Abstract
Background
We studied whether neonatal propofol anesthesia affects development of the endocrine and neural systems.
Methods
Sprague-Dawley rats were anesthetized using intraperitoneal propofol for 5 h on postnatal days (P) 4, 5, or 6. Pups that received either saline or intralipid, but not those in the negative control groups, were also maternally separated for 5 h. Serum levels of corticosterone were measured immediately after anesthesia and in adulthood after prepulse inhibition (PPI) of acoustic startle testing (≥P80), followed by measurement of hippocampal neuronal activity.
Results
Propofol acutely increased corticosterone levels to 146.6 ± 23.5 ng/ml (n=6) vs 16.4 ± 3.5 ng/ml (n=6) and 18.4 ± 3.2 ng/ml (n=6) in saline- and intralipd-treated pups, respectively. In adulthood, the propofol group exhibited exacerbated endocrine responses to stress in a form of increased corticosterone levels (1171.58 ± 149.17 ng/ml (n=15) vs 370.02 ± 36.01 ng/ml (n=10) in the saline group). The propofol group had increased the frequency of miniature inhibitory postsynaptic currents in CA1 neurons of male and female rats, but reduced PPI of startle was detected only in males. The Na+–K+–2Cl− co-transporter inhibitor bumetanide, administered to pups prior to propofol, alleviated long-term endocrine and PPI abnormalities. Exogenous corticosterone, administered to naïve pups, induced synaptic and endocrine, but not PPI effects, similar to those of propofol.
Conclusions
Propofol-caused acute increases in corticosterone levels and gamma-aminobutyric acid type A receptor-mediated excitation at the time of anesthesia may play mechanistic roles in development of exacerbated endocrine responses to stress and neurobehavioral abnormalities.
INTRODUCTION
One in every 50 children undergoes a surgical procedure in their first year of life.1 A large body of evidence, predominantly from laboratory studies of healthy animals and to a lesser extent from children that had surgical procedures under general anesthesia at an early postnatal age, suggests that general anesthesia administered during the early postnatal period may have negative long-term consequences for brain development (for review, see Sanders et al.).2 The mechanisms that mediate these developmental changes and the full spectrum of pathophysiological alterations induced by neonatal anesthesia, currently predominantly linked to the brain, are poorly understood.
We recently reported that adverse developmental effects of sevoflurane, a polyvalent anesthetic agent, whose actions include enhancement of γ-aminobutyric acid (GABA) type A receptor (GABAAR) activity, were associated with seizure-like electroencephalographic patterns, neuronal cell death, and abnormal behavior. These effects of sevoflurane were diminished by pretreatment with the Na+–K+–2Cl− (NKCC1) co-transporter inhibitor, bumetanide,3,4 pointing to a role of developmental GABAAR-mediated excitation in these effects.5 We have also observed that the effects of sevoflurane were accompanied by a prominent increase in serum levels of the mineralocorticoid hormone aldosterone and that exogenous aldosterone, administered at high doses, further enhanced these adverse effects of sevoflurane.4 Aldosterone together with the glucocorticosteroids corticosterone (in rodents) or cortisol (in humans), represent the family of corticosteroid hormones, secreted by the adrenal gland. The adrenal secretion of the corticosteroids is increased in response to stress-induced activation of the limbic-hypothalamic-pituitary-adrenal (LHPA) axis,6 though the renin-angiotensin-aldosterone system plays a primary role in the control of aldosterone production.7 Activation of the LHPA axis is also associated with the release of other neuroactive agents, such as the neurosteroids allopregnalonone and tetrahydrodeoxycorticosterone.8,9 These and other products of activation of the LHPA axis interacting with each other through complex signaling and metabolic pathways play important, unique, and sometimes opposing roles in the mechanisms that underlie behavioral adaptations to stress.8–11 Excessive stimulation of the LHPA axis early in life as a result of prolonged stress, such as repeated maternal separation, induces long-term heightened endocrine responses to stress, anxiety-like behavior and reduced prepulse inhibition (PPI) of startle,8–11 one of the main symptoms of schizophrenia in animal models and humans.12
In this study we test whether long-term developmental effects of prolonged anesthesia with propofol, similar to long-term developmental effects of exposure to prolonged and repeated neonatal stress, involve exacerbation in endocrine activity and neurobehavioral abnormalities.
MATERIALS AND METHODS
Animals
All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee. Sprague-Dawley rats were studied. Animals were housed under controlled illumination (12-h light/dark, lights on at 7:00 a.m.) and temperature (23–24 °C) with free access to food and water. Within 24 h of delivery, litters were culled to 12 pups. At the age of 21 days, pups were weaned and housed in sex-matched groups of two for the rest of the study. To control for litter variability, we used several pups from each litter for different treatment conditions. Multiple sets of animals were used in a given treatment condition.
Treatment groups
P4, P5, or P6 rat pups of both genders were kept in a temperature-controlled chamber (+37 °C) with a continuous supply of oxygen (1.5 L/min) during anesthesia. The anesthesia protocol with propofol (40 mg/kg intraperitoneally for induction for the first 60 min and then 20 mg/kg/h intraperitoneally for maintenance for 5 h in total) is a shorter version of the anesthesia protocol originally described by Briner et al.13 in neonatal rats consisting of six injections of propofol at the same doses and lasting for 6 h. Neither Briner et al. nor other authors using a single injection of propofol at 75 mg/kg intraperitoneally detected significant changes in blood gasses or glucose in neonatal rats.13,14 In order to study the role of GABAAR-mediated excitation in the effects of propofol, a subgroup of P4, P5 or P6 rats received the NKCC1inhibitor, bumetanide (1.82 mg/kg, intraperitoneally), 15 min prior to initiation of anesthesia with propofol for 5 h. Another subgroup of P4–P6 rats received a single injection of corticosterone (0.2 mg/kg, intraperitoneally) followed by intraperitoneal injections of saline at hours 2, 3 and 4. The animals in the corticosterone group were not exposed to anesthesia with propofol. This dose of corticosterone (0.2 mg/kg) is in the range of glucocorticoid doses administered to children in the early postnatal period to alleviate respiratory distress syndrome and to modulate the inflammatory response associated with cardiopulmonary bypass.15,16 There were two control groups in which animals received equal numbers and volumes of intraperitoneal injections of saline or intralipid (the vehicle for propofol). All rat pups were separated from the dams for 5 h, the time equal to the duration of anesthesia with propofol, except rats in the negative control groups, which were neither separated from their dams nor injected. Mortality in the range of 10% occurred in P4–P6 rats during anesthesia with propofol for 5 h. Investigators analyzing data were blind to the experimental conditions. The sample sizes in this study were based on previous experience with the same experimental techniques.
Measurement of serum corticosterone
Serum corticosterone was measured using commercial ELISA kits by following the manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI). In order to assess acute changes in serum levels of corticosterone, P4–P6 rats were sacrificed by decapitation without anesthesia 5 h after start of the treatments and trunk blood samples were collected.
To study long-term effects of stress on serum levels of corticosterone, trunk blood samples were collected from the >P80 rats 5 min after completion of the PPI of startle test (stressed condition, see Measurements of the acoustic startle response and PPI of startle). Serum corticosterone levels in blood samples collected 3 to 5 days after completion of the PPI test were assumed to represent baseline serum levels of corticosterone. Animals were sacrificed by decapitation without anesthesia.
Slice electrophysiology
Brain hippocampal slices were prepared from >P80 rats sacrificed for the determination of corticosterone levels. The brain was removed after decapitation and was placed into ice-cold sucrose buffer containing (in millimoles): 254 sucrose,10 D-glucose, 26 NaHCO3, 2 CaCl2, 2 MgSO4, 3 KCl, and 1.25 NaH2PO4, saturated with 95% O2/5% CO2, at pH 7.4. Transverse hippocampal slices (300 µM thick) were cut with a VT 1000S microtome (Leica Microsystems Inc., Buffalo Grove, IL). Slices were transferred immediately into a holding chamber and were incubated at 32 to 33°C for a 30-min recovery period in a mixture of 50% sucrose saline and 50% artificial cerebrospinal fluid (aCSF) containing (in millimoles): 128 NaCl, 10 D-glucose, 26 NaHCO3, 2 CaCl2, 2 MgSO4, 3 KCl, and 1.25 NaH2PO4. Slices were then placed on a nylon mesh, submerged in normal aCSF bubbled continuously with 95% O2/5% CO2, and maintained at room temperature (~21–24°C) until whole-cell patch-clamp recording, typically within 0.5 to 5 h.
Slices were transferred to a submersion-type recording chamber (Warner Instruments, Hamden, CT) on a Burleigh Gibraltar fixed-stage system (Burleigh Instruments, Fisher, NY), secured beneath a nylon harp, and perfused with aCSF heated to 30 to 33°C with an inline heater (Warner SC-20) at a rate of 2 to 3 mL per min. CA1 pyramidal cells and interneurons were identified visually by using a microscope (Leica DM LFS, Leica Microsystems Wetzlar GmbH, Wetzlar, Germany) equipped with a 40× water-immersion objective coupled with an infrared differential interference contrast camera system. Whole-cell patch-clamp recordings were established using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Membrane current and potential signals were digitized and analyzed with Digidata 1322A (Molecular Devices, Sunnyvale, CA) and pClamp 10.0 systems (Molecular Devices). Patch pipettes of ≈5 MΩ were pulled with a P-1000 puller (Sutter Instruments, Novato, CA). The pipette solution had the following composition (in millimoles) unless otherwise stated: 140 KCl, 0.1 CaCl2, 5 EGTA, 10 HEPES, 4 ATP-Mg2+, 0.4 GTP-2Na+, 1 QX314 (Lidocaine N-ethyl bromide), pH 7.2, and 290 mOsm. The diffusion potential (liquid junction potential) was 4 mV, calculated by Clampex software (Molecular Devices). QX314 was added to the pipette solution to block the GABABR-mediated currents and to prevent the generation of Na+-dependent action potentials. Under these conditions, total miniature postsynaptic currents (mPSCs) were acquired in aCSF containing tetrodotoxin (1 µM) at a holding potential of −70 mV. To record miniature inhibitory postsynaptic currents (mIPSCs), glutamate receptor antagonists DNQX (6,7-dinitroquinoxaline-2,3-dione, 20 µM) and AP5 (DL-2-amino-5-phosphonovaleric acid, 20 µM) were added to aCSF. Drugs were administered by bath application. Synaptic currents were collected for 5 min for each experimental condition. Access resistance (<25 MΩ) was regularly monitored during recordings, and cells were rejected if resistance changed >15% during the experiment. If the access resistance increased during the course of the experiment and caused significant reductions in the synaptic current amplitudes, efforts were made to improve access (such as applying additional suction or slight positive pressure); if this failed, the experiment was discontinued. Spontaneously occurring synaptic currents were filtered at 2 kHz and were digitized at 10 kHz using Digidata 1322A (Molecular Devices). Offline data analysis was performed using the MiniAnalysis software (version 6.0.7; Synaptosoft, Decatur, GA). Synaptic currents were screened automatically using an amplitude threshold of 3 pA. Events were then visually screened to ensure that the analysis was not distorted by changes in the noise level or by membrane fluctuations. If the background noise increased during the recording, the data from that cell were discarded. The miniature excitatory postsynaptic current (mEPSC) frequency was calculated by subtracting the mIPSC frequency from the total mPSC frequency.
Measurements of the acoustic startle response and PPI of startle
The PPI of startle tests were performed in young adulthood at ~P80 with a dual intent, first to create a standardized stressful condition and second to assess sensorimotor gating. PPI of startle tests were performed using the SR-Lab startle apparatus (San Diego Instruments, San Diego, CA) as previously described by our laboratory.4,17 Testing occurred during the light phase of the dark–light cycle. At the beginning of every testing session, each animal was placed in the cylindrical animal enclosure and was then exposed to a 75-dB white noise background for a 5-min acclimation period. The acclimation period was then followed by a test session consisting of five different types of trials: a 120-dB 40-ms pulse only; a 120-dB 40-ms pulse preceded by a prepulse of a 20-ms duration at 5, 10, and 15 dB above background; and a no-stimulus trial of background noise. The delay between the onset of the prepulse and the onset of the pulse was 100 ms. The trials were presented in pseudorandom order with variable inter-trial intervals averaging 15 s. The first four trials and last three trials consisted of 120-dB pulse-only trials. All five types of trials were presented eight times, each in pseudorandom order after the first four and before the last three pulse-only trials. The %PPI for each PPI was calculated using the following formula: %PPI =100× [(pulse alone) − (prepulse + pulse)]/pulse alone.18 Data were collected as Vmax amplitude. The entire test for a given animal lasted 28 min. The animal enclosure (20 cm in length, 9 cm in interior diameter) permitted the animal to turn around in the enclosure.
Drugs
Propofol was purchased from APP Pharmaceuticals, LLC (Schaumburg, IL). Corticosterone, TTX, and QX314 were acquired from Sigma-Aldrich (St. Louis, MO). Bumetanide (Ben Venue Laboratories, Inc., Bedford, OH) was purchased from Bedford Laboratories (Bedford, OH). AP5 and DNQX were purchased from Tocris Cookson, Inc. (Ellisville, MO).
Statistical Analysis
Values are reported as mean ± SEM. SigmaPlot 12.5 software (Systat Software, Inc., Point Richmond, CA) was used for statistical analyses. Single comparisons were tested using the t test, whereas multiple comparisons among groups were analyzed using ANOVA, followed by Holm-Sidak tests. All comparisons were run as two-tailed tests. P < 0.05 was considered significant.
RESULTS
Propofol, administered to neonatal rats, causes acute increase in serum levels of corticosterone at the time of anesthesia and development of increased endocrine activity both at rest and under stress in adulthood
The serum levels of corticosterone after 5 h of anesthesia with propofol and after an equal number of injections of saline and intralipid were 146.6 ± 23.5 ng/ml (n=6), 16.4 ± 3.5 ng/ml (n=6) and 18.4 ± 3.2 ng/ml (n=6), respectively, (F(2,15) = 29.094, P<0.001). The changes in serum levels of corticosterone were similar in male and female rat pups.
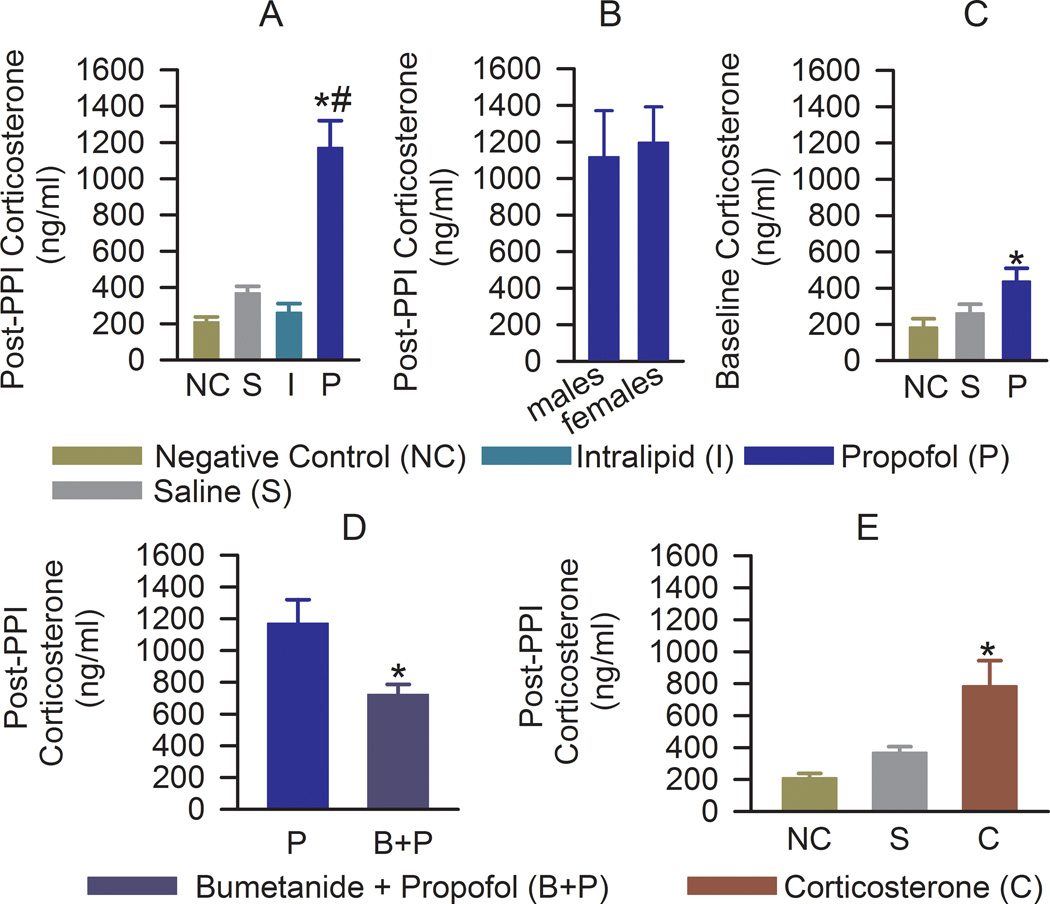
In early adulthood, rats in the propofol group responded to stress, caused by a PPI test, with a >550% increase in serum corticosterone levels beyond that of animals in the negative control group (F(3,30) = 13.07, P < 0.001; Fig. 1A). The changes in serum corticosterone levels of saline and intralipid treated rats were not statistically significant. There was no difference in the stressed corticosterone responses between male and female rats from the propofol group (t(13) = −0.241, P = 0.813, Fig. 1B). Baseline serum levels of corticosterone in the propofol group were also elevated (F(2,14) = 4.177, P = 0.038; Fig. 1C). In the propofol group serum levels of corticosterone under stress were higher when compared to their respective baseline values (t(18) = −3.371; P = 0.003), but not in rats from the negative control (t(6) = −0.473; P = 0.653) and saline (t(14) = −1.794; P = 0.094) groups.
Figure 1.
Anesthesia of postnatal days (P) 4, 5, or 6 rats with propofol results in long-term heightened endocrine activity. Histograms show serum levels of corticosterone in >P80 rats of both genders during stress (5 min after completion of the prepulse inhibition (PPI) test) and at rest (3 to 5 days after completion of the PPI test). Numbers of animals per treatment group: (A) negative control (n = 4), saline (n = 10), intralipid (n = 5), and propofol (n = 15). *P < 0.001 vs. all other groups. (B) Propofol-exposed males (n = 5), propofol-exposed females (n = 10). (C) Negative control (n=4), saline (n = 6), and propofol (n = 7). *P = 0.038 vs. other groups. (D) Histograms showing serum levels of corticosterone under stress in rats that received bumetanide (1.82 mg/kg, intraperitoneally) prior to anesthesia with propofol at P4–P6 (n = 13, *P = 0.015). The rats in the propofol group were the same as those in Fig. 1A. (E) Histograms showing serum levels of corticosterone under stress in rats that received corticosterone (0.2 mg/kg, intraperitoneally) at P4–P6 (n = 6). The rats in the negative control and saline groups were the same as those in Fig. 1A. *P < 0.01 vs. all other groups.
Rats, pretreated with bumetanide prior to propofol at P4–P6, responded to stress with lower increases in serum levels of corticosterone, when compared to rats that did not receive bumetanide prior to induction of anesthesia with propofol (t(24) = 2.71; P = 0.012, Fig. 1D). A single administration of 0.2 mg/kg corticosterone to P4–P6 rats increased serum levels of corticosterone at 1h and 5 h post-injection to 219.0±15.0 ng/ml (n=3) and 49.1±10.2 ng/ml (n=5), respectively. Exogenous corticosterone administered at the time of maternal separation at P4–P6 also resulted in an increase in the stressed (F(2,17) = 9.483, P = 0.002; Fig. 1E), but not the baseline F(2,13) = 1.595, P = 0.24) serum levels of corticosterone in early adulthood.
Exposure to propofol at an early age induces alterations in synaptic activity in hippocampal CA1 neurons of adult rats
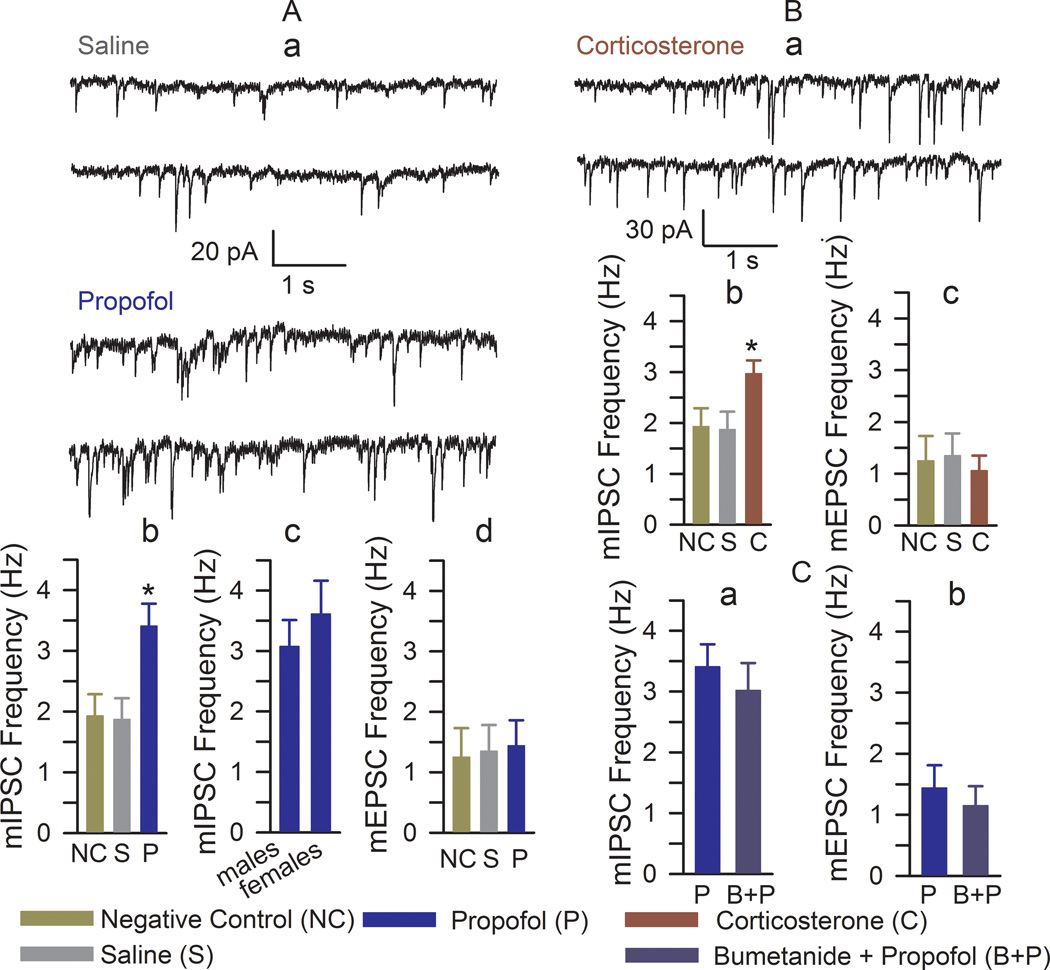
Rats of both genders that received propofol during maternal separation had increased mIPSC frequency when compared to all other treatment groups (F(2,27) = 6.02, P = 0.007; Fig. 2A,a,b), whereas the amplitude of mIPSCs remained unaltered between all treatment groups (not shown). There was no difference in the frequency of mIPSCs between male and female rats previously anesthetized with propofol (t(11) = 0.693, P = 0.502, Fig. 2A,c). No significant changes were detected in the parameters of mEPSCs (Fig. 2A,d).
Figure 2.
Anesthesia of postnatal days (P) 4, 5, or 6 rats with propofol induced an increase in the frequency of miniature inhibitory postsynaptic currents (mIPSCs). (A,a) Examples of mIPSC recordings in hippocampal CA1 neurons of the >P80 rats that were treated at P4–P6 with saline or propofol. (A,b and A,d) Histograms showing the frequencies of mIPSCs and miniature excitatory postsynaptic current (mEPSCs) in hippocampal CA1 neurons of the >P80 rats. Number of recorded cells for each treatment group: negative control (9), saline (8), and propofol (13). *P < 0.05 vs. all other treatment groups. (A,c) Histograms showing the frequencies of mIPSCs in hippocampal CA1 neurons of the male (n=5) and female (n=9) rats treated with propofol. (B,a) Examples of mIPSC recordings in hippocampal CA1 neurons of the >P80 rats that were treated at P4–P6 with corticosterone (0.2 mg/kg, intraperitoneally). (B,b and B,c) Histograms showing frequencies of mIPSCs and mEPSCs in hippocampal CA1 neurons of the >P80 rats. Number of recorded cells for each treatment group: negative control (9), saline (8), and corticosterone (10). The rats in the negative control and saline groups were the same as those in Fig. 2A,b,d. *P < 0.05 vs. all other treatment groups. (C) Histograms showing frequencies of mIPSCs (a) and mEPSCs (b) in hippocampal CA1 neurons of the >P80 rats pretreated with bumetanide (1.82 mg/kg, intraperitoneally) 15 min prior to induction of anesthesia with propofol. Number of recorded cells for each treatment group: propofol (n=13) and bumetanide plus propofol (n=7). The rats in the propofol group were the same as those in Fig. 2A.
Exogenous corticosterone administered to neonatal rats induced an increase in the frequency of mIPSCs, similar to that observed in rats anesthetized with propofol (F(2,24) = 3.894, P = 0.034; Fig. 2Ba,b) without significant changes in the mEPSC frequency (Fig. 2B,c). Pretreatment with bumetanide prior to induction of anesthesia with propofol did not alter the long-term effect of propofol on the frequency of mIPSCs (t(18) = 0.647, P = 0.526, Fig. 2C,a,b).
Exposure to propofol at an early age induced long-term impairment of sensorimotor gating in male rats
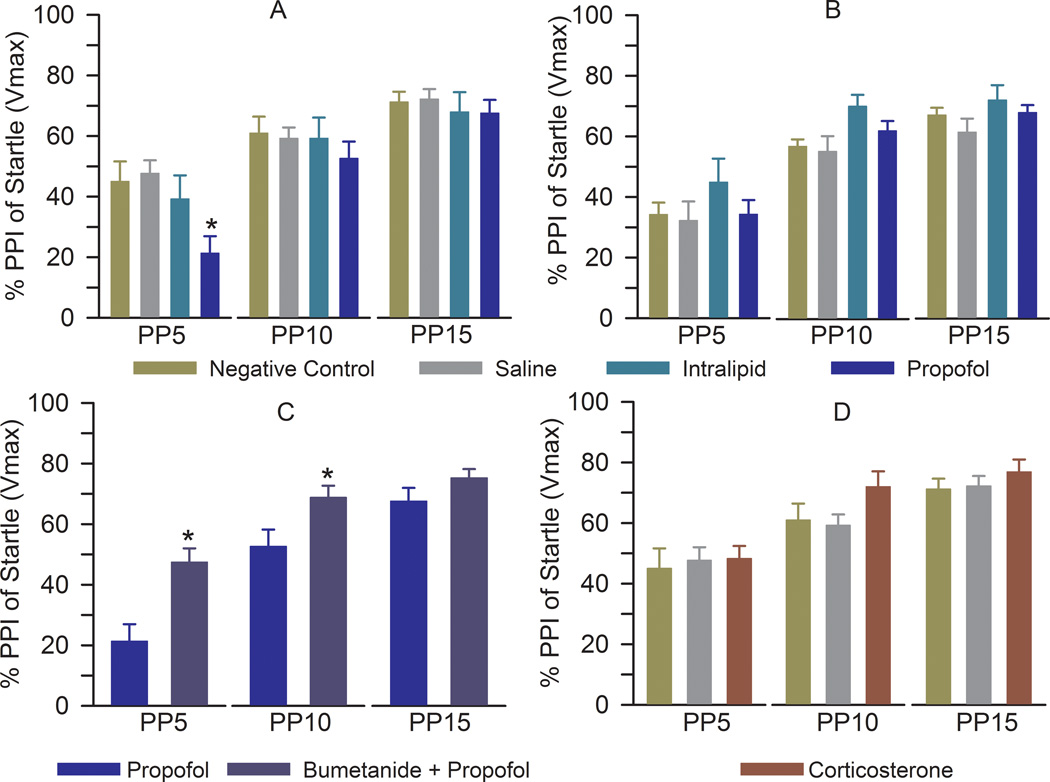
Young adult male rats that were anesthetized with propofol during the early postnatal period exhibited reduced PPI of startle responses at a prepulse intensity of 5 dB (F(3,47) = 4.548, P = 0.007; Fig. 3A). The PPI of startle responses in the saline- and intralipid-treated, maternally separated male rats were not different from those in the negative control group (F(2,34) = 0.489, P = 0.618; Fig. 3A). Startle response amplitudes in male rats were 285.7 ± 63.9, 286.7 ± 45.0, 247.9 ± 30.9, and 263.1 ± 64.7 in the negative control, saline, intralipid, and propofol groups, respectively (F(3,47) = 0.071, P = 0.975). Propofol did not affect the PPI of startle responses of female rats (F(3,51) = 0.908, P = 0.444; Fig. 3B).
Figure 3.
Anesthesia of neonatal male, but not female, rats with propofol results in impaired prepulse inhibition (PPI) of the startle response. (A) Histogram showing PPI of startle in different treatment groups of male rats: negative control (n = 12), saline (n = 15), intralipid (n = 10), and propofol (n = 15). *P = 0.007 vs. negative control. PP5-PP15: prepulse intensities in decibels above background. (B) Histogram showing PPI of startle in different treatment groups of female rats: negative control (n = 12), saline (n = 15), intralipid (n = 12), and propofol (n = 16). (C) PPI of startle in male rats that received bumetanide (1.82 mg/kg, intraperitoneally) 15 min prior to induction of anesthesia with propofol for 5 h at postnatal days (P) 4, 5, or 6 (n = 12). The rats in the propofol group were the same as those in Fig. 3A. *P < 0.05 vs. propofol. (D) PPI of startle in male rats that received one injection of corticosterone (0.2 mg/kg, intraperitoneally) at P4, P5 or P6 (n = 10). The rats in the negative control and saline groups were the same as those in Fig. 3A.
Treatment with bumetanide prior to anesthesia with propofol at P4–P6 completely reversed the propofol-induced impairment in the PPI of startle response at a prepulse intensity of 5 dB (F(1,25) = 12.114, P = 0.002) and further facilitated the PPI of startle response at a prepulse intensity of 10 dB (F(1,25) = 5.167, P = 0.032; Fig. 3C) in male rats. The male rats, treated with corticosterone at P4–P6, exhibited unaltered PPI of startle responses at ~P80 (Fig. 3D).
DISCUSSION
The major finding of this study is that propofol treatment during neonatal life induces long-term exacerbation of endocrine responsiveness to stress as well as endocrine activity at rest. These findings qualitatively widen the scope of abnormalities caused by neonatal anesthesia in rats beyond the relatively well studied neurobehavioral phenotypes so far described in animal models.2 The heightened baseline and stress-related levels of corticosterone months after exposure to propofol suggest that the functional consequences of exposure of neonates to propofol may result from a combination of the acute effects of the anesthetic at the time of anesthesia and subsequent continuous exacerbated endocrine responses to stress originally initiated by the neonatal anesthetic exposure. Our results suggest that propofol-enhanced GABAAR-mediated excitation and LHPA axis activity at the time of anesthesia are involved in mediation of the long-term developmental effects of the anesthetic.
Enhancement of GABAAR activity is considered the main mechanism mediating the anesthetic effect of propofol.19,20 The two long-term developmental effects of propofol, a heightened endocrine response to stress and impaired sensorimotor gating function, were mitigated by bumetanide given prior to propofol anesthesia. Bumetanide may modify GABAAR-mediated effects of propofol in neonatal cortical and hippocampal neurons by inhibiting NKCC1 activity and shifting GABA-initiated responses from excitatory to inhibitory.5 We previously demonstrated that bumetanide alleviated the reduction in PPI of startle caused by anesthesia of neonatal rats with sevoflurane and isoflurane,4,21 anesthetics whose mechanisms of action also include enhancement of GABAAR activity. Similarly neonatal rats treated with a single injection of allopregnanolone, a neurosteroid that enhances GABAAR activity,22 later developed a reduced PPI of startle reactivity.23,24 Given our findings that propofol stimulates the adrenal secretion of corticosterone in neonatal rats, it is plausible that propofol may enhance GABAAR activity not only through direct interaction with the receptor, but also through stimulation of the corticosterone secretion and an increase of corticosterone precursor-derived neurosteroids, allopregnanolone and tetrahydrodeoxycorticosterone. Furthermore, corticosterone may modulate the levels of neurosteroids in rats by increasing 3α-hydroxysteroid dehydrogenase expression, an enzyme involved in the synthesis of neurosteroids.25 An increase in systemic levels of corticosterone after a single administration of corticosterone without enhancement of GABAAR activity may not be sufficient to induce changes in the PPI of acoustic startle response.
Propofol also induced alteration in hippocampal synaptic activity, as evident from the increased frequency of mIPSCs in CA1 neurons. This effect could be simulated by administering exogenous corticosterone to neonatal rats. The synaptic effect of propofol was not sensitive to pretreatment with bumetanide. One potential mechanism of the observed increase in the frequency of hippocampal mIPSCs could be a compensatory change in synaptic activity in response to propofol- and exogenous corticosterone-elicited increases in serum levels of corticosterone both acutely and long-term. Corticosterone is known to enhance excitatory glutamatergic transmission by increasing presynaptic glutamate release, inhibiting glutamate uptake, potentiating expression of the N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors.26,27 Even small changes in the neuronal excitation/inhibition ratio may have important consequences for normal memory formation and cognition.28 Alterations at synaptic levels, caused by exposure of neonatal brain to different general anesthetics, were previously reported by many laboratories,29–31 pointing to potentially important neuronal mechanisms whereby neonatal anesthesia exerts its adverse effects.
Although propofol induced heightened endocrine activity in both genders, the impaired sensory motor gating function was observed only in male rats previously anesthetized with propofol. Others also report that female rats are more resistant to stress early in life than their male counterparts in terms of their behavior, but not their endocrine responses. For example, early maternal separation of infant rats lead to abnormal endocrine responses to stress in adulthood in both genders, while behavioral abnormalities were more prominent in male rats.25,32 Gonodactemy of adult female rats, previously subjected to maternal separation, revealed anxiety-like behavior similar to that seen in male counterparts, suggesting a protective role of female sex hormones.26 It will be important to evaluate the role of sex hormones in the behavioral effects of neonatal exposure to general anesthetics.
A large body of evidence indicates that an increase in glucocorticoid levels resulting from pathophysiological conditions, therapeutic interventions, or stress can have profound effects on cognitive and emotional functions.8–11,33–37 Severe stress may be especially detrimental during the early stages of development, resulting in long-lasting changes in neuroendocrine function and behavior.33,35,38 The results of this study lead us to speculate that the developmental effects of propofol administered during the early postnatal period mimic at least some aspects of the neuroendocrine and behavioral consequences of severe neonatal stress, such as long-term exacerbation of the LHPA axis activity15,35,38 and impairment of the PPI of startle response.11 The similarity between the developmental effects of propofol and exposure to severe postnatal stress could be even more pronounced when the effects of neonatal anesthetics are combined with those of acute diseases or surgical procedures, which are also known to cause a stress response.39,40 The developmental consequences of exposure to neonatal propofol can be further exaggerated in subjects with pathophysiological conditions characterized by elevated levels of corticoids, such as low birth weight or prematurity. Similar mechanisms may be involved in mediation of the developmental effects of other anesthetics.
In summary, the results of this study demonstrate that neonatal exposure to propofol results in acute and long-term exacerbation of endocrine system activity, alterations in hippocampal synaptic activity, and animal behavior later in life. The finding of a neonatal anesthetic-caused long-term alteration in the LHPA axis activity opens the possibility that there may be other yet to be described neonatal anesthesia-induced developmental consequences in which alterations in the LHPA axis activity play etiological roles.
Acknowledgment
We would like to acknowledge technical contribution of Dyanet L. Puentes (Student, University of Florida, Gainesville, Florida).
Sources of financial support: Supported by grant No. R01 GM93036-01A1 from the National Institute of Health/ National Institute of General Medical Sciences, Bethesda, Maryland (to AEM), and the Jerome H. Modell, M.D., F.A.H.A. Endowed Professorship, Gainesville, Florida (to NG).
Footnotes
Meetings at which the work has been presented: the International Anesthesia Research Society Annual Meeting, May 23, 2013, San Diego, CA.
The authors declare no competing interests
REFERENCES
- 1.Tzong KYS, Han S, Roh A, Ing C. Epidemiology of Pediatric Surgical Admissions in US Children: Data From the HCUP Kids Inpatient Database. J Neurosur Anesthesiol. 2012;24:391–395. doi: 10.1097/ANA.0b013e31826a0345. [DOI] [PubMed] [Google Scholar]
- 2.Sanders RD, Hassell J, Davidson AJ, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: An update. Br J Anaesth. 2013;110(Suppl 1):i53–i72. doi: 10.1093/bja/aet054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards DA, Shah HP, Cao W, Gravenstein N, Seubert CN, Martynyuk AE. Bumetanide alleviates epileptogenic and neurotoxic effects of sevoflurane in neonatal rat brain. Anesthesiology. 2010;112:567–575. doi: 10.1097/ALN.0b013e3181cf9138. [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Pavlinec C, Gravenstein N, Seubert CN, Martynyuk AE. Roles of aldosterone and oxytocin in abnormalities caused by sevoflurane anesthesia in neonatal rats. Anesthesiology. 2012;117:791–800. doi: 10.1097/ALN.0b013e318266c62d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Löscher W, Puskarjov M, Kaila K. Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 2013;69:62–74. doi: 10.1016/j.neuropharm.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubzansky LD, Adler GK. Aldosterone: A forgotten mediator of the relationship between psychological stress and heart disease. Neurosci Biobehavioral Rev. 2010;34:80–86. doi: 10.1016/j.neubiorev.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joëls M, Lucassen PJ, Krugers H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Xue X, Shao S, Shao F, Wang W. Cognitive, emotional and neurochemical effects of repeated maternal separation in adolescent rats. Brain Res. 2013;1518:82–90. doi: 10.1016/j.brainres.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008;199:331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–293. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- 14.Yu D, Jiang Y, Gao J, Liu B, Chen P. Repeated exposure to propofol potentiates neuroapoptosis and long-term behavioral deficits in neonatal rats. Neurosci Lett. 2013;534:41–46. doi: 10.1016/j.neulet.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 15.O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG, 3rd, Dillard RG. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: Outcome of study participants at 1-year adjusted age. Pediatrics. 1999;104:15–21. doi: 10.1542/peds.104.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Graham EM, Atz AM, Butts RJ, Baker NL, Zyblewski SC, Deardorff RL, DeSantis SM, Reeves ST, Bradley SM, Spinale FG. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: Results from a randomized trial. J Thorac Cardiovasc Surg. 2011;142:1523–1529. doi: 10.1016/j.jtcvs.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao W, Shah HP, Glushakov AV, Mecca AP, Shi P, Sumners C, Seubert CN, Martynyuk AE. Efficacy of 3,5-dibromo-L-phenylalanine in rat models of stroke, seizures and sensorimotor gating deficit. Br J Pharmacol. 2009;158:2005–2013. doi: 10.1111/j.1476-5381.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geyer MA, Dulawa SC. Assessment of murine startle reactivity, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2003;Chapter 8(Unit 8.17) doi: 10.1002/0471142301.ns0817s24. [DOI] [PubMed] [Google Scholar]
- 19.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta 3 subunit. FASEB J. 2003;17:250–266. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 20.Zeller A, Arras M, Lazaris A, Jurd R, Rudolph U. Distinct molecular targets for the central respiratory and cardiac actions of the general anesthetics etomidate and propofol. FASEB J. 2005;19:1677–1692. doi: 10.1096/fj.04-3443fje. [DOI] [PubMed] [Google Scholar]
- 21.Seubert CN, Zhu W, Pavlinec C, Gravenstein N, Martynyuk AE. Developmental effects of neonatal isoflurane and sevoflurane exposure in rats. Anesthesiology. 2013;119:358–364. doi: 10.1097/ALN.0b013e318291c04e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunn BG, Brown AR, Lambert JJ, Belelli D. Neurosteroids and GABA(A) Receptor Interactions: A Focus on Stress. Front Neurosci. 2011;5:131. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darbra S, Modol L, Vallée M, Pallarès M. Neonatal neurosteroid levels are determinant in shaping adult prepulse inhibition response to hippocampal allopregnanolone in rats. Psychoneuroendocrinology. 2013;38:1397–1406. doi: 10.1016/j.psyneuen.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Darbra S, Mòdol L, Llidó A, Casas C, Vallée M, Pallarès M. Neonatal allopregnanolone levels alteration: Effects on behavior and role of the hippocampus. Prog Neurobiol. 2014;113:95–105. doi: 10.1016/j.pneurobio.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Mitev YA, Darwish M, Wolf SS, Holsboer F, Almeida OF, Patchev VK. Gender differences in the regulation of 3 alpha-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–549. doi: 10.1016/s0306-4522(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 26.Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prager EM, Johnson LR. Stress at the synapse: Signal transduction mechanisms of adrenal steroids at neuronal membranes. Sci Signal. 2009;2:re5. doi: 10.1126/scisignal.286re5. [DOI] [PubMed] [Google Scholar]
- 28.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 29.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile anesthetics rapidly increase dendritic spine density in the rat medial prefrontal cortex during synaptogenesis. Anesthesiology. 2010;112:546–556. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez V1, Feinstein SD, Lunardi N, Joksovic PM, Boscolo A, Todorovic SM, Jevtovic-Todorovic V. General Anesthesia Causes Long-term Impairment of Mitochondrial Morphogenesis and Synaptic Transmission in Developing Rat Brain. Anesthesiology. 2011;115:992–1002. doi: 10.1097/ALN.0b013e3182303a63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patchev VK, Montkowski A, Rouskova D, Koranyi L, Holsboer F, Almeida OF. Neonatal treatment of rats with the neuroactive steroid tetrahydrodeoxycorticosterone (THDOC) abolishes the behavioral and neuroendocrine consequences of adverse early life events. J Clin Invest. 1997;99:962–966. doi: 10.1172/JCI119261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 34.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 36.Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 37.Hunter RG, McEwen BS. Stress and anxiety across the lifespan: Structural plasticity and epigenetic regulation. Epigenomics. 2013;5:177–194. doi: 10.2217/epi.13.8. [DOI] [PubMed] [Google Scholar]
- 38.Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Lv L, Ma Y, Hu X. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci USA. 2011;108:14312–14337. doi: 10.1073/pnas.1010943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobsen KR, Kalliokoski O, Teilmann AC, Hau J, Abelson KS. The effect of isoflurane anaesthesia and vasectomy on circulating corticosterone and ACTH in BALB/c mice. Gen Comp Endocrinol. 2012;179:406–413. doi: 10.1016/j.ygcen.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Gibbison B, Angelini GD, Lightman SL. Dynamic output and control of the hypothalamic-pituitary-adrenal axis in critical illness and major surgery. Br J Anaesth. 2013;111:347–360. doi: 10.1093/bja/aet077. [DOI] [PubMed] [Google Scholar]