| |||||
| c-index | 0.546 | 0.72 | |||
HAS-BLED = Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile International Normalized Ratio, Elderly, Drugs/alcohol; ROCKET AF = Rivaroxaban Once-daily oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
Table 5.
ATRIA Score in ROCKET AF Trial and Original ATRIA Cohort
| Score | ROCKET-AF
|
ATRIA Cohort
|
|||
|---|---|---|---|---|---|
| Major Bleedings | Patient-Yrs of Follow-Up | Bleeds/100 Patient-Yrs | Major Bleedings | Bleeds/100 Patient-Yrs | |
| 0 | 30 | 1,264.4 | 2.37 | 9 | 0.48 |
|
| |||||
| 1 | 234 | 9,905.8 | 2.36 | 14 | 0.58 |
|
| |||||
| 2 | 38 | 760.9 | 4.99 | 14 | 0.78 |
|
| |||||
| 3 | 259 | 6,849.5 | 3.78 | 38 | 1.27 |
|
| |||||
| 4 | 81 | 1,443.4 | 5.61 | 18 | 2.41 |
|
| |||||
| 5 | 19 | 166.3 | 11.42 | 13 | 4.18 |
|
| |||||
| 6 | 96 | 1,262.3 | 7.61 | 31 | 5.11 |
|
| |||||
| 7 | 15 | 89.6 | 16.74 | 5 | 3.56 |
|
| |||||
| 8 | — | — | — | 4 | 23.11 |
|
| |||||
| 9 | 0 | 9.9 | 0.00 | 6 | 10.13 |
|
| |||||
| 10 | 0 | 1.2 | 0.00 | 2 | 16.34 |
|
| |||||
| Any score | 772 | 21,753.5 | 3.55 | 154 | 1.40 |
|
| |||||
| c-index | 0.605 | 0.74 | |||
ATRIA = AnTicoagulation and Risk factors In Atrial fibrillation; ROCKET AF = Rivaroxaban Once-daily oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
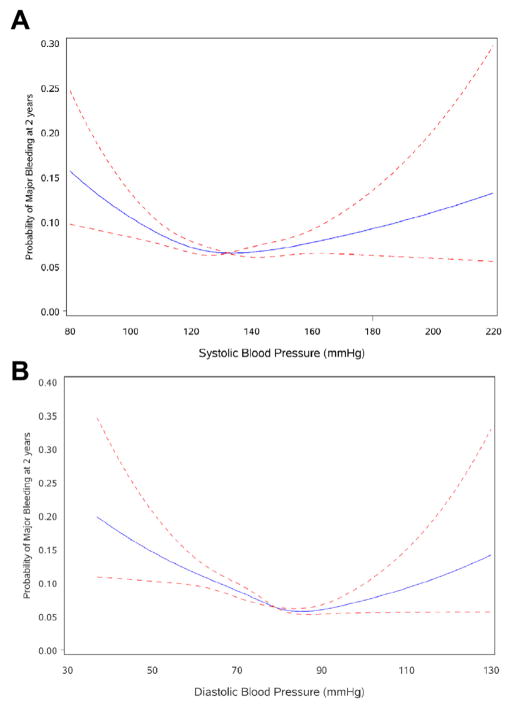
Table 6 presents the multivariable model examining factors associated with major bleeding derived from the ROCKET AF cohort. Increasing age, baseline DBP ≥90 mm Hg, history of COPD, GI bleeding, prior ASA use, and anemia were associated with an increased risk, whereas female sex and DBP <90 mm Hg were associated with a decreased risk for major bleeding. Figure 3 illustrates the “v-shaped” univariate relationship of both baseline SBP and DBP with the risk of major bleeding. Study treatment was not independently associated with major bleeding (p = 0.38) when added to the model; however, when we investigated a separate model restricted to patients randomized to rivaroxaban, female sex and DBP <90 mm Hg were not independently associated with major bleeding, whereas body mass index and peripheral artery disease were of borderline significance (data not shown). None of the independent predictors for bleeding interacted with study treatment, with the exception of prior history of GI bleeding (p = 0.002). Patients on a regimen of rivaroxaban compared with warfarin were at higher risk of major bleeding if they had a prior history of GI bleeding (HR: 2.33; 95% CI: 1.39 to 3.88), whereas there was a similar risk for major bleeding if there was no prior history of GI bleeding (HR: 1.00; 95% CI: 0.86 to 1.16). However, prior history of GI bleeding was included in the major bleeding model, and adding the treatment by prior GI bleeding interaction term to the prediction model minimally improved the c-index from 0.6445 to 0.6455.
Table 6.
Multivariable Model Predicting Major Bleeding in the ROCKET AF Cohort
| Independent Predictor | HR | 95% CIs | Chi-Square | p Value |
|---|---|---|---|---|
| Age (per 5-yr increase) | 1.17 | 1.12–1.23 | 53.0 | <0.0001 |
| Sex (female vs. male) | 0.82 | 0.70–0.95 | 6.7 | 0.009 |
| DBP <90 mm Hg (per 5-mm Hg increase) | 0.92 | 0.89–0.96 | 17.7 | <0.0001 |
| DBP ≥90 mm Hg (per 5-mm Hg increase) | 1.28 | 1.11–1.47 | 12.0 | 0.0005 |
| History of COPD | 1.29 | 1.05–1.58 | 5.8 | 0.016 |
| History of GI bleeding | 1.88 | 1.44–2.45 | 21.9 | <0.0001 |
| Prior use of aspirin | 1.42 | 1.23–1.64 | 22.8 | <0.0001 |
| Anemia at baseline | 1.88 | 1.59–2.22 | 53.8 | <0.0001 |
Figure 3. Blood Pressure at Randomization and Major Bleeding.
Univariate relationship between systolic (SBP) and diastolic (DBP) blood pressure at randomization and major bleeding (at 2 years). Solid lines are predicted probabilities of major bleeding at 2 years; dashed lines are 95% confidence intervals. p values for linearity test in both figures >0.0001. SBP (2 linear segments, <135, ≥135): chi-square: 16.53, p = 0.0003, c-index: 0.5326; DBP (2 linear segments, <90, ≥90): chi-square: 51.71, p < 0.0001, c-index: 0.5678.
Discussion
In this large population of patients with nonvalvular AF at moderate-to-high risk for stroke, there were no significant differences in the rates of major bleeding between rivaroxaban and warfarin. A multivariable model derived from the ROCKET AF cohort demonstrated that several baseline clinical characteristics—age, female sex, DBP, history of COPD, GI bleeding, prior ASA use, and anemia—were associated with major bleeding risk. This model was more predictive of major bleeding in the ROCKET AF cohort than the HAS-BLED or ATRIA bleeding risk scores. Apart from prior GI bleeding, which was significantly associated with major bleeding in patients randomized to rivaroxaban but not warfarin, the other risk factors had the same association with bleeding in both randomized oral anticoagulation groups.
Other studies comparing new oral anticoagulants with warfarin
Although ROCKET AF patients were at increased risk for both stroke (e.g., 55% with prior stroke, TIA, or systemic embolism and a median CHADS2) risk score of 3 and bleeding (e.g., median age 73 years, 91% with hypertension), the major bleeding rates were similar to those in recent studies (14–18). However, cross-trial comparisons are challenging, because the patient populations and associated risk factors for stroke and bleeding, definitions of bleeding, and newer anticoagulants were clearly different in these trials. Of note, Eikelboom et al. (17) reported an important age × treatment interaction with the 2 different dabigatran doses compared with warfarin, suggesting that the risk of extra- but not intracranial bleeding was increased with the 150-mg twice-daily dose in those ≥75 years of age. In the ROCKET AF trial, we did not observe an age × treatment interaction with rivaroxaban compared with warfarin; the relative risk for major bleeding was similar, regardless of age, whereas intra-cranial bleeding risk was lower with rivaroxaban (statistically in those <75 years and numerically in those ≥75 years with a nonsignificant p value for interaction).
Bleeding location
Although mucosal bleeding (e.g., epistaxis, hematuria, GI) was increased, intracranial hemorrhage with rivaroxaban was decreased. The consistently lower rates of intracranial bleeding observed with the newer oral anticoagulants versus warfarin might be due to the effect on a single target in the hemostatic system (e.g., factor Xa inhibition) compared with the multiple targets by warfarin (17). In addition, it has been speculated that the presence of large amounts of tissue factor in the cerebral vascular beds could modulate vascular hemostatic activity within brain vessels whereby warfarin decreases factor VII activity, but the newer agents do not affect the tissue factor-factor VIIa complex (17). It has been postulated that higher rates of GI bleeding with rivaroxaban (and dabigatran) relative to warfarin could be due to exacerbation of surface bleeding by the presence of active anticoagulant in the gut. Whereas warfarin has over 99% bioavailability and unabsorbed warfarin is inactive, all of the new oral anticoagulants are partially excreted in the feces as active drug (19).
Application of established bleeding risk scores in ROCKET AF
Although the performance of the ATRIA and HAS-BLED predictive bleeding risk scores in the ROCKET AF population was limited, a number of qualifications must be taken into account. For example, the ROCKET AF trial did not record and/or measure in a different manner some of the variables (e.g., hypertension). In addition, the ROCKET AF trial specifically excluded some patients with hemorrhagic risk-related criteria and/or concomitant conditions. Furthermore, although HAS-BLED requires determination of “labile INR” status (on the basis of TTR <60%) (7) while on a regimen of vitamin K antagonist therapy, the ROCKET AF study design (requirement for discontinuation of warfarin and an INR <3.0) precluded accurate INR assessment pre-randomization. Therefore, although this cohort described 781 major bleeding events over a median 19-month time period, the low predictive value of the HAS-BLED and ATRIA risk scores in the ROCKET AF population might be more a reflection of incomplete variable information than a lack of validity of these schemas. Furthermore, the discriminatory capacity of bleeding risk scores seems relatively limited (e.g., c-indexes in the 0.70 range) in both original model development and subsequent validation cohorts (20). Finally, no trials have shown the value of withholding stroke prevention therapy in patients with a high bleeding risk score, and it remains unclear as to what bleeding risk threshold would lead to an alternative (or no) antithrombotic strategy employment.
Factors associated with major bleeding in ROCKET AF
We identified increasing age, prior (GI) bleeding, anemia at baseline, and (pre-randomization) ASA use as independent predictors of major bleeding, consistent with findings from several studies examining risk factors for anticoagulation-related bleeding in patients with AF (2–4,6,7,21). Many studies have also identified hypertension, including SBP elevation, as a predictor of major bleeding; in the ROCKET AF trial we observed a dichotomous relationship between the risk of bleeding and DBP such that a baseline DBP ≥90 mm Hg was an independent predictor of increased risk, whereas a baseline DBP <90 mm Hg was associated with a significantly lower risk. Female sex has also been noted in some studies to be associated with an increased risk of bleeding with warfarin in patients with either AF (2,22) or acute venous thromboembolism (5); in contrast, in the ROCKET AF trial women were at significantly lower risk for major bleeding. Finally, we identified that a history of COPD, presumably a marker for important comorbidity (23), was associated with an increased risk for major bleeding.
Some differences in major bleeding rates were noted according to race and region with associated significant interaction p values (Fig. 2). However, race was not a significant predictor of major bleeding in univariate analysis, and regional differences are confounded by multiple differences in baseline characteristics. For example, North American patients were much older and had a higher proportion of comorbidities associated with higher bleeding rates (e.g., hypertension, anemia). Alternatively, these subgroup findings of a single component of the primary safety endpoint might be simply due to chance; this potential explanation is supported, because no difference in region was observed when examining the composite endpoint of major bleeding and clinically relevant nonmajor bleeding. Thus, we did not include race or region in our multivariable model but rather all of the previously established baseline characteristics associated with bleeding.
Study limitations
We did not identify other predictors such as renal dysfunction, liver disease, or prior stroke as independent predictors of major bleeding, in contrast to some other studies. Although warfarin has no renal metabolism, rivaroxaban has a dual mode of elimination. Approximately one-third of rivaroxaban is eliminated unchanged by the kidneys with the remaining approximately two-thirds of the drug being metabolized by the liver, after which one-half of the metabolized fraction is excreted in urine and the other one-half is excreted in feces (11). Therefore, patients were excluded from the ROCKET AF trial if their estimated creatinine clearance was <30 ml/min at the screening visit, and those included in the trial with moderate renal dysfunction (creatinine clearance 30 to 49 ml/min; n = 2,950 [20.7%]) received a reduced dose of rivaroxaban (15 mg instead of 20 mg) on the basis of extensive pharmacodynamic data and pharmacokinetic modeling (24). Although ROCKET AF patients with moderate renal dysfunction had higher rates of stroke and bleeding than those with normal renal function, there was no evidence of heterogeneity in treatment effect across dosing groups, and the findings with rivaroxaban were consistent with the overall trial, in comparison with dose-adjusted warfarin (e.g., fatal bleeding occurred less often with rivaroxaban) (24). The ROCKET AF trial is unique among contemporary studies comparing novel anticoagulants with warfarin in AF with respect to overall stroke risk on the basis of the inclusion of 55% of patients with prior stroke, TIA, or systemic embolism. Therefore, although prior stroke has been identified in some studies of AF patients on a regimen of oral anticoagulation as an independent risk factor for major bleeding (3,4), more than 1 in 2 patients in the ROCKET AF trial had a prior event, and this might have impacted the potential independent predictive value of previous stroke in our population.
Conclusions
Our analyses of bleeding from the ROCKET AF trial indicate that rivaroxaban compared with warfarin provides important safety benefits in patients with AF at moderate-to-high risk for stroke or systemic embolism. Despite higher rates of bleeding from GI sites and bleeding that led to a drop in hemoglobin level or was treated with transfusion, rivaroxaban compared with warfarin led to a similar overall risk of major and nonmajor clinically relevant bleeding and a lower risk of intracranial hemorrhage and fatal bleeding. We identified, consistent with previous studies, several baseline factors associated with the risk of major bleeding, including age, sex, DBP, prior (GI) bleeding and ASA use, and anemia. Careful assessment of bleeding risk in patients with AF is required to support clinical decision making for stroke prevention therapy. With the exception of prior GI bleeding, which was significantly associated with major bleeding in patients randomized to rivaroxaban but not warfarin, the identified risk factors for bleeding had the same association in both oral anticoagulation groups. Finally, it is important to recognize that risk of major bleeding must be placed in the context of stroke prevention demonstrated with anticoagulation therapy, especially because some factors associated with an increased risk of bleeding are also associated with the risk of stroke; indeed, no study has demonstrated improved outcome on the basis of bleeding risk assessment.
Acknowledgments
The ROCKET AF trial was supported by Johnson & Johnson Pharmaceutical Research and Development and Bayer HealthCare. The Duke Clinical Research Institute coordinated the trial, managed the database, and performed the analyses independently of the sponsors. The Executive Committee designed the trial, was responsible for overseeing the conduct of the study, retained the ability to independently analyze and present the data, made the decision to submit the manuscript for publication, and takes responsibility for the accuracy and completeness of the data and all analyses. Dr. Goodman has received consulting fees/honoraria and/or research grant support from Bayer, Johnson & Johnson, Boehringer Ingelheim, Bristol-Myers Squibb, Sanofi-Aventis. Dr. Piccini has received consulting fees/honoraria and/or research grant support from Johnson & Johnson, GE Healthcare, Resmed, ARCA Biopharma, BMS/Pfizer, Sanofi-Aventis, Medtronic, Inc., Spectranetics, and Forest Laboratories. Dr. White has received consulting fees/honoraria and/or research grant support from Sanofi-Aventis, Eli Lilly, The Medicines Company, National Institutes of Health, Pfizer, Roche, Johnson & Johnson, Schering-Plough, Merck & Co., Inc. Sharp and Dohme, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, Bristol-Myers Squibb, and Regado. Dr. Paolini was employed by and has held stock options for Bayer HealthCare. Dr. Nessel is employed by and owns stock in Johnson & Johnson. Dr. Berkowitz is employed by Bayer HealthCare. Dr. Mahaffey has received consulting fees/honoraria and/or research grant support from Johnson & Johnson, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pozen, Regado Biosciences, Sanofi-Aventis, Schering-Plough, The Medicines Company, Daiichi Sankyo, and Pfizer. Dr. Patel has received consulting fees/honoraria and/or research grant support from Johnson & Johnson, AstraZeneca, AHRQ, NHBLI, Bayer, Otsuka Pharmaceutical Co., Ltd., and Ortho McNeil Janssen. Dr. Becker has received consulting fees/honoraria and/or research grant support from Johnson & Johnson, Regado, Boehringer Ingelheim, Astra-Zeneca, Daiichi Sankyo, and Bayer. Dr. Halperin has received consulting fees/honoraria and/or research grant support from AstraZeneca, Bayer AG HealthCare, Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, Pfizer, Sanofi-Aventis, Biotronik, Inc., Duke Clinical Research Institute, Boston Scientific, and Ortho-McNeil-Janssen Pharmaceuticals. Dr. Hacke has received consulting fees/honoraria and/or research grant support from Bayer, Johnson & Johnson, and Boehringer Ingelheim. Dr. Singer has received consulting fees/honoraria and/or research grant support from Bayer, Bristol-Myers Squibb, CSL Behring, Johnson & Johnson, Merck & Co., Inc. Pfizer, Sanofi-Aventis, CVS Caremark, Boehringer Ingelheim, Daiichi Sankyo, Bristol-Myers Squibb, CSL Behring, and Pfizer. Dr. Hankey has received consulting fees/honoraria and/or research grant support from Johnson & Johnson, Bayer, and theheart.org. Dr. Breithardt has received consulting fees/honoraria and/or research grant support from Bayer HealthCare, Johnson & Johnson, Meda Pharma, Sanofi-Aventis, St. Jude, Boehringer Ingelheim, Boston Scientific, Otsuka Pharmaceutical Co., Ltd., MSD, and 3M. Dr. Fox has received consulting fees/honoraria and/or research grant support from Bayer, Johnson & Johnson, Eli Lilly and Company, Sanofi-Aventis, AstraZeneca, and Boehringer Ingelheim. Dr. Califf has received consulting fees/honoraria and/or research grant support from Johnson & Johnson, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi Sankyo. Dr. Mahaffey is now affiliated with the Department of Medicine, Stanford University, Stanford, California. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
A complete listing of steering committee members and trial investigators in the ROCKET AF trial is provided in the Supplementary Appendix to Patel et al. (1).
Abbreviations and Acronyms
- AF
atrial fibrillation
- ASA
acetylsalicylic acid
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- DBP
diastolic blood pressure
- GI
gastrointestinal
- HR
hazard ratio
- INR
international normalized ratio
- SBP
systolic blood pressure
- TIA
transient ischemic attack
- TTR
time in the therapeutic (INR) range
References
- 1.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 2.Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant-antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke. 2004;35:2362–7. doi: 10.1161/01.STR.0000141933.75462.c2. [DOI] [PubMed] [Google Scholar]
- 3.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–9. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–9. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 5.Kuijer PM, Hutten BA, Prins MH, Büller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159:457–60. doi: 10.1001/archinte.159.5.457. [DOI] [PubMed] [Google Scholar]
- 6.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 8.Loewen P, Dahri K. Risk of bleeding with oral anticoagulants: an updated systematic review and performance analysis of clinical prediction rules. Ann Hematol. 2011;90:1191–200. doi: 10.1007/s00277-011-1267-3. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Andreotti F, Fauchier L, et al. Bleeding risk assessment and management in atrial fibrillation patients. Executive Summary of a Position Document from the European Heart Rhythm Association (EHRA), endorsed by the European Society of Cardiology (ESC) Working Group on Thrombosis. Thromb Haemost. 2011;106:997–1011. doi: 10.1160/TH11-10-0690. [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser SH. Stroke prevention versus bleeding risk in atrial fibrillation: a clinical dilemma. J Am Coll Cardiol. 2011;57:181–3. doi: 10.1016/j.jacc.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 11.The Executive Steering Committee, on behalf of the ROCKET AF Study Investigators. Rivaroxaban-Once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340–7. doi: 10.1016/j.ahj.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S, Kearon C the Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial. J Am Heart Assoc. 2013;2:e000067. doi: 10.1161/JAHA.112.000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SPORTIF Executive Steering Committee for the SPORTIF V Investigators. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. JAMA. 2005;293:690–8. doi: 10.1001/jama.293.6.690. [DOI] [PubMed] [Google Scholar]
- 15.The ACTIVE Writing Group on behalf of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903–12. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 16.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation. Circulation. 2011;123:2363–72. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 19.Weitz JI. New oral anticoagulants: a view from the laboratory. Am J Hematol. 2012;87:S133–6. doi: 10.1002/ajh.23139. [DOI] [PubMed] [Google Scholar]
- 20.Apostolakis S, Lane DA, Guo Y, Buller H, Lip GYH. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk-prediction scores in patients with atrial fibrillation undergoing anticoagulation. The AMADEUS (Evaluating the Use of SR34006 Compared to Warfarin or Acenocoumarol in Patients With Atrial Fibrillation) study. J Am Coll Cardiol. 2012;60:861–7. doi: 10.1016/j.jacc.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Hughes M, Lip GY on behalf of the Guideline Development Group for the NICE national clinical guideline for management of atrial fibrillation in primary and secondary care. Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. QJM. 2007;100:599–607. doi: 10.1093/qjmed/hcm076. [DOI] [PubMed] [Google Scholar]
- 22.Sam C, Massaro JM, D’Agostino RB, et al. Warfarin and aspirin use and the predictors of major bleeding complications in atrial fibrillation (The Framingham Heart Study) Am J Cardiol. 2004;94:947–51. doi: 10.1016/j.amjcard.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 23.Sode BF, Dahl M, Nordestgaard BG. Myocardial infarction and other comorbidities in patients with chronic obstructive pulmonary disease: a Danish Nationwide Study of 7.4 million individuals. Eur Heart J. 2011;32:2365–75. doi: 10.1093/eurheartj/ehr338. [DOI] [PubMed] [Google Scholar]
- 24.Fox KAA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32:2387–94. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]