Abstract
Background
Guidelines recommend ICD candidates have an estimated longevity of at least 1 year. Longevity can be affected by CKD.
Methods and Results
Using the National Cardiovascular Data Registry ICD registry linked with the Social Security Death Master File, we assessed the rate of death after primary prevention ICD placement between January 1, 2006 and December 31, 2007, according to CKD stage. Using Cox models, we identified factors associated with death among CKD patients. Compared with patients without CKD (n=26,056), those with CKD (n=21,226) were older, less commonly male, more often white, and more frequently had comorbid illness. Compared with patients without CKD, patients with a glomerular filtration rate (GFR) 30–60, GFR <30, and end-stage renal disease on dialysis had a higher risk of death after ICD placement (hazard ratio (HR) 2.08, 95% confidence interval (CI) 1.99–2.18, p<0.0001; HR 4.20, 95% CI 3.92–4.50, p<0.0001; HR 4.80, 95% CI 4.46–5.17, p<0.0001, respectively). Corresponding one-year death rates were 4.4%, 9.1%, 20.2%, and 22.4%. Among patients with CKD, factors associated with increased risk of death included CKD severity, age > 65 years, heart failure symptoms, diabetes mellitus, lung disease, serum sodium < 140 mEq/L, a trial fibrillation or flutter, and a lower ejection fraction.
Conclusions
The risk of death after primary prevention ICD placement is proportional to CKD severity. Among CKD patients, several factors are prognostically significant and could inform clinical decision making regarding primary prevention ICD candidacy.
Keywords: implanted cardioverter defibrillator, chronic kidney disease
Introduction
Heart failure afflicts more than 5 million Americans,1 approximately half of whom have concomitant chronic kidney disease (CKD).2 Both of these diseases are increasing in prevalence1, 3 and both elevate the risk of all-cause death.1, 4 The implantable cardioverter-defibrillator (ICD) has a survival benefit in individuals with left ventricular dysfunction at risk for arrhythmic death;5, 6 however, CKD is associated with an increased risk of non-arrhythmic death.7 Further, patients with advanced CKD were underrepresented in the clinical trials establishing the efficacy and cost-effectiveness of the ICD.5, 6, 8
An association between CKD and death has been observed in the general population9, 10 as well as among primary prevention ICD recipients.11, 12 A graded relationship between CKD severity and death has also been observed in the general population and high-risk cohorts.10 However, this relationship has been incompletely characterized in recipients of primary prevention ICDs. Prior studies were limited to one or two centers and hampered by modest sample sizes.13–17 Defining the relationship between CKD severity and death and identifying factors associated with an increased risk of death in such patients are particularly important, as professional guidelines recommend that ICD candidates have an estimated longevity of at least 1 year.18 Accordingly, we used the National Cardiovascular Data Registry’s (NCDR) ICD registry linked with the Social Security Death Master File to (1) examine the unadjusted risk and temporal pattern of death after primary prevention ICD placement according to CKD severity, and (2) identify factors associated with death among patients with an ICD and CKD.
Methods
Data Sources
Data for this study were derived from the NCDR ICD Registry and the Social Security Death Master File. A collaboration of the Heart Rhythm Society and the American College of Cardiology Foundation, the NCDR ICD Registry was established in 2005 and became the only repository for ICD placement data for Medicare beneficiaries on April 1, 2006. The Centers for Medicare & Medicaid Services mandate data input on its beneficiaries receiving a primary prevention ICD into this Registry. Nonetheless, approximately 78% of participating hospitals enter data on all ICD implantations irrespective of payer or indication.19 These data include patient demographics, medical history, and clinical information, including preprocedure creatinine levels and whether patients are dialysis-dependent. Vital status was available for patients via the Social Security Death Master file through December 31, 2009. Patients without a file during the study period were assumed alive. For patients with several device implants in the registry, the index implant was selected for analysis.
Study Cohort
We selected patients who underwent primary prevention ICD placement and were discharged home alive between January 1, 2006, and December 31, 2007. Patients with a history of myocardial infarction and a left ventricular ejection fraction ≤ 30% or a history of congestive heart failure with a left ventricular ejection fraction ≤ 35% were included. Patients with New York Heart Association Class IV symptoms, a myocardial infarction within 40 days prior to implant or coronary artery bypass grafting within 90 days prior to implant, new-onset heart failure within 3 months of diagnosis, or inducible sustained ventricular tachycardia on electrophysiology study as the indication for device placement were excluded. Those receiving a biventricular device, or who were missing covariates necessary for determining eligibility for inclusion in this analysis, were also excluded.
Statistical Analysis
Glomerular filtration rate (GFR) was estimated from the preprocedure creatinine using the Chronic Kidney Disease Epidemiology Collaboration equation.20 Baseline characteristics of patients with renal disease (estimated GFR ≤ 60 mL/min per 1.73 m2) were compared with those without renal disease (estimated GFR >60 mL/min per 1.73 m2). Continuous variables are presented as medians with 25th and 75th percentiles, and categorical variables are presented as percentages and counts. Differences between groups were assessed using the Wilcoxon rank sum test and Pearson chi-square test as appropriate. Patients were then categorized according to GFR and whether they were dialysis-dependent: >60 mL/min per 1.73 m2, 30–60 mL/min per 1.73m2, <30 mL/min per 1.73 m2 but not on dialysis, and dialysis-dependent. Unadjusted event rates of death and in-hospital complications and comparisons were identified for various levels of renal function. Unadjusted Kaplan-Meier 1-year and 3-year all-cause death rates and corresponding curves of cumulative risk of death were generated for each renal function group. An unadjusted Cox proportional hazards model was used to test for a difference between the group without renal disease and each of the other three groups. As our primary interest was in assessing death risk among patients with CKD, we examined whether the relationship between candidate predictors and death risk varied with renal function, by testing interactions between each variable and the presence or absence of CKD, one at a time in otherwise unadjusted models. The substantial number of significant interactions indicated that explanation of death risk among patients with CKD might be considerably different than among patients without CKD, and that development of the model in the subgroup of CKD patients was warranted. A multivariable Cox proportional hazards model assessing the impact of renal disease among those with an estimated GFR < 60 mL/min per 1.73 m2 was then fitted. The linearity of continuous variables’ relationships to death was assessed, and cubic-polynomial splines were used as needed. For candidate predictors in the model, missing data were imputed to the median for continuous variables and the mode for categorical variables. Rates of “missingness” were <1% for any variable under consideration. We performed a sensitivity analysis in which patients were weighted by the inverse of their propensity to have missing covariates,21 to confirm that the simple imputation method did not introduce bias; results from this analysis were very similar to the original analysis. Covariates were entered into the model using forward stepwise selection. Renal disease terms were retained in the model regardless of their significance. The proportional hazards assumption for three major covariates that might be expected to violate the assumption—age, gender, and renal disease group—was tested using a stratified multivariable Cox model with those 3 covariates as strata; there were no substantive changes in estimated hazard ratios (HRs) and 95% confidence intervals (CIs).
Results are presented as HRs and 95% CIs, and an alpha level of 0.05 was used for statistical significance. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC). The Duke University Health System institutional review board approved the study and determined that informed consent was not applicable to data collected by the ICD Registry.
Results
After applying our exclusion criteria, the final study cohort consisted of 47,282 patients from 1134 sites. Compared with patients without CKD disease, those with CKD disease were older; less commonly male; more often white; and more frequently covered by Medicare (Table 1). They had more advanced heart failure symptoms and more frequently had other comorbid illness, including atrial fibrillation or flutter, abnormal sinus node function, ischemic heart disease and prior revascularization, cerebrovascular disease, diabetes mellitus, and hypertension. They also more commonly had electrocardiographic abnormalities, including a wide QRS complex, left bundle-branch block, and atrioventricular conduction delay.
Table 1.
Baseline Patient Characteristics*
| Baseline characteristic | All Patients (n=47,282) | Chronic kidney disease† (n=21,226) | No chronic kidney disease (n=26,056) | P‡ |
|---|---|---|---|---|
|
| ||||
| Age, years | 67 (57, 75) | 72 (64, 78) | 62 (53, 70) | <.0001 |
|
| ||||
| Male | 74.8 (35374) | 70.3 (14926) | 78.5 (20448) | <.0001 |
|
| ||||
| White race | 78.6 (37138) | 80.5 (17089) | 76.9 (20049) | <.0001 |
|
| ||||
| Insurance | <.0001 | |||
| Medicare | 62.0 (29064) | 75.0 (15818) | 51.3 (13246) | |
| Medicaid | 5.6 (2638) | 3.5 (733) | 7.4 (1905) | |
| Commercial | 21.2 (9943) | 13.5 (2846) | 27.5 (7097) | |
| Health maintenance organization | 8.5 (3984) | 6.6 (1383) | 10.1 (2601) | |
| Other/None | 2.7 (1249) | 1.4 (301) | 3.7 (948) | |
|
| ||||
| New York Heart Association Class | <.0001 | |||
| I | 9.4 (4437) | 7.4 (1572) | 11.0 (2865) | |
| II | 54.1 (25586) | 51.7 (10981) | 56.1 (14605) | |
| III | 36.5 (17259) | 40.9 (8673) | 33.0 (8586) | |
|
| ||||
| Other medical conditions | ||||
|
| ||||
| Atrial fibrillation or flutter | 27.2 (12834) | 33.2 (7045) | 22.2 (5789) | <.0001 |
|
| ||||
| Non-sustained ventricular tachycardia | 19.8 (9370) | 20.5 (4347) | 19.3 (5023) | 0.0011 |
|
| ||||
| Sinus node dysfunction | 22.8 (10760) | 27.5 (5821) | 19.0 (4939) | <.0001 |
|
| ||||
| Nonischemic cardiomyopathy | 32.7 (15463) | 27.7 (5881) | 36.8 (9582) | <.0001 |
|
| ||||
| Ischemic heart disease | 71.4 (33755) | 76.6 (16264) | 67.1 (17491) | <.0001 |
|
| ||||
| Prior myocardial infarction | 61.3 (28967) | 63.9 (13571) | 59.1 (15396) | <.0001 |
|
| ||||
| Prior coronary artery bypass grafting | 36.9 (17446) | 42.8 (9080) | 32.1 (8366) | <.0001 |
|
| ||||
| Prior percutaneous intervention | 35.9 (16968) | 35.6 (7551) | 36.2 (9417) | 0.20 |
|
| ||||
| Prior valvular surgery | 5.7 (2708) | 6.8 (1439) | 4.9 (1269) | <.0001 |
|
| ||||
| Cerebrovascular disease | 14.5 (6859) | 17.4 (3684) | 12.2 (3175) | <.0001 |
|
| ||||
| Chronic lung disease | 21.9 (10370) | 23.1 (4900) | 21.0 (5470) | <.0001 |
|
| ||||
| Diabetes mellitus | 37.5 (17741) | 43.6 (9251) | 32.6 (8490) | <.0001 |
|
| ||||
| Hypertension | 75.8 (35852) | 79.8 (16939) | 72.6 (18913) | <.0001 |
|
| ||||
| Renal failure with dialysis | 3.8 (1812) | 8.5 (1812) | 0 | <.0001 |
|
| ||||
| Left ventricular ejection fraction, mean (SD) | 24.9 (6.1) | 25.0 (6.1) | 24.8 (6.2) | 0.0099 |
|
| ||||
| QRS duration, ms | <.0001 | |||
| < 120 | 69.2 (32731) | 65.2 (13840) | 72.5 (18891) | |
| ≥ 120 and < 140 | 13.5 (6369) | 14.4 (3067) | 12.7 (3302) | |
| ≥ 140 | 17.3 (8182) | 20.3 (4319) | 14.8 (3863) | |
|
| ||||
| Intraventricular conduction delay | 10.5 (4934) | 11.0 (2327) | 10.0 (2607) | 0.0007 |
|
| ||||
| Left bundle branch block | 13.1 (6191) | 14.7 (3121) | 11.8 (3070) | <.0001 |
|
| ||||
| Atrioventricular block | <.0001 | |||
| None | 81.5 (38395) | 77.7 (16498) | 84.6 (21979) | |
| 1st degree | 16.1 (7566) | 19.2 (4050) | 13.5 (3516) | |
| 2nd or 3rd degree | 2.5 (1160) | 3.2 (678) | 1.9 (482) | |
|
| ||||
| Sodium, mEq/L, mean (SD) | 138.7 (3.4) | 138.8 (3.6) | 138.7 (3.3) | 0.0063 |
|
| ||||
| Systolic blood pressure | 129 (114, 144) | 130 (115, 146) | 128 (114, 142) | <.0001 |
|
| ||||
| Dual-chamber ICD | 56.9 (26920) | 59.7 (12674) | 54.7 (14246) | <.0001 |
Data are presented as median (25th, 75th percentiles) for continuous variables and % (n) for categorical variables unless otherwise noted.
Chronic kidney disease is defined as GFR ≤ 60 or on dialysis.
P-values are from Pearson chi-square test for categorical variables or the Wilcoxon rank sum test for continuous variables.
Patients with advanced CKD had higher rates of hematoma after ICD placement but not lead dislodgement, pneumothorax, cardiac perforation or pericardial tamponade (Table 2). After a median follow-up of 2.9 years, 9,676 patients (20.5%) died. Death rates varied according to the CKD severity (Table 2). Compared with patients without CKD, patients with a GFR 30–60, GFR <30, and end-stage renal disease on dialysis had a higher risk of death after ICD placement (hazard ratio (HR) 2.08, 95% confidence interval (CI) 1.99–2.18, p<.0001; HR 4.20, 95% CI 3.92–4.50, p<.0001; HR 4.80, 95% CI 4.46–5.17, p<.0001, respectively). One-year unadjusted Kaplan-Meier death rates among patients without CKD and the aforementioned CKD categories were 4.4%, 9.1%, 20.2%, and 22.4%, respectively (Table 2). Approximately one in two patients with a GFR<30 or on dialysis died within 3 years of ICD placement. The cumulative risk of death among patients with mild CKD (GFR 30–60) diverged from that of patients without CKD immediately after ICD placement (Figure 1). The greater cumulative risk of death among patients on dialysis compared with those with advanced CKD (GFR<30) was evident by 6 months after ICD placement.
Table 2.
Unadjusted Rates of In-Hospital Complications and Death According to Severity of Chronic Kidney Disease
| No renal disease | GFR 30–60 | GFR < 30 | ESRD on dialysis | |
|---|---|---|---|---|
|
| ||||
| (n=26,056) | (n=16,994) | (n=2,420) | (n=1,812) | |
|
| ||||
| In-hospital complications rates, % | ||||
| Hematoma | 0.7 | 0.8 | 0.8 | 1.5 |
| Lead dislodgment | 0.7 | 0.7 | 0.7 | 0.5 |
| Pneumothorax | 0.4 | 0.5 | 0.2 | 0.3 |
| Cardiac perforation or pericardial tamponade | 0.1 | 0.1 | 0.1 | 0.1 |
|
| ||||
| Follow-up duration among survivors, years (median, IQR) | 2.9 (2.4, 3.3) | 2.9 (2.4, 3.3) | 2.8 (2.4, 3.3) | 2.9 (2.4, 3.3) |
|
| ||||
| Death rate at 1 year, % | 4.4 | 9.1 | 20.2 | 22.4 |
|
| ||||
| Death rate at 3 years, % | 13.5 | 26.2 | 44.7 | 49.0 |
Figure 1.
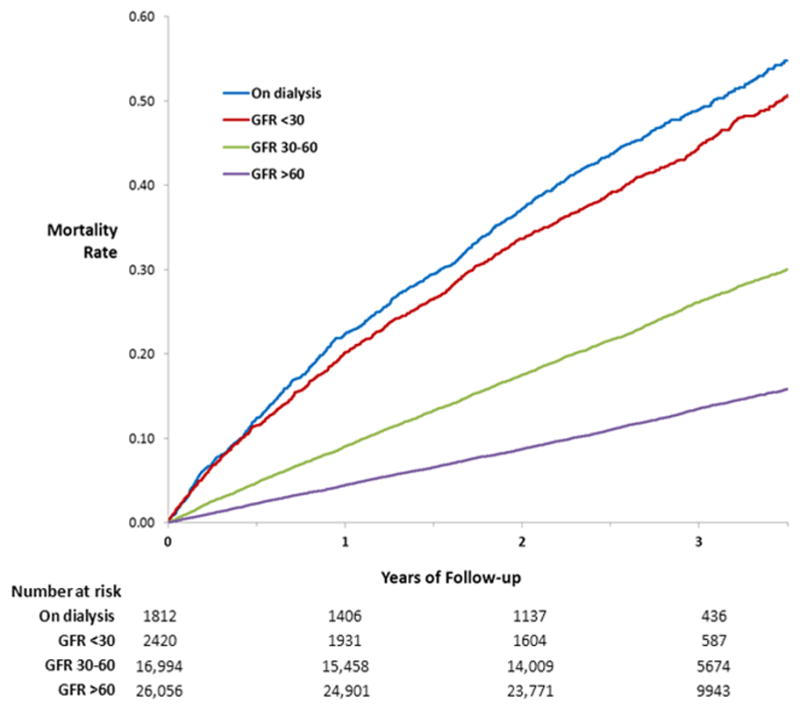
Cumulative risk of death by severity of chronic kidney disease. The risk of death among patients with mild kidney disease (glomerular filtration rate of 30–60) differed from that of patients without kidney disease (glomerular filtration rate > 60) immediately after placement of a primary prevention implantable cardioverter-defibrillator. A higher risk of death among patients on dialysis compared with those with advanced chronic kidney disease (glomerular filtration rate < 30) was evident by 6 months after device placement.
Among patients with CKD, factors most strongly associated with risk of death were severity of CKD (end-stage renal disease on dialysis vs GFR 30–60, HR 2.29, 95% CI 2.12–2.46, p<.0001); GFR < 30 vs GFR 30–60, HR 1.77, 95% CI 1.66–1.90, p<.0001), older age (HR 1.19, 95% CI 1.17–1.21 per 5-year increase above age 65, p<.0001), the degree of heart failure symptoms (New York Heart Association class III vs I, HR 1.63, 95% CI 1.45–1.83; New York Heart Association class II vs I, HR 1.18, 95% CI 1.05–1.32, p<.0001), diabetes mellitus (HR 1.38, 95% CI 1.31–1.45, p<.0001), chronic lung disease (HR 1.38, 95% CI 1.31–1.45, p<.0001), a lower serum sodium (HR 1.28, 95% CI 1.22–1.34 per 5 mEq/L decrease from 140, p<.0001), atrial fibrillation or flutter (HR 1.30, 95% CI 1.23–1.36, p<.0001), and a lower left ventricular ejection fraction (HR 1.10, 95% CI 1.08–1.12 per 5% decrease, p<.0001). Several additional factors were also identified (Table 3).
Table 3.
Factors Associated with Death among Patients with Chronic Kidney Disease in Multivariable Analysis
| Factor | Hazard ratio (95% confidence interval) | χ2 | P | |
|---|---|---|---|---|
|
| ||||
| Severity of kidney disease | 632.2 | <.0001 | ||
| ESRD - on dialysis vs. GFR 30–60 (no dialysis) | 2.29 (2.12, 2.46) | |||
| GFR<30 (no dialysis) vs. GFR 30–60 (no dialysis) | 1.77 (1.66, 1.90) | |||
|
| ||||
| Age, per 5 year increase above 65 | 1.19 (1.17, 1.21) | 390.1 | <.0001 | |
|
| ||||
| NYHA Class | 180.3 | <.0001 | ||
| III vs. I | 1.63 (1.45, 1.83) | |||
| II vs. I | 1.18 (1.05, 1.32) | |||
|
| ||||
| Diabetes mellitus | 1.38 (1.31, 1.45) | 151.7 | <.0001 | |
|
| ||||
| Chronic lung disease | 1.38 (1.31, 1.46) | 131.6 | <.0001 | |
|
| ||||
| Serum sodium, per 5 mEq/L decrease below 140 | 1.28 (1.22, 1.34) | 115.4 | <.0001 | |
|
| ||||
| Atrial fibrillation or flutter | 1.30 (1.23, 1.36) | 95.6 | <.0001 | |
|
| ||||
| Left ventricular ejection fraction, per 5% decrease | 1.10 (1.07, 1.12) | 77.7 | <.0001 | |
|
| ||||
| Systolic blood pressure, per 5 mm Hg decrease below 120 | 1.06 (1.05, 1.08) | 58.7 | <.0001 | |
|
| ||||
| QRS duration, per 10 ms increase up to 120 | 1.07 (1.05, 1.09) | 44.9 | <.0001 | |
|
| ||||
| Nonsustained ventricular tachycardia | 1.21 (1.14, 1.28) | 41.5 | <.0001 | |
|
| ||||
| Ischemic heart disease | 1.24 (1.16, 1.33) | 38.4 | <.0001 | |
|
| ||||
| Medicare or Medicaid, vs. health maintenance organization/commercial/other/none | 1.22 (1.14, 1.31) | 31.2 | <.0001 | |
|
| ||||
| Cerebrovascular disease | 1.17 (1.10, 1.24) | 23.9 | <.0001 | |
|
| ||||
| Non-white race | 1.16 (1.09, 1.24) | 20.2 | <.0001 | |
|
| ||||
| Prior percutaneous coronary intervention | 0.90 (0.85, 0.95) | 15.0 | 0.0001 | |
|
| ||||
| Atrioventricular block, any (1st, 2nd, or 3rd degree) vs. none | 1.11 (1.05, 1.17) | 12.3 | 0.0004 | |
|
| ||||
| Male sex | 1.08 (1.02, 1.14) | 6.9 | 0.0085 | |
Discussion
This is the largest study to examine the survival pattern of CKD patients with a primary prevention ICD as well as factors associated with survival. It has three major findings. First, the risk of death after ICD placement varied according to the severity of CKD. Patients with end-stage renal disease on dialysis have the worst prognosis; however, even mild reductions in GFR are associated with a significant reduction in survival. Second, compared with patients without CKD, those with CKD have a greater burden of comorbid illness and thus are more predisposed to comorbidity-related death. Third, in addition to CKD severity, several other factors are associated with a higher risk of death among CKD patients with an ICD, including older age, the degree of heart failure symptoms, and diabetes mellitus.
A secondary evaluation of the Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II) indicated that the ICD was not beneficial among patients with an eGFR <35 ml/min/1.73 m2 (all-cause death HR 1.09, p=0.84).22 A subgroup analysis of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) found that the ICD was less effective among patients with a GFR < 60 compared with those with a higher GFR (HR 0.74, 95% CI 0.39–1.39 vs. HR 0.27, 95% CI 0.16–0.46, difference p=0.011).23 A meta-analysis of the Multicenter Automatic Defibrillator Implantation Trial-I, the Multicenter Automatic Defibrillator Implantation Trial-II, and Sudden Cardiac Death in Heart Failure Trial indicates ICD efficacy varies according to GFR.24 ICD placement may not significantly alter the natural course of these patients. CKD per se or associated comorbidities may predispose patients to death. Arrhythmic death refractory to defibrillation such as that associated with acute metabolic disarray25 or competing causes of non-arrhythmic death such as pump-failure7 may play significant roles in this regard. In the absence of further studies, professional guidelines do not explicitly address the role of CKD in the selection of ICD candidates. They nonetheless specify that recipients should have an estimated life expectancy of ≥ 1 year.18 Our findings indicate that CKD, even in its most advanced form, is not a strict contraindication to ICD placement based on this criterion. However, among patients with a GFR < 30, approximately 1 in 5 died by 1 year and 1 in 2 by 3 years after ICD placement. Factors associated with survival in our analysis, including older age, the degree of heart failure symptoms, and diabetes mellitus among others, may in fact aid in the selection of candidates most likely to derive a survival benefit from an ICD. CKD is associated with a higher risk of in-hospital complications after ICD placement.26 The current analysis indicates the most clinically relevant in-hospital complication ICD recipients with CKD experience is hematoma, and this is largely limited to those with advanced disease.
It is noteworthy that a prior NCDR analysis examined patients with CKD; however, that analysis was restricted to end-stage renal disease and only examined in-hospital outcomes.27 Previous studies of patients in various stages of CKD were performed in one or two centers and limited by modest sample sizes.13–17 The current analysis characterizes the association between CKD severity and long-term survival on a national scale with a considerable degree of granularity, identifies additional factors associated with death not previously observed in this patient subgroup, and extends the findings to an expansive population of 1134 sites.
Implications of the current analysis are clear. Life expectancy after ICD placement among patients with a GFR < 30 irrespective of whether dialysis has been initiated is sufficiently limited to give patients and physicians pause before proceeding with placement, particularly since these patients are also predisposed to procedural risk11, 27 and infection. A comparison group of ICD non-recipients was not included in the current analysis. The observed mortality rates are nonetheless comparable to those of high-risk patients with multiple comorbidities unlikely to benefit from an ICD.28, 29 Discussions between physicians and patients regarding the potential benefits of the ICD that take into account clinical factors associated with death and competing causes of death may be worthwhile before proceeding with placement. However, the efficacy, effectiveness, and cost-effectiveness of ICD therapy in this patient subgroup remain unknown. Further study in each of these areas is required.
Limitations
The current analysis has several limitations. First, CKD staging for non-dialysis patients was based on creatinine levels obtained just prior to ICD placement, and they might not reflect steady state levels. However, it is expected that because these implants are elective, most creatinine values were at or close to baseline. Second, participation in the ICD Registry is mandatory for ICD recipients with Medicare and thus our findings may not be generalized to other patient populations. However, the majority of participating hospitals enter data on all implantations regardless of insurance, and 38% of the patients in the current analysis had non-Medicare insurance. The NCDR ICD Registry data are susceptible to data entry errors. However, periodic audits suggest that more than 90% of fields accurately reflect the data in medical charts. 30 Finally, our analyses were observational and thus subject to residual and unmeasured confounding.
Conclusions
The risk of death after ICD placement is proportional to CKD severity. Patients with CKD have a higher burden of comorbidities than those without CKD. Consequently, they are more predisposed to comorbidity-related death. Among patients with CKD, several factors are prognostically significant, including CKD severity, older age, the degree of heart failure symptoms, and diabetes mellitus. Further studies regarding the efficacy, effectiveness, and cost-effectiveness of ICD therapy among patients with CKD, particularly those with advanced disease, are needed.
Supplementary Material
Acknowledgments
The National Heart, Lung, and Blood Institute had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Views expressed in this article are those of the authors and do not necessarily represent the official view of the National Heart, Lung, and Blood Institute. The manuscript was reviewed by the American College of Cardiology-NCDR ICD Registry Research and Publications Committee.
Funding Sources: This analysis was funded by a grant (1R01-HL093071-01A1) from the National Heart, Lung, and Blood Institute. P.L.H. was funded by the National Institutes of Health (T-32 training grant HL069749-09).
Footnotes
Conflict of Interest Disclosures: Drs. Curtis and Anstrom received research grants from Medtronic, Inc. Authors reported no other relevant conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: Prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, van Lente F, Levey AS. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308:2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall J, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 8.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–1480. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS. Comparison of risk prediction using the ckd-epi equation and the mdrd study equation for estimated glomerular filtration rate. JAMA. 2012;307:1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Khatib SM, Greiner MA, Peterson ED, Hernandez AF, Schulman KA, Curtis LH. Patient and implanting physician factors associated with mortality and complications after implantable cardioverter-defibrillator implantation, 2002–2005. Circ Arrhythm Electrophysiol. 2008;1:240–249. doi: 10.1161/CIRCEP.108.777888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. doi: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wase A, Basit A, Nazir R, Jamal A, Shah S, Khan T, Mohiuddin S, McCullough PA. Impact of chronic kidney disease upon survival among implantable cardioverter-defibrillator recipients. J Interv Card Electrophysiol. 2004;3:199–204. doi: 10.1023/B:JICE.0000048570.43706.34. [DOI] [PubMed] [Google Scholar]
- 14.Turakhia MP, Varosy PD, Lee K, Tseng ZH, Lee R, Badhwar N, Scheinman M, Lee BK, Olgin JE. Impact of renal function on survival in patients with implantable cardioverter-defibrillators. PACE. 2007;30:377–384. doi: 10.1111/j.1540-8159.2007.00678.x. [DOI] [PubMed] [Google Scholar]
- 15.Cuculich PS, Sanchez JM, Kerzner R, Greenberg SL, Sengupta J, Chen J, Faddis MN, Gleva MJ, Smith TW, Lindsay BD. Poor prognosis for patients with chronic kidney disease despite icd therapy for the primary prevention of sudden death. PACE. 2007;30:207–213. doi: 10.1111/j.1540-8159.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 16.Levy R, DellaValle A, Atav AS, ur Rehman A, Sklar AH, Stamato NJ. The relationship between glomerular filtration rate and survival in patients treated with an implantable cardioverter defibrillator. Clin cardiol. 2008;31:265–269. doi: 10.1002/clc.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hager CS, Jain S, Blackwell J, Culp B, Song J, Chiles CD. Effect of renal function on survival after implantable cardioverter defibrillator placement. Am J Cardiol. 2010;106:1297–1300. doi: 10.1016/j.amjcard.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 18.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. Acc/aha/hrs 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:e1–62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Hammill SC, Kremers MS, Stevenson LW, Kadish AH, Heidenreich PA, Lindsay BD, Mirro MJ, Radford MJ, Wang Y, Curtis JP, Lang CM, Harder JC, Brindis RG. Review of the registry’s second year, data collected, and plans to add lead and pediatric icd procedures. Heart rhythm. 2008;5:1359–1363. doi: 10.1016/j.hrthm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Therneau TM, Grambsch PM. Modeling survival data: Extending the cox model. Springer-Verlag; New York: 2000. [Google Scholar]
- 22.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, Greenberg H, Case RB. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson MG, Ip JH, Hellkamp AS, Anderson J, Poole JE, Sharma AD, Johnson GW, Freudenberger RS, Mark DB, Lee KL, Bardy GH. Decreased impact of implantable defibrillators on sudden cardiac death in patients with renal insufficiency and heart failure: An analysis of the scd-heft. Heart Rhythm. 2006;3:S38. [Google Scholar]
- 24.Pun PH, Al-Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, Buxton AE, Moss AJ, Lee KL, Steinman R, Dorian P, Hallstrom A, Cappato R, Kadish AH, Kudenchuk PJ, Mark DB, Hess PL, Inoue LYT, Sanders GD. Implantable cardioverter defibrillators for primary prevention of sudden cardiac death in ckd: A meta-analysis of patient-level data from 3 randomized trials. Am J Kid Dis. doi: 10.1053/j.ajkd.2013.12.009. Epub Feb 8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- 26.Dodson JA, Reynolds MR, Bao H, Al-Khatib SM, Peterson ED, Kremers MS, Mirro MJ, Curtis JP. Developing a risk model for in-hospital adverse events following icd implantation: A report from the ncdr(r) registry. J Am Coll Cardiol. doi: 10.1016/j.jacc.2013.09.079. Epub March 4, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal A, Wang Y, Rumsfeld JS, Curtis JP, Heidenreich PA. Clinical characteristics and in-hospital outcome of patients with end-stage renal disease on dialysis referred for implantable cardioverter-defibrillator implantation. Heart Rhythm. 2009;6:1565–1571. doi: 10.1016/j.hrthm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole-Wilson PA, Fishbein DP, Johnson G, Anderson J, Mark DB, Bardy GH. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barsheshet A, Moss AJ, Huang DT, McNitt S, Zareba W, Goldenberg I. Applicability of a risk score for prediction of the long-term (8-year) benefit of the implantable cardioverter-defibrillator. J Am Coll Cardiol. 2012;59:2075–2079. doi: 10.1016/j.jacc.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 30.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA. The national cardiovascular data registry (ncdr) data quality brief: The ncdr data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


