Abstract
Satellite cells (SCs) are the muscle stem cells responsible for longitudinal and cross-sectional postnatal growth, repair after injury and which provide new myonuclei when needed. Here we review their morphology, contribution to development, and their role in sarcomere and myonuclear addition. SCs, similar to other tissue stem cells, cycle through different states such as quiescence, activation, and self-renewal and thus we consider the signaling mechanisms involved in maintenance of these states. The role of the SC niche, their interactions with other cells such as fibroblasts and the extracellular matrix are all emerging as important factors that affect aging and disease. Interestingly, children with cerebral palsy appear to have a reduced SC number, which could play a role in their reduced muscular development and even in muscular contracture formation. Finally we review the current information on SC dysfunction in children with muscular dystrophy and emerging therapies that target promotion of myogenesis and reduction of fibrosis.
Keywords: Postnatal development, satellite cells, muscle stem cells, cerebral palsy, myonuclei
Introduction
Skeletal muscles are composed primarily of multinucleated myofibers with their basic contractile elements, sarcomeres, arranged in series to provide length and in parallel to provide cross-sectional area. Alexander Mauro1 first named the satellite cell (SC) in frog skeletal muscle based on its peripheral location in the myofiber, sandwiched between the sarcolemma and basal lamina. In terms of location, SCs are similar in placement to skeletal muscle myonuclei, i.e. on the periphery of the myofiber but are outside the sarcolemma whereas myonuclei are just under the sarcolemma. In other words, SCs represent a distinct cell type from myofibers. While the postnatal increase in myonuclear number has been known since the 1960s2, the source of these nuclei during growth and repair has been thought to be SCs. This is because adult myonuclei are terminally differentiated, i.e. unable to divide, proliferate, or regenerate. Consequently there must be other myogenic tissue specific stem cells that can make skeletal muscle. Indeed, isotope tracing experiments strongly suggested that the source for postnatal increase in myonuclei comes from mitosis of mononucleated SCs3,4.
By definition, an adult tissue stem cell has 2 properties—the ability to differentiate to create new tissue and the ability to self-renew5. However, it was not until the 2000s, after molecular markers such as the Pax7 transcription factor were identified6, that it was convincingly shown that the SCs are indeed the primary muscle stem cells capable of self-renewal and differentiation7-9. In this review, we will focus on postnatal development of muscle, SCs, their functional and regulatory mechanisms and their dysfunction and therapeutic implications. A number of excellent recent reviews on specific topics related to satellite stem cell biology and their role in myogenesis have been published10-17. For a state-of-the-art review on the molecular biology of SCs, see Yin et al.17; for a historical perspective on the experiments that led to the discovery of the SC as well as recent developments, see Yablonka-Reuveni16.
While SCs were initially identified based on their sublaminar anatomical location, specific cell surface membrane glycoproteins and intracellular markers have now been identified. The most common surface markers are NCAM (CD56), M-cadherin, and CD34 while Pax7 is nuclear (for a detailed list see Yin et al.17) and is considered the classic SC transcription factor6. Currently these markers have been used in human and animal studies to evaluate SCs by immunohistochemistry, fluorescence microscopy, fluorescence-activated cell sorting (FACS) and/or in vitro cell culture.
Satellite cell quiescence, activation, self-renewal and return to quiescence
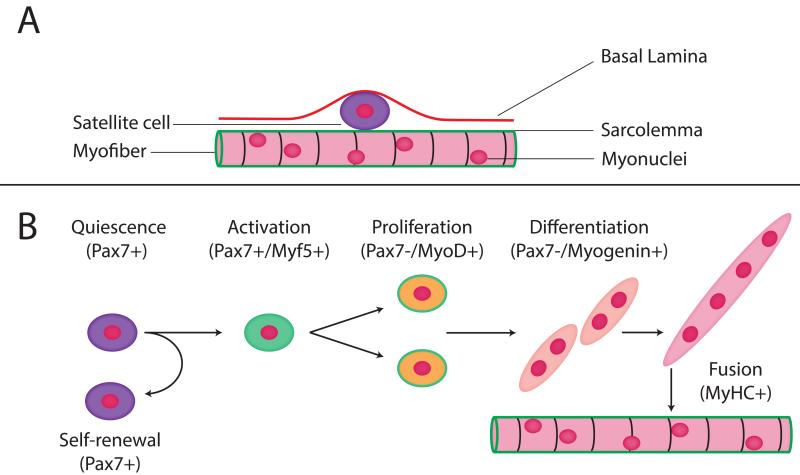
The transition from SC to myofiber consists of specific steps that proceed from quiescence, to activation, proliferation, differentiation and finally, cell fusion (Fig. 1). During quiescence, SCs are located in their microenvironment and express Pax7. Upon activation, they enter the cell cycle to progress down the myogenic pathway by expressing myogenic regulatory factors (MRFs)18, starting with Myf5, then proliferate (amplify cell number) and are considered to be “committed” to the myogenic lineage after they express MyoD, and form myoblasts. At this stage, myogenin is expressed, differentiated myoblasts fuse to create myotubes and express myosin heavy chain, the major muscle contractile protein. Myotubes fuse with existing myofibers in both growth and repair (reviewed in13). At the stages of proliferation, differentiation and fusion, cells no longer express purely SC markers but may also express myogenic markers. The signaling pathways mediating myoblast fusion are multifactorial and complex (for review, see Hindi et al.19).
Figure 1.
Satellite cell (SC) location and function. A) The SC is anatomically located between the myofiber basal lamina and sarcolemma. B) The relationship between gene expression and SC activation, self-renewal, proliferation, differentiation and fusion with existing myofiber.
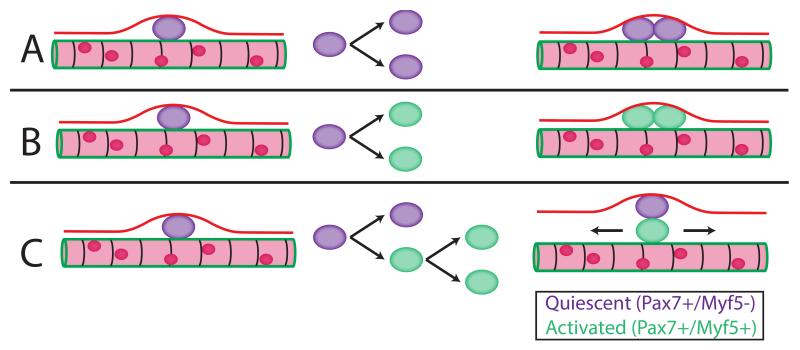
While SCs participate in growth and repair, they must also meet the other functional requirement of being a tissue-stem cell, i.e. self-renewal, without which the SC pool would be exhausted over time. SCs, like other stem cells, can undergo either symmetric or asymmetric division (Fig. 2). Using lineage tracing it was demonstrated that 90% of SCs had expressed Myf5 at some point, indicating they had previously undergone commitment to the myogenic lineage and then returned to quiescence—in other words, they had self-renewed20. Importantly, since 10% of the SCs never expressed Myf5, this indicated that this subgroup’s function was to self-renew rather than differentiate. It was also shown that cellular orientation during division critically determined SC fate. Specifically, if the SC division were planar (both daughter cells in the traditional position between basal lamina and sarcolemma, Figs. 2A, 2B) division would result in two identical cells (symmetrical division), which could either increase satellite cell number or create two activated cells that proceed down the myogenic path. However, if orientation were apicobasal, (one daughter cell touching the basal lamina and the other touching the sarcolemma, Fig. 2C) development was likely to split with one cell becoming a myoblast and the other creating a new SC (asymmetrical division)20.
Figure 2.
Satellite cell division, quiescence, and activation. A) Quiescent SC symmetrically divides into two quiescent cells, B) Quiescent SC symmetrically divides into two activated cells C) Quiescent SC asymmetrically divides into one quiescent cell and one activated cell, which can then continue down the myogenic path. Pax7+/Myf5− indicates quiescence and Pax7+/Myf5+ indicates activation. Using lineage-tracing experiments, it was shown that 10% of SCs never express Myf5 indicating that this subset functions to maintain self-renewal. If the situation shown in B were the only process occurring, it would lead to depletion of the SC pool.
Postnatal muscle development
SC nuclear length is 8-12 μm across various species21,22. SC number reduces with age during the early postnatal period to reach a steady state value of 5-6% of the myonuclei23 with greatest number soon after birth (~30% of the myonuclei23,24). At the extreme end of life, there is a reported SC number decline24-26. While the functional consequences of SC number decline are unclear, it is becoming clear that the SC function changes profoundly with aging27. The intrinsic differentiation potential of the aged SCs can remain unaltered but they may decline in proliferation potential, which can be rescued with the use of systemic factors such as Fibroblast growth factor (FGF)25 and exposure to young serum28.
Sarcomere addition
Postnatal muscle development is characterized by both longitudinal and radial muscle fiber growth29. Longitudinal growth increases the range over which a muscle functions while radial growth increases muscle contractile force30. During postnatal development in mammalian muscles, the number of myofibers does not increase2,31. In a series of seminal murine studies32-35, Williams and Goldspink measured skeletal muscle myofiber longitudinal and cross-sectional area increases during development. They reported increased myofiber cross-sectional area (Fig. 3A) by addition of myofibirils29,35 which occurred by fusion of the myoblasts from SCs into existing myofibers. We have recently shown29 that between postnatal day 1 and day 28 there was an almost twofold increase in myofibrillar packing, a sevenfold increase in myofiber cross-sectional area and a fourfold increase in muscle mass29. Longitudinal myofiber length increased fivefold primarily by addition of sarcomeres-in-series during the first 4-6 postnatal weeks 29,32,33. It was suggested that this sarcomere addition occurred primarily at the ends of the growing myofibers. Importantly, this distal region has been shown to be associated with the greatest number and concentration of SCs in developing muscles in chicks which was the basis of the suggestion22. Additional experiments demonstrated that growing muscle, prevented from increasing in length by maintaining a shortened position for 4 weeks, did not increase serial sarcomere number to the same extent. However, if it was then allowed to recover33 by removing the immobilization, subsequent stretch and growth resulted in rapid serial sarcomere number increase. These experiments demonstrate that the postnatal period is particularly plastic for adaptation and suggest that SCs may be involved in the process of serial sarcomere addition33
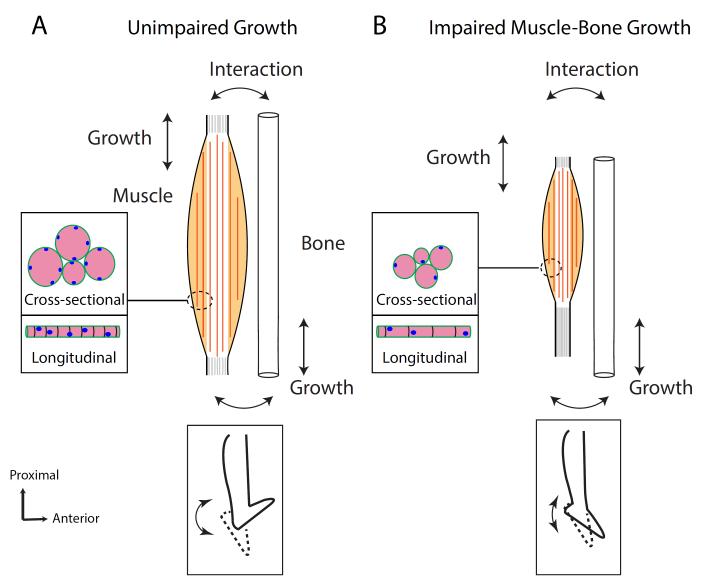
Figure 3.
Schematic of postnatal muscle growth. A) Unimpaired muscle growth resulting from bone growth. Myofiber length increase is associated with adding sarcomeres, myonuclei and girth by adding myofibrils (Inset, Cross-sectional or Longitudinal growth). This allows normal joint range of motion (Bottom Inset) B) In developmental disorders such as cerebral palsy (CP), longitudinal sarcomere growth may be impaired leading to overstretched sarcomeres as well as reduced cross-sectional growth of myofibers (Inset). In the case of children with CP this can lead to contracture development (Bottom Inset).
Myonuclear number and domain
In parallel with increased serial sarcomere number (i.e., increased fiber length) and myofiber area during the postnatal period there is also an increase in nuclear number2,36 in growing myofibers. After the initial 6 postnatal weeks of increase in sarcomere number in mouse solei and biceps brachii, the myonuclear addition continues for another 10 weeks suggesting a role for myonuclear accretion for radial growth33. However the exact relationship between myonuclear addition, cross-sectional and longitudinal growth is not entirely clear36. White et al.36 report a significant increase in both myofiber cross-sectional area and length in mouse EDL without accretion of any addition myonuclei beyond the first 21 postnatal days.
Myonuclear domain, i.e. the volume of cytoplasm per myonucleus might be a relevant parameter that relates myonuclear number to fiber length and size37. It has been suggested that myonuclear domain is maintained during atrophy by myonuclear loss38-40 or during hypertrophy by myonuclear accretion41 but this is not universally agreed upon24,42. The precise relationship between myonuclear domain and fiber atrophy and hypertrophy is thus not fully defined (see below).
Satellite cell function
As stated, postnatal muscle development is critically dependent on SCs and Pax743,44. Pax7 null mice demonstrated a dramatic reduction in both myofiber size and in SC number during the postnatal period44. Using a transgenic mouse that allowed conditional inactivation of Pax7, Lepper et al. 43 showed that myoblasts from Pax7 lineage fuse into myofibers and are indispensible during the postnatal period.
In contrast, the “obligatory” role of SCs for adult hypertrophy and regrowth after atrophy is not clear49-51. A 90% reduction in SCs does not prevent fiber hypertrophy in a synergistic ablation mouse model, in which the gastrocnemius and soleus are surgically excised and compensatory hypertrophy of the plantaris is measured52. However, it is important to note that, in control animals, where SCs were not knocked down, there was robust SC activation and participation in hypertrophy, indicating that, if present, they certainly do appear to participate in the hypertrophic response. This hypertrophy was not different between SC depleted and control groups. Similarly, recovery from atrophy was not hampered by a 90% knockdown of SCs53. Interestingly, the most recent results54 show that, while hypertrophy is possible initially, long-term hypertrophy is blunted and associated changes in the extracellular matrix and fibroblasts are observed, implying that hypertrophy in adult muscle is not completely independent of SCs.
Conditional SC inactivation during the postnatal period resulted in severely compromised muscle regeneration after injury43. A number of studies45-48 have conclusively shown that Pax7 expressing SCs are critical for long-term muscle repair capability even in adult muscle. While Lepper et al. reported that Pax7-inactivated-SCs could still regenerate normally in the short term, suggesting an additional non-Pax7 dependence for SC function in adults, this interpretation was confounded by an incomplete understanding of the timelines of deletion48. Günther et al.48 using a similar mouse model demonstrated that conditional inactivation of the Pax7 gene led to a delayed but significant loss of SCs and continued inactivation of Pax7 led to impaired muscle regeneration even in adults.
Signaling pathways for satellite cell activation, quiescence and self-renewal
Satellite cells have a large number of activation factors55 including mechanical stretch, which are important in the postnatal period during bone mediated muscle growth (Fig. 3). Local signaling factors such as nitric oxide56, growth factors such as fibroblast growth factor (FGF) and insulin-like growth factor (IGF)57 play an important role in SC activation and muscle growth. Mechanical stretch is strong regulator of SC activation through hepatocyte growth factor (HGF) and nitric oxide58. SCs can be activated after only brief period of stretching of 2 hours59 and this mechanical stretch can induce increased expression of the matrix metalloproteinases (MMPs) which are responsible for extracellular matrix remodeling60. MMPs play an important role in promoting migration of activated satellite cells permitting robust regeneration61 (see section on satellite cell niche below).
Maintaining the balance amongst the states of quiescence, activation, proliferation, differentiation and self-renewal is critical for continued myogenic potential. Lack of maintenance of quiescence or self-renewal leads to depletion of the stem cell pool while prevention of activation leads to impaired regeneration. There are many signaling mechanisms involved in this dynamic balance. Notch is an extrinsic signaling pathway whose influence on cell function has been studied extensively. SCs in the quiescent state (Fig. 1) are maintained actively rather than it being in a default inactive state. Active notch signaling is required to maintain this state62,63. Transgenic mice, in which canonical notch signaling was conditionally ablated, demonstrated increased propensity for spontaneous differentiation and dramatic depletion of the satellite cell pool62. Interestingly, SCs with impaired notch signaling progressed immediately to differentiation without initially dividing and simply fused to existing myofibers63. During aging, muscle regenerative potential decreases, which can be improved via notch-mediated pathways64. Recently it was shown that the quiescent state might not actually be a single homogenous state, rather it is heterogeneous, and progresses from a mitotically quiescent G0 phase to a GAlert (pre-activation) phase, where it is primed for activation65.
Wnt signaling plays an important role during SC activation, proliferation and helps determine stem cell fate66. Symmetric expansion likely occurs through wnt7a utilizing the planar cell polarity pathway, i.e. a non-canonical pathway not involving β-catenin67. This pathway might be critical for maintaining the SC pool, since muscles without wnt7a show decreased SC number after regeneration21. After activation, while Notch-1 signaling has been implicated in SC proliferation68, to progress from proliferation to differentiation, a switch in signaling from notch to wnt is required69. Wnt and notch signaling interact to create sufficient proliferation prior to differentiation to maintain efficient repair and appropriate progression down the myogenic lineage. Furthermore, it has been shown that, in aging, increased canonical wnt signaling during SC proliferation can convert the SC myogenic lineage to a fibrogenic one resulting in aberrant fibrosis70. Importantly, this conversion can be suppressed with wnt inhibitors, suggesting a critical role of wnt signaling in altering SC fate.
An emerging research area is SC self-renewal and how activated SCs revert to quiescence to maintain the SC pool. Elevated activity of p38 mitogen-activated protein kinase (MAPK) in aging was shown to be involved in loss of self-renewal71 while transient inhibition of this pathway expanded the stem cell population72. Sprouty1 (spry1), a tyrosine kinase inhibitor, is expressed during SC quiescence, downregulated during proliferation and re-expressed during return to quiescence73. Conditional ablation of spry1 in SCs led to a dramatic reduction in SC pool after activation due to injury. This indicates that spry1 is required for restoring the muscle stem cell pool after activation, i.e. for reversible quiescence and self-renewal73. Homeodomain transcription factor six1 has also been implicated to play a role in limiting SC self-renewal, as evidenced by the increased SC pool observed in Six1 knockout mice74. Recently it was shown that forkhead box O3 (foxo3), which is most widely known as the factor controlling the skeletal muscle atrophy pathway75,76, also promotes quiescence during self-renewal77. Together, these results point to an intricate interaction among signaling pathways to control the state of muscle and its resident stem cells. A number of signaling pathways are required to maintain the dynamic balance between quiescence, activation, return to quiescence and self-renewal, all of which are necessary to maintain a healthy and functional SC pool (Table 1).
Table 1.
Various signaling pathways involved in the dynamic balance among maintaining quiescence, activation, proliferation, differentiation, return to quiescence and self-renewal. Numbers refer to respective references.
| Signaling Pathway | SC function | References |
|---|---|---|
| Nitric oxide | Activation | 56,58 |
| Growth factors (IGF, FGF) | Activation | 57 |
| Notch | Maintenance of quiescence, proliferation, SC fate |
62,63,64,67 |
| Wnt | Differentiation, SC fate | 66,68,69 |
| MAPK | Self-renewal | 70,71 |
| Spry1 | Quiescence, return to quiescence after Self-renewal |
72 |
| Six1 | Self-renewal | 73 |
| Foxo3 | Return to quiescence after self- renewal |
76 |
Satellite cell niche and interactions
Unlike the initial impression that a SC had a fairly simple extracellular localization, it is becoming clear that the SC “niche” (defined as the microenvironment where the quiescent SC resides) is complex, important and thus, highly regulated. The niche composition and structure is critical because extrinsic environmental cues are important determinants of SC functional state, i.e. maintenance of quiescence, activation, and return to quiescence. SCs have a complex interaction with their associated extracellular matrix (ECM). During quiescence, they interact with both the basal lamina and sarcolemma, but, once activated, actively remodel the local extracellular matrix via increased levels of matrix metalloproteinases (MMPs)78. This interaction apparently is partly responsible for SC quiescence since simple mechanical disruption of this contact results in immediate activation. During postnatal development79,42 and in adulthood80 proliferating myoblasts are fairly motile and can rather easily cross the ECM between fibers depending on where they are needed. Biomechanical and biochemical properties of the niche may influence SC response. In vitro methods have demonstrated that the elastic stiffness of the extracellular matrix might be important for functional differentiation81 and for self-renewal of SCs82.
Furthermore, tamoxifen-induced experimental ablation of 90% of the SCs 83 revealed that SCs have a regulatory effect on fibroblasts (identified as Tcf4+) since their ablation and chemical injury to the muscles led to increased fibrosis along with poor regeneration. Similarly, experimental ablation of 42% of fibroblasts and chemical muscle injury impaired SC ability to proliferate, leading to premature SC differentiation and smaller regenerated myofibers83. Connective tissue fibroblasts have also been shown to regulate myogenesis and expression of myosin heavy chain (MyHC) isoform84. In another study evaluating the role of SCs on adult muscle hypertrophy and ECM remodeling54 it was shown that after ~90% SC ablation and synergistic ablation of the gastrocnemius and soleus, while compensatory hypertrophy of the plantaris was possible, there was dysregulation of the connective tissue with an increased accumulation of the ECM and an expansion of the fibroblasts. Additionally, in vitro studies suggested that activated SCs exerted negative regulation of fibroblasts. Together, these studies show that ECM, fibroblasts and SCs interact to regulate their function and myogenesis. Interestingly, in other organ systems such as in the liver, similar feedback processes are at play wherein hepatic stellate cells interact intimately with other cells such as hepatocytes and endothelial cells, particularly after injury and inflammation, to ensure appropriate repair. Inappropriate interactions amongst these cells leads to fibrosis85-88.
Apart from the ECM, quiescent SCs are present in close proximity with muscle microvasculature89. On activation, interaction with endothelial cells is facilitated, which is important for angiogenesis to coordinate myogenesis. Furthermore, change in factors associated with the niche can influence SC function. With aging, aberrant signaling within the niche can lead to increased loss of quiescence and SC depletion90. Our current understanding of the composition, cellular interactions and molecular control of the SC niche is quickly evolving.
Cerebral Palsy Palsy & Muscular Dystrophy
Our laboratory has a particular interest in cerebral palsy (CP), which is the most common developmental motor disorder affecting 2-4 children per 1000 every year91. After perinatal brain injury, these children present with significant clinical problems that are related to impaired longitudinal and cross-sectional muscle growth. A most obvious impairment is weakness, that is due in part, to decreased muscle fiber size92 and decreased neural drive93,94. However, more complex musculoskeletal changes are also noted95,96, specifically, the formation of contractures97,98. Paradoxically, these shortened muscles are accompanied by overstretched sarcomeres within the tissue98. Changes in contracture extracellular matrix97, i.e. fibrotic changes and transcriptional profile99,100 also highlight the deranged nature of the muscle tissue. Grossly, there may be changes in the fascicle length95 and tendon length101. Overall it is clear that CP muscle has reduced capacity for longitudinal and cross-sectional growth during the postnatal period. It is our hypothesis that this inability to accommodate growth, because of SC loss and dysfunction leads to contractures (Fig. 3B).
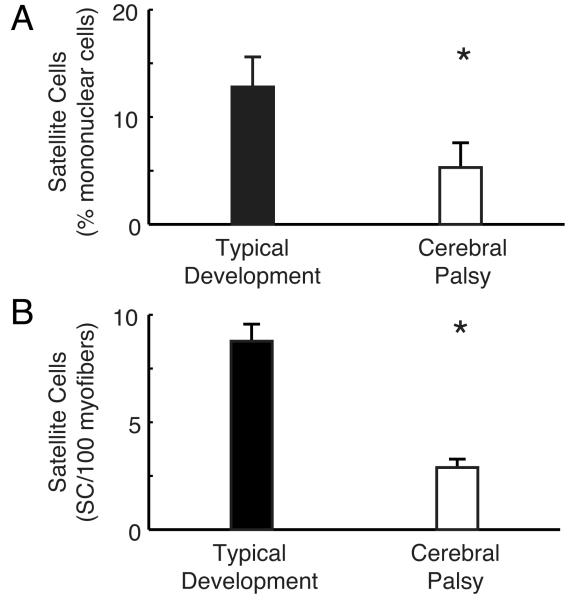
In light of the discussion above where we presented evidence that SCs play a significant role in muscle growth, we speculated that contracture formation may, in part, be due to SC dysfunction. Using flow cytometry of muscle biopsies from children with CP102, we showed that, indeed, compared to typically developing children, children with CP had a significantly reduced (~60%) SC population, expressed as a percentage of all mononuclear cells (Fig. 4A). However, as noted, children with CP have extracellular matrix abnormalities that may systematically bias flow cytometry results in that it may be more difficult to extract SCs from CP muscle. To test this idea, we used the more labor intensive in situ immunohistochemistry method to quantify SCs103 using antibodies for SCs (anti-Pax7) the basal lamina (anti-Laminin) and a nuclear stain (DAPI). By systematically sampling large volumes of tissue, we quantified SC number in situ without significant tissue manipulation (Fig. 5). It turned out that SC number quantified from these sections, as number per 100 myofibers, similarly showed a 70% decrease compared to age-appropriate controls (Fig. 4B). Since total nuclear number was not different between groups, we speculate that SC reduction occurred later during development. Together, these two studies using different methods and different human subjects, demonstrate that it is highly likely that there are significant changes in the SC population in children with CP and suggest possible future avenues for therapeutic intervention using regenerative medicine.
Figure 4.
Satellite cell populations in children with typical development (TD) and cerebral palsy (CP) measured using two different methods. (A) Satellite cell percentage measured by flow cytometry (B) Satellite cell percentage measured by immunohistochemistry. Both of these methods, using different human subjects, demonstrate a decreased number of SCs with in CP muscle contractures. Data are replotted from Smith et al.102 (A) or Dayanidhi et al.103 (B). Asterisks indicate significant difference, p<0.05.
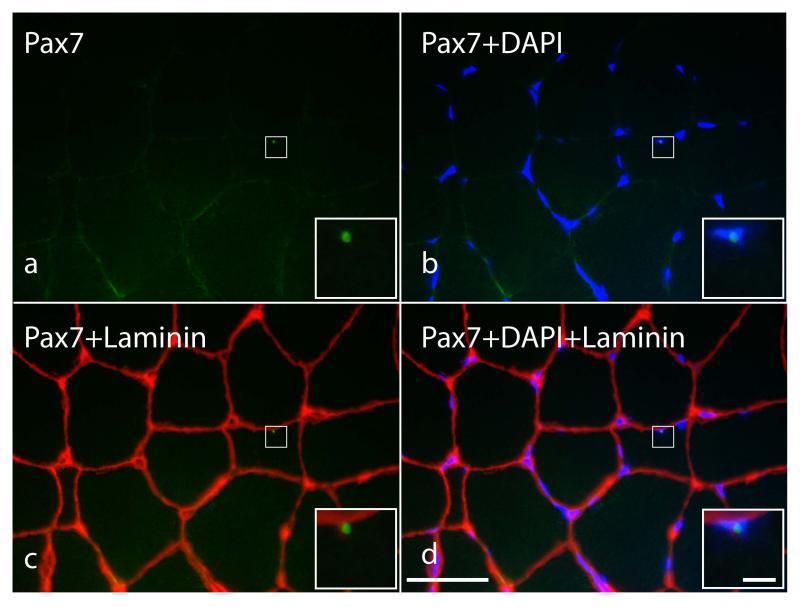
Figure 5.
High-resolution immunohistochemical identification of satellite cells in a representative gracilis cross-section from a child with cerebral palsy. Because satellite cells are indistinguishable from myonuclei based on morphology at the light microscope level, it is necessary to use immunohistochemistry for the basal lamina (laminin, red), a transcription factor (Pax7, green) and DNA stain (DAPI, blue) to unambiguously distinguish satellite cells from myonuclei and other mononuclear cells within muscle tissue. Satellite cells identified based on a positive label for Pax7 (a), co-localization with DAPI (b) and location outside of the basal lamina (c). The merged image is shown in d. Square box in a, b, c and d show the location of the satellite cell. Inset shows magnified view. Scale bar is 50μm except for inset which is 5 μm.
Duchenne muscular dystrophy (DMD) is the most common form of muscular dystrophy that results from a genetic defect leading to a lack of the cytoskeletal muscle protein dystrophin104. Becker muscular dystrophy (BMD) is also associated with a defect in the dystrophin gene but is functionally milder. Clinically, children with DMD have progressive weakness starting at age 3-5 that leads to premature death by late adolescence/young adulthood105,104. The impairment in dystrophin and its associated proteins causes an interruption between the myofiber, its sarcolemma and extracellular matrix (ECM)106 leading to altered mechanical stress, reduced stiffness, inflammation, and consequent muscle regeneration107,108. Transgenic mouse models for DMD (mdx, mdx/mTR, mdx/utrophin−/−) have defined the phenotype of muscle dysfunction in DMD and have been used to evaluate the role of SCs in the progressive weakness associated with DMD109. Sacco et al.110 showed that pathological muscle progression in DMD could be related to an inability of the SCs to maintain the capacity to repair following multiple damage-repair cycles, i.e. an early exhaustion of the stem cell pool. Consistent with the idea that dystrophic muscle experiences multiple cycles of regeneration, it has been shown that telomere shortening110 occurs in DMD patients’ muscles111. Most interestingly, somewhat similar to the extracellular matrix changes in CP, there are marked fibrotic changes observed in DMD patients’ muscles and in transgenic mouse models110,112,113. Currently, novel therapies targeting muscle stem cell dysfunction are being evaluated as a means to improve muscle function in dystrophies114-116. Recently focal treatment in mdx mice with wnt7a results in structural improvements such as increased SC number and fiber hypertrophy111. In addition, as would be expected, antifibrotic therapies can improve muscle function by reducing fibrosis and improving muscle regeneration capacity109.
Conclusions
In summary, we have reviewed the role that skeletal muscle stem cells, SCs play during postnatal development and repair. SCs, on activation participate in myogenic function and provide the biological basis for longitudinal and cross-sectional growth. Their activation and subsequent return to quiescence is controlled by complex molecular mechanisms that ensure participation in growth, regeneration and repair while maintaining the SC pool. The SC niche facilitates intimate interaction between SCs and other cell types and allows feedback control among cells to coordinate participation in myogenic function. Children with cerebral palsy have significant problems with longitudinal and cross-sectional muscle growth. Our recent work showed that SC dysfunction could explain that finding and provide new therapeutic directions to enhance muscle function. Children with muscular dystrophies also show similar features of SC depletion and fibrotic changes that may also lend themselves to novel therapeutic treatments.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award number NIH grants R24 HD05083, and the Department of Veterans Affairs Grant A9028-R. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BMD
Becker muscular dystrophy
- CD
Cluster of differentiation
- CP
Cerebral Palsy
- DAPI
4′, 6-diamidino-2-phenylindole
- DMD
Duchenne muscular dystrophy
- ECM
Extracellular matrix
- FACS
Fluorescence- activated cell sorting
- FGF
Fibroblast growth factor
- FOXO3
Forkhead box O3
- HGF
Hepatocyte growth factor
- IGF
Insulin-like growth factor
- NCAM
Neural cell adhesion molecule
- MAPK
Mitogen-activated protein kinase
- MMP
Metalloproteinases
- MRF
Myogenic regulatory factor
- MYF5
Myogenic factor 5
- MYHC
Myosin heavy chain
- PAX7
Paired box 7
- SC
Satellite cell
- SPRY1
Sprouty1
References
- 1.Mauro A. Satellite Cell Of Skeletal Muscle Fibers. The Journal of Biophysical and Biochemical Cytology. 1961;9(2):493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enesco M, Puddy D. Increase in the Number of Nuclei and Weight in Skeletal Muscle of Rats of Various Ages. The American journal of anatomy. 1964;114:235–244. doi: 10.1002/aja.1001140204. [DOI] [PubMed] [Google Scholar]
- 3.Macconnachie HF, Enesco M, Leblond CP. The Mode of Increase in the Number of Skeletal Muscle Nuclei in the Postnatal Rat. The American journal of anatomy. 1964;114:245–253. doi: 10.1002/aja.1001140205. [DOI] [PubMed] [Google Scholar]
- 4.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. The Anatomical record. 1971;170(4):421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- 5.Weissman IL. Stem Cells: Units of Development, Units of Regeneration, and Units in Evolution. Cell. 2000;100(1):157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 6.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell. 2000;102(6):777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 7.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem Cell Function, Self-Renewal, and Behavioral Heterogeneity of Cells from the Adult Muscle Satellite Cell Niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? The Journal of cell biology. 2004;166(3):347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halevy O, Piestun Y, Allouh MZ, Rosser BWC, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Developmental Dynamics. 2004;231(3):489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- 10.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends in cell biology. 2005;15(12):666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Sambasivan R, Tajbakhsh S. Skeletal muscle stem cell birth and properties. Seminars in Cell & Developmental Biology. 2007;18(6):870–882. doi: 10.1016/j.semcdb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Pannérec A, Marazzi G, Sassoon D. Stem cells in the hood: the skeletal muscle niche. Trends in Molecular Medicine. 2012 doi: 10.1016/j.molmed.2012.07.004. (0) [DOI] [PubMed] [Google Scholar]
- 13.Wang YX, Rudnicki MA. Satellite cells, the engines of muscle repair. Nature reviews Molecular cell biology. 2012;13(2):127–133. doi: 10.1038/nrm3265. [DOI] [PubMed] [Google Scholar]
- 14.Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO reports. 2013;14(12):1062–1072. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nature reviews Molecular cell biology. 2013;14(6):329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yablonka-Reuveni Z. The Skeletal Muscle Satellite Cell. Journal of Histochemistry & Cytochemistry. 2011;59(12):1041–1059. doi: 10.1369/0022155411426780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin H, Price F, Rudnicki MA. Satellite Cells and the Muscle Stem Cell Niche. Physiological Reviews. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pownall ME, Gustafsson MK, Emerson CP. Myogenic Regulatory Factors And The Specification Of Muscle Progenitors In Vertebrate Embryos. Annual Review of Cell and Developmental Biology. 2002;18(1):747–783. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- 19.Hindi SM, Tajrishi MM, Kumar A. Signaling mechanisms in mammalian myoblast fusion. Science signaling. 2013;6(272):re2. doi: 10.1126/scisignal.2003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins SC, Cullen MJ. A quantitative study of myonuclear and satellite cell nuclear size in Duchenne’s muscular dystrophy, polymyositis and normal human skeletal muscle. The Anatomical record. 1988;222(1):6–11. doi: 10.1002/ar.1092220103. [DOI] [PubMed] [Google Scholar]
- 22.Allouh MZ, Yablonka-Reuveni Z, Rosser BWC. Pax7 Reveals a Greater Frequency and Concentration of Satellite Cells at the Ends of Growing Skeletal Muscle Fibers. Journal of Histochemistry & Cytochemistry. 2008;56(1):77–87. doi: 10.1369/jhc.7A7301.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schultz E. A quantitative study of the satellite cell population in postnatal mouse lumbrical muscle. The Anatomical record. 1974;180(4):589–595. doi: 10.1002/ar.1091800405. [DOI] [PubMed] [Google Scholar]
- 24.Neal A, Boldrin L, Morgan JE. The Satellite Cell in Male and Female, Developing and Adult Mouse Muscle: Distinct Stem Cells for Growth and Regeneration. PLoS ONE. 2012;7(5):e37950. doi: 10.1371/journal.pone.0037950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Developmental Biology. 2006;294(1):50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson MC, Schultz E. Age-related differences in absolute numbers of skeletal muscle satellite cells. Muscle & Nerve. 1983;6(8):574–580. doi: 10.1002/mus.880060807. [DOI] [PubMed] [Google Scholar]
- 27.Brack A, Rando T. Intrinsic Changes and Extrinsic Influences of Myogenic Stem Cell Function During Aging. Stem Cell Rev. 2007;3(3):226–237. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- 28.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 29.Gokhin DS, Ward SR, Bremner SN, Lieber RL. Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse. Journal of Experimental Biology. 2008;211(6):837–843. doi: 10.1242/jeb.014340. [DOI] [PubMed] [Google Scholar]
- 30.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23(11):1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery RD. Growth of human striated muscle. Nature. 1962;195:194–195. doi: 10.1038/195194a0. [DOI] [PubMed] [Google Scholar]
- 32.Griffin GE, Williams PE, Goldspink G. Region of longitudinal growth in striated muscle fibres. Nature: New biology. 1971;232(27):28–29. doi: 10.1038/newbio232028a0. [DOI] [PubMed] [Google Scholar]
- 33.Williams PE, Goldspink G. Longitudinal growth of striated muscle fibres. Journal of cell science. 1971;9(3):751–767. doi: 10.1242/jcs.9.3.751. [DOI] [PubMed] [Google Scholar]
- 34.Williams PE, Goldspink G. The effect of immobilization on the longitudinal growth of striated muscle fibres. J Anat. 1973;116(Pt 1):45–55. [PMC free article] [PubMed] [Google Scholar]
- 35.Goldspink G. The Proliferation of Myofibrils During Muscle Fibre Growth. Journal of cell science. 1970;6(2):593–603. doi: 10.1242/jcs.6.2.593. [DOI] [PubMed] [Google Scholar]
- 36.White R, Bierinx A-S, Gnocchi V, Zammit P. Dynamics of muscle fibre growth during postnatal mouse development. BMC Developmental Biology. 2010;10(1):21. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle & Nerve. 1999;22(10):1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 38.Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. The Journal of clinical investigation. 2008;118(4):1450–1457. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? The Journal of Physiology. 2008;586(11):2675–2681. doi: 10.1113/jphysiol.2008.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. Journal of Applied Physiology. 1995;78(5):1969–1976. doi: 10.1152/jappl.1995.78.5.1969. [DOI] [PubMed] [Google Scholar]
- 41.Roy RR, Monke SR, Allen DL, Edgerton VR. Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers. Journal of Applied Physiology. 1999;87(2):634–642. doi: 10.1152/jappl.1999.87.2.634. [DOI] [PubMed] [Google Scholar]
- 42.Van der Meer SF, Jaspers RT, Degens H. Is the myonuclear domain size fixed? Journal of musculoskeletal & neuronal interactions. 2011;11(4):286–297. [PubMed] [Google Scholar]
- 43.Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460(7255):627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. The EMBO Journal. 2004;23(16):3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proceedings of the National Academy of Sciences. 2013;110(41):16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138(17):3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 48.Günther S, Kim J, Kostin S, Lepper C, Fan C-M, Braun T. Myf5-Positive Satellite Cells Contribute to Pax7-Dependent Long-Term Maintenance of Adult Muscle Stem Cells. Cell stem cell. 2013 doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Connor RS, Pavlath GK. Point:Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. Journal of Applied Physiology. 2007;103(3):1099–1100. doi: 10.1152/japplphysiol.00101.2007. [DOI] [PubMed] [Google Scholar]
- 50.O’Connor RS, Pavlath GK, McCarthy JJ, Esser KA. Last Word on Point:Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. Journal of Applied Physiology. 2007;103(3):1107. doi: 10.1152/japplphysiol.00502.2007. [DOI] [PubMed] [Google Scholar]
- 51.McCarthy JJ, Esser KA. Counterpoint: Satellite cell addition is not obligatory for skeletal muscle hypertrophy. Journal of Applied Physiology. 2007;103(3):1100–1102. doi: 10.1152/japplphysiol.00101.2007a. [DOI] [PubMed] [Google Scholar]
- 52.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138(17):3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. American Journal of Physiology - Cell Physiology. 2012;303(8):C854–C861. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. The FASEB Journal. 2014;28(4):1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuang S, Gillespie MA, Rudnicki MA. Niche Regulation of Muscle Satellite Cell Self-Renewal and Differentiation. Cell stem cell. 2008;2(1):22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Wozniak AC, Kong J, Bock E, Pilipowicz O, Anderson JE. Signaling satellite-cell activation in skeletal muscle: Markers, models, stretch, and potential alternate pathways. Muscle & Nerve. 2005;31(3):283–300. doi: 10.1002/mus.20263. [DOI] [PubMed] [Google Scholar]
- 57.Allen RE, Rankin LL. Regulation of Satellite Cells during Skeletal Muscle Growth and Development. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 1990;194(2):81–86. doi: 10.3181/00379727-194-43060. [DOI] [PubMed] [Google Scholar]
- 58.Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. American Journal of Physiology - Cell Physiology. 2006;290(6):C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- 59.Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res. 2001;267(1):107–114. doi: 10.1006/excr.2001.5252. [DOI] [PubMed] [Google Scholar]
- 60.Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: Possible mechanism of stretch-induced activation of resident myogenic stem cells. Animal Science Journal. 2010;81(1):11–20. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- 61.Nishimura T, Nakamura K, Kishioka Y, Kato-Mori Y, Wakamatsu J-i, Hattori A. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. Journal of muscle research and cell motility. 2008;29(1):37–44. doi: 10.1007/s10974-008-9140-2. [DOI] [PubMed] [Google Scholar]
- 62.Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A Critical Requirement for Notch Signaling in Maintenance of the Quiescent Skeletal Muscle Stem Cell State. Stem Cells. 2012;30(2):243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 63.Bjornson CRR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch Signaling Is Necessary to Maintain Quiescence in Adult Muscle Stem Cells. Stem Cells. 2012;30(2):232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 65.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai C-R, Goodell MA, Rando TA. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to GAlert. Nature. 2014 doi: 10.1038/nature13255. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends in cell biology. 2012;22(11):602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Grand F, Jones AE, Seale V, ScimË A, Rudnicki MA. Wnt7a Activates the Planar Cell Polarity Pathway to Drive the Symmetric Expansion of Satellite Stem Cells. Cell stem cell. 2009;4(6):535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conboy IM, Rando TA. The Regulation of Notch Signaling Controls Satellite Cell Activation and Cell Fate Determination in Postnatal Myogenesis. Developmental Cell. 2002;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 69.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis. Cell stem cell. 2008;2(1):50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt Signaling During Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science. 2007;317(5839):807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 71.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20(3):265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20(3):255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell stem cell. 2010;6(2):117–129. doi: 10.1016/j.stem.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le Grand F, Grifone R, Mourikis P, Houbron C, Gigaud C, Pujol J, Maillet M, Pagès G, Rudnicki M, Tajbakhsh S, Maire P. Six1 regulates stem cell repair potential and self-renewal during skeletal muscle regeneration. The Journal of cell biology. 2012;198(5):815–832. doi: 10.1083/jcb.201201050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. The International Journal of Biochemistry & Cell Biology. 2005;37(10):1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 76.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 Controls Autophagy in Skeletal Muscle In Vivo. Cell Metabolism. 2007;6(6):458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Gopinath Suchitra D, Webb Ashley E, Brunet A, Rando Thomas A. FOXO3 Promotes Quiescence in Adult Muscle Stem Cells during the Process of Self-Renewal. Stem Cell Reports. 2014;2(4):414–426. doi: 10.1016/j.stemcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, Montarras D, Buckingham M. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem cell research. 2010;4(2):77–91. doi: 10.1016/j.scr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 79.Hughes SM, Blau HM. Migration of myoblasts across basal lamina during skeletal muscle development. Nature. 1990;345(6273):350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- 80.Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DDW. 3D Timelapse Analysis of Muscle Satellite Cell Motility. Stem Cells. 2009;27(10):2527–2538. doi: 10.1002/stem.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. The Journal of cell biology. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science. 2010;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138(17):3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138(2):371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedman SL. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiological Reviews. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman SL. Mechanisms of Hepatic Fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. Journal of Gastroenterology and Hepatology. 2007;22:S73–S78. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 88.Lieber RL, Ward SR. Cellular Mechanisms of Tissue Fibrosis. 4. Structural and functional consequences of skeletal muscle fibrosis. American Journal of Physiology - Cell Physiolog. 2013;305(3):C241–C252. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Christov C, Chrétien F, Abou-Khalil R, Bassez G, Vallet G, Authier F-J, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle Satellite Cells and Endothelial Cells: Close Neighbors and Privileged Partners. Molecular Biology of the Cell. 2007;18(4):1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490(7420):355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 92.Castle ME, Reyman TA, Schneider M. Pathology of spastic muscle in cerebral palsy. Clinical orthopaedics and related research. 1979;(142):223–232. [PubMed] [Google Scholar]
- 93.Mockford M, Caulton JM. The Pathophysiological Basis of Weakness in Children With Cerebral Palsy. Pediatric Physical Therapy. 2010;22(2):222–233. doi: 10.1097/PEP.0b013e3181dbaf96. 210.1097/PEP.1090b1013e3181dbaf1096. [DOI] [PubMed] [Google Scholar]
- 94.Rose J, McGill KC. Neuromuscular activation and motor-unit firing characteristics in cerebral palsy. Developmental Medicine & Child Neurology. 2005;47(5):329–336. doi: 10.1017/s0012162205000629. [DOI] [PubMed] [Google Scholar]
- 95.Barrett RS, Lichtwark GA. Gross muscle morphology and structure in spastic cerebral palsy: a systematic review. Developmental Medicine & Child Neurology. 2010;52(9):794–804. doi: 10.1111/j.1469-8749.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- 96.Foran JR, Steinman S, Barash I, Chambers HG, Lieber RL. Structural and mechanical alterations in spastic skeletal muscle. Dev Med Child Neurol. 2005;47(10):713–717. doi: 10.1017/S0012162205001465. [DOI] [PubMed] [Google Scholar]
- 97.Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. 2011;589(Pt 10):2625–2639. doi: 10.1113/jphysiol.2010.203364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lieber RL, Friden J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve. 2002;25(2):265–270. doi: 10.1002/mus.10036. [DOI] [PubMed] [Google Scholar]
- 99.Smith LR, Ponten E, Hedström Y, Ward SR, Chambers HG, Subramaniam S, Lieber RL. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC medical genomics. 2009;2:44. doi: 10.1186/1755-8794-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith LR, Chambers HG, Subramaniam S, Lieber RL. Transcriptional Abnormalities of Hamstring Muscle Contractures in Children with Cerebral Palsy. PLoS ONE. 2012;7(8):e40686. doi: 10.1371/journal.pone.0040686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wren TAL, Cheatwood AP, Rethlefsen SA, Hara R, Perez FJ, Kay RM. Achilles Tendon Length and Medial Gastrocnemius Architecture in Children With Cerebral Palsy and Equinus Gait. Journal of Pediatric Orthopaedics. 2010;30(5):479–484. doi: 10.1097/BPO.0b013e3181e00c80. [DOI] [PubMed] [Google Scholar]
- 102.Smith LR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. Developmental Medicine & Child Neurology. 2013;55(3):264–270. doi: 10.1111/dmcn.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dayanidhi S, Dykstra P, Lyubasyuk V, McKay BR, Chambers HG, Lieber RL. Reduced satellite cell population may lead to contractures in children with cerebral palsy. 2014. Submitted.
- 104.Emery AEH. The muscular dystrophies. The Lancet. 2002;359(9307):687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 105.Blake DJ, Weir A, Newey SE, Davies KE. Function and Genetics of Dystrophin and Dystrophin-Related Proteins in Muscle. Physiological Reviews. 2002;82(2):291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 106.Patel TJ, Lieber RL. Force transmission in skeletal muscle: from actomyosin to external tendons. Exerc Sport Sci Rev. 1997;25:321–363. [PubMed] [Google Scholar]
- 107.Matsumura K, Campbell KP. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994;17(1):2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- 108.Pasternak C, Wong S, Elson EL. Mechanical function of dystrophin in muscle cells. The Journal of cell biology. 1995;128(3):355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu A, Poddar M, Tang Y, Proto JD, Sohn J, Mu X, Oyster N, Wang B, Huard J. Rapid depletion of muscle progenitor cells in dystrophic mdx/utrophin−/− mice. Human molecular genetics. 2014 doi: 10.1093/hmg/ddu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143(7):1059–1071. doi: 10.1016/j.cell.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Decary S, Ben Hamida C, Mouly V, Barbet JP, Hentati F, Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscular Disorders. 2000;10(2):113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 112.Zhou L, Lu H. Targeting Fibrosis in Duchenne Muscular Dystrophy. Journal of Neuropathology & Experimental Neurology. 2010;69(8):771–776. doi: 10.1097/NEN.0b013e3181e9a34b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kharraz Y, Guerra J, Pessina P, Serrano AL, Muñoz-Cánoves P. Understanding the Process of Fibrosis in Duchenne Muscular Dystrophy. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/965631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Maltzahn J, Renaud JM, Parise G, Rudnicki MA. Wnt7a treatment ameliorates muscular dystrophy. Proc Natl Acad Sci U S A. 2012;109(50):20614–20619. doi: 10.1073/pnas.1215765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bertoni C. Emerging gene editing strategies for Duchenne muscular dystrophy targeting stem cells. Frontiers in Physiology. 2014:5. doi: 10.3389/fphys.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilschut KJ, Ling VB, Bernstein HS. Bernstein HS. Concise Review: Stem Cell Therapy for Muscular Dystrophies. Stem Cells Translational Medicine. 2012;1(11):833–842. doi: 10.5966/sctm.2012-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]