Abstract
Background
Coronary artery bypass grafting (CABG) success is limited by vein graft failure (VGF). Understanding factors associated with VGF may improve patient outcomes.
Methods and Results
We examined 1828 participants in the PREVENT IV trial undergoing protocol-mandated follow-up angiography 12–18 months post-CABG or earlier clinically-driven angiography. Outcomes included patient- and graft-level angiographic VGF (≥75% stenosis or occlusion). Variables were selected using Fast False Selection Rate methodology. We examined relationships between variables and VGF in patient- and graft-level models using logistic regression without and with generalized estimating equations. At 12–18 months post-CABG, 782 of 1828 (42.8%) patients had VGF, and 1096 of 4343 (25.2%) vein grafts had failed. Demographic and clinical characteristics were similar between patients with and without VGF, though VGF patients had longer surgical times, worse target artery quality, longer graft length, and more frequently underwent endoscopic vein harvesting. After multivariable adjustment, longer surgical duration (odds ratio [OR] per 10-minute increase 1.05, 95% confidence interval [CI] 1.03–1.07), endoscopic vein harvesting (OR 1.41, 95% CI 1.16–1.71), poor target artery quality (OR 1.43, 95% CI 1.11–1.84), and postoperative use of clopidogrel or ticlopidine (OR 1.35, 95% CI 1.07–1.69) were associated with patient-level VGF. The predicted likelihood of VGF in the graft-level model ranged from 12.1–63.6%.
Conclusions
VGF is common and associated with a number of patient and surgical factors. These findings may help identify patients with risk factors for VGF and inform the development of interventions to reduce VGF.
Keywords: coronary disease, revascularization, bypass, surgery
Coronary artery bypass grafting (CABG) is one of the most frequently performed surgical procedures in the United States, with over 400,000 procedures performed annually.1 Although CABG improves survival and symptoms in selected patients,1-3 surgical success depends on the continued patency of grafts, and graft failure has been associated with worse outcomes.4,5 Saphenous vein grafts remain the most widely used conduit during CABG, and rates of vein graft failure (VGF) during the first 12 to 18 months after surgery have been reported to be as high as 25%.6-10
Many studies have examined factors associated with VGF and have inconsistently reported associations between multiple clinical and surgical characteristics and VGF.11-15 These previous efforts have been limited by the absence of systematic angiographic follow-up. In addition, results from these studies may be outdated, given advances in surgical techniques and adjunctive medical therapies that could impact graft failure. We therefore sought to examine factors associated with VGF assessed by coronary angiography 12–18 months after CABG using data from the PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) trial.
METHODS
Data source and patient population
We used data from the PREVENT IV trial (ClinicalTrials.gov: NCT00042081), the design and results of which have been previously described.16 Briefly, PREVENT IV was a phase 3 randomized, double-blind, placebo-controlled trial of ex-vivo vein graft treatment with edifoligide in patients undergoing primary CABG with ≥2 planned vein grafts. A total of 3014 patients were enrolled between August 2002 and October 2003 at 107 centers across the U.S., the first 2400 of whom were scheduled for follow-up angiography between 12–18 months after CABG. The PREVENT IV protocol was approved by institutional review boards of all participating sites and all enrolled patients provided written informed consent.
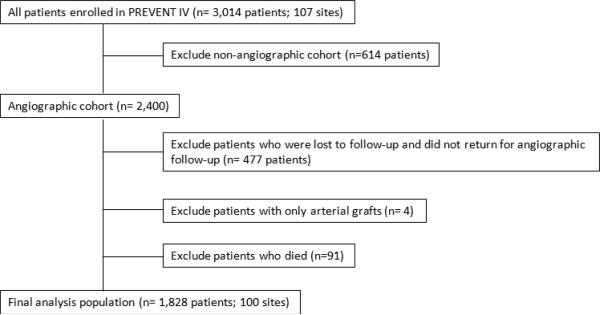
We included patients in the angiographic cohort who were scheduled to undergo follow-up angiography 12–18 months after the index CABG (n=2400). Patients in the angiographic cohort who had VGF documented during earlier angiography for clinical indications in place of (n=64) or in addition to (n=107) routine protocol angiography were included. We excluded patients who did not undergo angiographic follow-up (n=477), who received only arterial grafts (n=4), or who died prior to their 12–18 month repeat angiogram (n=91). Our final analysis population consisted of 1828 patients enrolled at 100 sites (Figure 1).
Figure 1.
Flowchart of patient selection for the final analysis population.
Definitions and outcomes
VGF was defined as ≥75% stenosis or occlusion detected at follow-up angiography 12–18 months after CABG or earlier angiography performed for clinical indications. All angiograms were analyzed at a core laboratory (PERFUSE Angiographic Core Laboratory, Boston, MA). For grafts with multiple distal anastomoses (m-SVG), failure of any component was considered VGF.17 Outcomes for our analyses were defined as failure of 1 or more vein grafts (patient-level angiographic VGF) and graft-level angiographic VGF.
Statistical analysis
Baseline patient and procedure characteristics were examined according to patient-level absence or presence of VGF at 12–18 months post-CABG. Continuous variables were summarized using medians and interquartile ranges (IQR), while categorical variables were presented as frequencies and percentages. Comparisons within continuous and categorical variable groups were performed using Wilcoxon 2-sample test and Chi-square test, respectively.
We analyzed surgical features at both the patient- and graft-levels. When describing patient-level characteristics, we used the “worst” status to describe procedure characteristics for patients with multiple vein grafts. The following hierarchies (worst status listed first) were used: target artery quality= poor, fair, good; graft quality= poor, fair, good; distal connection technique= non-suture, suture; graft length= longest measurement; graft source= arm vein, lesser saphenous vein, greater saphenous vein; vein harvest technique= endoscopic, open; and m-SVG use= yes, no.
We developed patient- and graft-level models to determine factors associated with VGF. For the main analysis, patient-level variables were created by assessing graft-level data for each patient and, for patients with multiple grafts, determining the worst status for each characteristic among all grafts. We also performed a secondary analysis to examine graft-level variables associated with VGF. For both models, variables associated with VGF were selected using Fast False Selection Rate (Fast FSR).18 Fast FSR is a conservative variable selection method that accounts for the percentage of variables incorrectly identified as associated with the outcome of interest. Logistic regression models were then fit using the chosen variables to estimate the association of each factor with VGF and odds ratios (OR) with associated 95% confidence intervals (CI) were reported. For graft-level analyses, in order to account for the correlation among multiple grafts within the same patient, generalized estimating equations were used to fit a generalized linear logistic model that allows for an exchangeable correlation matrix between grafts within a single patient.
The following candidate variables were chosen based on clinical judgment and considered for inclusion in both patient- and graft-level models: age, female sex, weight, race, smoking status, chronic lung disease, hypertension, dyslipidemia, prior myocardial infarction, prior percutaneous coronary intervention, prior cancer, history of liver disease, peripheral artery disease, cerebrovascular disease, prior congestive heart failure, current New York Heart Association class, diabetes (no history, non-insulin therapy, insulin therapy), renal failure, atrial fibrillation/flutter, ejection fraction, type of CABG procedure (emergent/salvage, urgent, elective), use of cardiopulmonary bypass (CPB), CPB time, aortic cross-clamp time, surgical time, graft source (greater saphenous, lesser saphenous), vein harvest technique (endoscopic, open), graft quality, maximum stenosis of target vessel (<75%, ≥75%), target artery quality, proximal anastomosis connection technique (suture, non-suture), graft length, and use of m-SVG. For both patient- and graft-level models, linear splines were used to determine appropriate knot points for the following non-linear variables (see Online supplement for knot points): aortic cross-clamp time, ejection fraction, graft length (patient-level model only), and CPB time (graft-level model only). Significant (p<0.1) levels were then included as candidate variables (see Online supplement). We hypothesized that chronic use of certain medications might be associated with VGF. In PREVENT IV, data regarding medication use were collected at the discrete time points at baseline, discharge, 30 days, and 1 year. We chose to examine 30-day medication use as covariates, as these were thought to best represent chronic postoperative use following the initial surgery. However, since medication use at 30 days is a post-baseline variable, it was included in models as a sensitivity analyses. Rates of missingness for data in our models were ≤1.5%, and no imputation was performed for missing data. Multivariable models were derived from complete cases. For the Fast FSR method, the desired false selection rate was set to 0.05. All analyses were performed at the Duke Clinical Research Institute using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient and procedure characteristics
Among a total of 1828 patients included in our study, 782 (42.8%) had VGF at 12–18 months after CABG. At the graft-level, 1096 (25.2%) of the 4343 grafts placed during the index CABG had failed at 12–18 months after CABD. Demographic characteristics and comorbid conditions were similar between patients with and without VGF with the exception of cerebrovascular disease, which was more prevalent among patients with VGF (Table 1).
Table 1.
Baseline patient characteristics according to presence or absence of VGF
| Characteristic | With VGF (n=782) | Without VGF (n=1046) | P Value |
|---|---|---|---|
| Age, median (IQR), yrs | 63.0 (55.0-69.0) | 63.0 (55.0-70.0) | 0.62 |
| Female sex | 158 (20.2) | 184 (17.6) | 0.16 |
| Weight, median (IQR), kg | 88.7 (77.0-100.0) | 88.0 (78.0-100.0) | 0.57 |
| Race: White | 701 (89.6) | 954 (91.2) | 0.26 |
| AF/flutter | 54 (6.9) | 60 (5.7) | 0.31 |
| Cancer | 72 (9.2) | 77 (7.4) | 0.15 |
| Prior CHF | 52 (6.6%) | 69 (6.6%) | 0.96 |
| Cerebrovascular disease | 90 (11.5%) | 88 (8.4%) | 0.03 |
| Diabetes mellitus | 0.07 | ||
| No diabetes | 489 (62.5%) | 678 (64.9%) | |
| Diabetes, no current treatment | 14 (1.8%) | 23 (2.2%) | |
| Diabetes, insulin treatment | 85 (10.9%) | 77 (7.4%) | |
| Diabetes, non-insulin treatment | 194 (24.8%) | 267 (25.6%) | |
| EF, median (IQR), % | 50.0 (40.0-60.0) | 52.5 (43.0-60.0) | 0.30 |
| Hypercholesterolemia | 169 (21.6) | 254 (24.3) | 0.18 |
| Hypertension | 574 (73.4) | 760 (72.7) | 0.72 |
| History of liver disease | 16 (2.0) | 17 (1.6) | 0.50 |
| Chronic lung disease | 101 (12.9) | 146 (14.0) | 0.52 |
| NYHA class | 0.95 | ||
| I | 312 (40.4) | 427 (41.1) | |
| II | 271 (35.1) | 353 (33.9) | |
| III | 131 (17.0) | 177 (17.0) | |
| IV | 58 (7.5) | 83 (8.0) | |
| PAD | 87 (11.1) | 114 (10.9) | 0.88 |
| History of renal failure | 6 (0.8) | 17 (1.6) | 0.10 |
| Smoking status | 0.62 | ||
| Never | 257 (32.9) | 339 (32.4) | |
| Former | 345 (44.1) | 483 (46.2) | |
| Current | 180 (23.0) | 224 (21.4) | |
| Prior MI | 343 (43.9) | 432 (41.3) | 0.27 |
| Prior PCI | 220 (28.1) | 279 (26.7) | 0.49 |
Data presented as no. (%), unless otherwise indicated.
AF indicates atrial fibrillation; CHF, congestive heart failure; EF, ejection fraction; IQR, interquartile range; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; VGF, vein graft failure.
Patient-level CABG procedure characteristics among patients with and without VGF are shown in Table 2. Compared with patients without VGF, those with VGF had longer surgical and cross-clamp times and worse target artery quality. Patients with VGF also more frequently underwent endoscopic versus open vein graft harvest and had slightly longer graft length than patients without VGF. At 30 days after the index CABG, patients with subsequent VGF were more frequently taking clopidogrel or ticlopidine (26.1% vs. 19.2%, p<0.001) and had similar use of warfarin (9.1% vs. 8.5%, p=0.66) and statins (74.6% vs. 74.9%, p=0.88) than patients who did not have subsequent VGF.
Table 2.
Baseline procedural characteristics at the patient-level according to presence or absence of VGF
| Characteristic | With VGF (n=782) | Without VGF (n=1046) | P Value |
|---|---|---|---|
| Angiographic classification | |||
| Per protocol angiography only | 655 (83.8) | 1002 (95.8) | |
| Early angiography only | 64 (8.2) | 0 (0.0) | |
| Early and per protocol angiographies | 63 (8.1) | 44 (4.2) | |
| Maximum stenosis of any target vessel ≥75% | 790 (72.3) | 2317 (71.5) | 0.61 |
| Endoscopic vein harvest technique | 468 (60.1) | 531 (50.9) | <0.001 |
| Any use of composite graft | 286 (36.6) | 344 (32.9) | 0.10 |
| Longest graft length, median (IQR), cm | 17.0 (14.3-19.3) | 16.0 (14.0-19.0) | 0.02 |
| Any proximal (non-suture) | 21 (2.7) | 19 (1.8) | 0.21 |
| Any distal (non-suture) | 23 (2.9) | 27 (2.6) | 0.65 |
| Graft source* | 0.32 | ||
| Arm vein | 0 (0.0) | 2 (0.2) | |
| Lesser saphenous | 12 (1.5) | 22 (2.1) | |
| Greater saphenous | 770 (98.5) | 1022 (97.7) | |
| Worst target artery quality | <0.01 | ||
| Good | 308 (39.4) | 484 (46.3) | |
| Fair | 281 (36.0) | 363 (34.7) | |
| Poor | 192 (24.6) | 198 (18.9) | |
| Worst graft quality | 0.12 | ||
| Good | 537 (68.7) | 764 (73.1) | |
| Fair | 206 (26.3) | 237 (22.7) | |
| Poor | 39 (5.0) | 44 (4.2) | |
| Use of cardiopulmonary bypass | 617 (78.9) | 825 (78.9) | 0.99 |
| Pump time, median (IQR), min | 95.0 (62.0-123.0) | 86.0 (51.0-111.0) | <.0001 |
| Cross-clamp time, median (IQR), min | 60.0 (33.0-78.0) | 53.0 (30.0-72.0) | 0.01 |
| Surgical time, median (IQR), min | 240.0 (201.0-284.0) | 221.0 (186.0-261.0) | <.0001 |
| Type of procedure | 0.66 | ||
| Emergent/salvage | 20 (2.6) | 32 (3.1) | |
| Urgent | 373 (47.7) | 480 (45.9) | |
| Elective | 389 (49.7) | 533 (51.0) |
Data presented as no. (%), unless otherwise indicated.
IQR indicates interquartile range; VGF, vein graft failure.
For patients with multiple graft sources, the “worst” source according to the following hierarchy was used (worst status listed first): arm vein, lesser saphenous vein, greater saphenous vein
Factors associated with VGF
We first examined patient-level factors associated with VGF at 12–18 months after CABG. Longer duration of surgery (OR per 10-minute increase 1.05; 95% CI 1.03–1.07; p<0.01), endoscopic vein graft harvest technique (OR 1.44; 95% CI 1.19–1.75; p<0.01), and poor target artery quality (OR 1.45; 95% CI 1.13–1.87; p<0.01) were significantly associated with VGF. Adding medications continued at 30 days after CABG to the variable selection model revealed that the use of clopidogrel or ticlopidine was significantly associated with VGF (OR 1.35; 95% CI 1.07–1.69; p=0.01); addition of clopidogrel or ticlopidine to the model did not substantially change the relationship between the other significant predictors and VGF (Table 3). Goodness of fit of the model as measured by the Hosmer-Lemeshow statistic indicated that the model fits the data well (p = 0.85). The c-statistic for the model was 0.61.
Table 3.
Factors associated with patient-level VGF
| Variable | Chi-Square | OR | 95% CI | P Value |
|---|---|---|---|---|
| Without 30-day medications* | ||||
| Duration of surgery (per 10-min increase) | 34.66 | 1.05 | 1.03-1.07 | <0.0001 |
| Endoscopic harvest technique (vs. open) | 14.07 | 1.44 | 1.19-1.75 | <0.0001 |
| Worst target artery quality (vs. good) | ||||
| Fair | 3.72 | 1.24 | 1.00-1.53 | 0.05 |
| Poor | 8.35 | 1.45 | 1.13-1.87 | <0.01 |
| Including 30-day medications | ||||
| Duration of surgery (per 10-min increase) | 32.51 | 1.05 | 1.03-1.07 | <0.0001 |
| Endoscopic harvest technique (vs. open) | 12.16 | 1.41 | 1.16-1.71 | <0.001 |
| Worst target artery quality (vs. good) | ||||
| Fair | 3.13 | 1.22 | 0.98-1.51 | 0.08 |
| Poor | 7.55 | 1.43 | 1.11-1.84 | <0.01 |
| Clopidogrel or ticlopidine use | 6.62 | 1.35 | 1.07-1.69 | 0.01 |
CI indicates confidence interval; OR, odds ratio.
1817 patients with non-missing covariates were included in the “without 30-day medications” model, and 1812 patients were included in the “30-day medications” model.
Next, we assessed the relationship of graft-level variables with VGF (Table 4). Factors that were significantly associated with per-graft VGF (Table 4) included fair or poor target artery quality (OR 1.31; 95% CI 1.11–1.56; p<0.01 and OR 2.34; 95% CI 1.89–2.91; p<0.01, respectively), longer duration of surgery (OR per 10-minute increase 1.04; 95% CI 1.02–1.05; p<0.01), endoscopic vein harvest technique (OR 1.37; 95% CI 1.16–1.62; p<0.01), and history of cerebrovascular disease (OR 1.39; 95% CI 1.06–1.81; p=0.02). After including 30-day medication use, clopidogrel or ticlopidine use was again associated with VGF (OR 1.30; 95% CI 1.07–1.58; p<0.01).
Table 4.
Factors associated with graft-level VGF
| Variable | Chi-Square | OR | 95% CI | P Value |
|---|---|---|---|---|
| Without 30-day medications* | ||||
| Duration of surgery (per 10-min increase) | 27.3 | 1.04 | 1.02-1.05 | <0.0001 |
| Endoscopic harvest technique (vs. open) | 14.03 | 1.37 | 1.16-1.62 | <0.001 |
| Target artery quality (vs. good) | ||||
| Fair | 9.85 | 1.31 | 1.11-1.56 | <0.01 |
| Poor | 59.19 | 2.34 | 1.89-2.91 | <0.0001 |
| History of cerebrovascular disease | 5.82 | 1.39 | 1.06-1.81 | 0.02 |
| Including 30-day medications | ||||
| Duration of surgery (per 10-min increase) | 25.30 | 1.03 | 1.02-1.05 | <0.0001 |
| Endoscopic harvest technique (vs. open) | 12.17 | 1.35 | 1.14-1.59 | <0.001 |
| Target artery quality (vs. good) | ||||
| Fair | 9.35 | 1.31 | 1.10-1.55 | <0.01 |
| Poor | 58.29 | 2.34 | 1.88-2.91 | <0.0001 |
| History of cerebrovascular disease | 4.92 | 1.35 | 1.04-1.77 | 0.03 |
| Clopidogrel or ticlopidine use | 7.10 | 1.30 | 1.07-1.58 | <0.01 |
CI indicates confidence interval; OR, odds ratio.
4288 grafts over 1813 patients with non-missing covariates were included in the “without 30-day medications” model, and 4279 grafts over 1808 patients were included in the “30-day medications” model.
Distribution of predicted VGF risk
We examined the distribution of predicted VGF risk using the full (including 30-day medication use) graft-level model of VGF. Predicted probability of VGF at 12–18 months post-CABG ranged from a low of 12.1% to a high of 63.6%. The median predicted risk of VGF among our patient cohort was 23.4% (interquartile range 19.5% to 29.2%) (Figure 2).
Figure 2.
Distribution of predicted VGF risk. Shown is the distribution of predicted risk of VGF using the full (including 30-day medication use) graft-level VGF model among the patient cohort. Listed above each bar is the observed probability of VGF. IQR, interquartile range; VGF, vein graft failure.
DISCUSSION
In this analysis from PREVENT IV which included over 1800 patients, more than 4300 implanted vein grafts, and systematic 12–18 month angiographic follow-up, we found that longer duration of surgery, endoscopic vein graft harvesting, poor target artery quality, and the use of clopidogrel or ticlopidine at 30 days post-CABG were factors associated with VGF in both per-patient- and per-graft-level models. The broad range of predicted VGF using our per-graft-level model (12.1–63.6%) suggests that VGF is prevalent and hence, these data may be clinically useful to inform efforts to reduce VGF.
Interest in understanding factors associated with VGF after CABG has been longstanding, but prior efforts have been limited.15 Previous studies have consistently reported 1 year VGF rates of 10–20%, with another 5–10% of vein grafts failing between 1–5 years after CABG.10,19-24 These studies have identified patient characteristics, including younger age,11,12 female sex,12,13 prior heart failure or low ejection fraction,12,13 and increased serum cholesterol,11,25 as predictors of VGF. Surgical factors, including temperature of graft solution,25 multiple distal anastamoses,13,26 poor distal vessel,13,26 target artery stenosis,12 and endoscopic harvest technique,26,27 have also been identified as predictive of VGF. Importantly, these analyses were based on data from patients undergoing CABG several decades ago, prior to the widespread use of antiplatelet therapy and the introduction of newer surgical CABG techniques.28-30 Some prior reports were also based on single-center studies, reducing the generalizability of their results, or analyzed data at either the patient- or graft-level, which may account for some of the inconsistency in previous findings. Furthermore, a number of prior studies examined patients undergoing clinically-driven coronary angiography, which may under or overestimate the rate and influence of factors associated with VGF.
Our study extends knowledge in the field in several ways. First, this analysis represents one of the largest analyses of factors associated with VGF to date and includes data from over 100 sites. Second, our study included patients undergoing angiography for clinical reasons as well as relatively complete, protocol-mandated follow-up angiography, allowing for a more unbiased assessment of VGF and the factors associated with it. Third, our analysis was based on data representing more contemporary practice and was strengthened by the detailed clinical and procedural data that were collected for PREVENT IV. Finally, whereas prior studies have assessed VGF at either the graft- or patient-level, we examined both, as each provides useful and potentially different information. We found that the factors associated with VGF in patient-and graft-level models were almost identical.
We found a number of surgical factors that were associated with VGF. Pathologic studies have demonstrated that atherosclerosis is the main etiology of late (more than 12 months) VGF, whereas early (less than 1 month) and subacute (up to 12 months) graft failure is due to thrombosis, surgical technical errors, and intimal hyperplasia.31 Intraoperative processes of vein graft harvesting, graft manipulation, and graft implantation can all lead to endothelial dysfunction, inflammation, and ultimately thrombosis and graft occlusion.15 Accordingly, there is mechanistic feasibility to explain our study results. Longer duration of surgery may reflect technical difficulty, thus contributing to risk of VGF. Endoscopic vein graft harvesting, though less invasive than open vein graft harvesting, can damage vein graft endothelium, causing inflammation and thrombosis with early graft failure or increased intimal hyperplasia and subacute VGF. Observational data regarding the benefits of endoscopic vein harvesting are mixed, with some studies reporting associations of this technique with VGF and worse outcomes,26,27,32 while others have not confirmed these findings.33,34 Definitively determining whether endoscopic graft harvesting is associated with VGF will require a prospective randomized clinical study. The Randomized Endo-Vein Graft Prospective (REGROUP) Trial (ClinicalTrials.gov: NCT01850082) which is currently under development will provide important insight into this topic.
We also found that poor target artery quality was associated with VGF. In PREVENT IV, assessments of target artery quality were based on qualitative surgeon judgment and not systematic classification. However, this qualitative rating likely incorporates the elements of smaller vessel diameter that might reflect challenging surgical anatomy and poor distal run-off, which has been previously associated with VGF.7
Two of the factors significantly associated with VGF in our analyses were not related to the surgical procedure. The first was a clinical history of cerebrovascular disease, which was associated with VGF in the graft-level model. Cerebrovascular disease may represent a marker of both more advanced vascular disease and also poor target vessel distal run-off. We also found that use of clopidogrel or ticlopidine at 30 days was associated with an increased risk of VGF. Given the pathologic contribution of thrombosis to early VGF, antiplatelet therapy would be expected to reduce VGF, and randomized data support the use of aspirin to reduce graft failure.35,36 In this study, since use of antiplatelet therapy was not randomized, we hypothesize that the relationship between antiplatelet therapy and VGF is likely due to confounding. Data to support the use of clopidogrel to improve early venous graft patency after CABG are limited,29,37 and clopidogrel is more frequently prescribed to patients with acute coronary syndrome, patients undergoing off-pump CABG, or patients with extensive coronary artery disease.38,39
In our study, the majority of VGF events were clinically silent. Only 7.1% of the patients with VGF had VGF identified during early repeat angiography for clinical indications. However, studies have demonstrated that VGF identified either during clinically-driven or routine follow-up angiography is associated with significant morbidity.4,5,10,40,41 Thus, reducing overall VGF after CABG is an important goal that may improve patient outcomes and the durability of CABG surgery.
Research efforts to date have focused on a multifaceted approach to prevent VGF, including modifications in patient behavior, especially smoking cessation, and exploration of optimal postoperative antiplatelet regimens, as a large proportion of CABG patients are resistant to aspirin.15 Given the wide range of predicted VGF risk of our model, these data might help to identify patients at higher risk for VGF who might be considered for CABG with non-vein graft conduits and who should be followed more closely for post-CABG VGF events. However, some of the factors associated with VGF in our study are non-modifiable, suggesting that the greatest use of our data may be to help direct further research into strategies to prevent VGF. The high rate of VGF also emphasizes the importance of investigational surgical techniques to reduce vein graft injury, such as external vein graft support through either stenting or fibrin glue, exploration of novel gene-based molecular therapies to reduce VGF, and the development of synthetic, non-vein graft conduits.15
Limitations
This is a retrospective, post-hoc analysis. We assessed VGF at routine angiography 12–18 months after CABG, and the predictors of VGF may change over time. We were not able to assess VGF in patients who died prior to angiography or who did not return for protocol-mandated angiography and have excluded these patients from the analysis. We chose to study VGF and did not include arterial conduits in our analysis. The factors associated with arterial graft failure may differ.19,20,42 Some other factors that have previously been associated with vein graft patency were not collected in PREVENT IV.11,28,30,35 PREVENT IV only included patients undergoing first-time CABG, and the vein graft handling techniques and pressurized delivery system used in PREVENT IV were unique to the trial. Although our models fit the data well (Hosmer-Lemeshow p=0.85), there was low discriminatory power (C-statistic 0.61). We also included use of clopidogrel and ticlopidine in sensitivity analyses, though these were post-baseline variables that might be associated with non-VGF factors. We were not able to account for clustering by specific surgeon, as these data were not available. Finally, it should be recognized that both the study timeframe and identification of VGF based on routine angiography impacted the selection of collected data elements, and strategies to reduce VGF have evolved since the time of this study15; all of these factors may limit the generalizability of our results.
Conclusions
VGF is common and associated with both patient and surgical factors including, poor target artery quality, longer duration of surgery, use of endoscopic vein harvesting, use of clopidogrel or ticlopidine, and cerebrovascular disease. These data may be useful in identifying patients with risk factors for VGF and to inform the development of strategies to prevent VGF. Further investigation of VGF should be pursued in contemporary datasets.
Supplementary Material
Acknowledgments
Funding Sources: PREVENT IV was funded by Corgentech, Inc, San Francisco, CA. Dr. Hess and Ms. Hager are supported by the National Institutes of Health (CNH: grant 5T32HL069749-09, RH: grant T32HL079896). Dr. Alexander is supported in part by grant U01-HL088953 from the National Institutes of Health Cardiothoracic Surgical Trials Network. The authors are solely responsible for the design and conduct of this study, study analyses the drafting and editing of the manuscript, and its final contents.
Footnotes
Clinical Trial Registration Information: ClinicalTrials.gov. Identifier: NCT00042081.
Conflict of Interest Disclosures: Dr. Lopes reports institutional research funding from Bristol-Myers Squibb and GlaxoSmithKline; consulting for AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, and Pfizer. Dr. Califf's disclosures are available at https://www.dcri.org/about-us/conflict-of-interest/COI-Califf_Jan-Mar2013.pdf. Dr. Peterson reports research funding from Eli Lilly & Company, Ortho-McNeil-Janssen Pharmaceuticals, Inc., Society of Thoracic Surgeons, American Heart Association, American College of Cardiology (all significant); consulting for AstraZeneca, Boehringer Ingelheim, Genentech, Johnson & Johnson, Ortho-McNeil-Janssen Pharmaceuticals, Inc., Pfizer, Sanofi-Aventis, and WebMD (all modest). Dr. Alexander reports consulting for Sohmalution and Moerae Matrix (all modest). The remaining authors have no conflicts to disclose.
References
- 1.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD. 2011 ACCF/AHA Guideline for coronary artery bypass graft surgery. Circulation. 2011;124:2610–2642. doi: 10.1161/CIR.0b013e31823b5fee. [DOI] [PubMed] [Google Scholar]
- 2.Yusuf S, Zucker D, Peduzzi P, Fisher LD, Takaro T, Kennedy JW, Davis K, Killip T, Passamani E, Norris R, Morris C, Mathur V, Varnauskas E, Chalmers TC. Effect of coronary artery bypass graft surgery on survival: Overview of 10-year results from randomised trials by the coronary artery bypass graft surgery trialists collaboration. Lancet. 1994;344:563–570. doi: 10.1016/s0140-6736(94)91963-1. [DOI] [PubMed] [Google Scholar]
- 3.Davis KB, Chaitman B, Ryan T, Bittner V, Kennedy JW. Comparison of 15-year survival for men and women after initial medical or surgical treatment for coronary artery disease. J Am Coll Cardiol. 1995;25:1000–1009. doi: 10.1016/0735-1097(94)00518-u. [DOI] [PubMed] [Google Scholar]
- 4.Halabi AR, Alexander JH, Shaw LK, Lorenz TJ, Liao L, Kong DF, Milano CA, Harrington RA, Smith PK. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol. 2005;96:1254–1259. doi: 10.1016/j.amjcard.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 5.Lopes RD, Mehta RH, Hafley GE, Williams JB, Mack MJ, Peterson ED, Allen KB, Harrington RA, Gibson CM, Califf RM, Kouchoukos NT, Ferguson TB, Jr, Alexander JH. Relationship between vein graft failure and subsequent clinical outcomes after coronary artery bypass surgery. Circulation. 2012;125:749–756. doi: 10.1161/CIRCULATIONAHA.111.040311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen K, Cheng D, Cohn W, Connolly M, Edgerton J, Falk V, Martin J, Ohtsuka T, Vitali R. Endoscopic vascular harvest in coronary artery bypass grafting surgery: A consensus statement of the international society of minimally invasive cardiothoracic surgery (ISMICS) 2005. Innovations. 2005;1:51–60. doi: 10.1097/01.gim.0000196315.32179.82. [DOI] [PubMed] [Google Scholar]
- 7.Bjork VO, Ekestrom S, Henze A, Ivert T, Landou C. Early and late patency of aortocoronary vein grafts. Scand J Thorac Cardiovasc Surg. 1981;15:11–21. doi: 10.3109/14017438109101020. [DOI] [PubMed] [Google Scholar]
- 8.Cataldo G, Braga M, Pirotta N, Lavezzari M, Rovelli F, Marubini E. Factors influencing 1-year patency of coronary artery saphenous vein grafts. Circulation. 1993;88:II93–98. [PubMed] [Google Scholar]
- 9.Roth JA, Cukingnan RA, Brown BG, Gocka E, Carey JS. Factors influencing patency of saphenous vein grafts. Ann Thorac Surg. 1979;28:176–183. doi: 10.1016/s0003-4975(10)63777-0. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 11.Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG, Thottapurathu L, Krasnicka B, Ellis N, Anderson RJ, Henderson W, Group VACS Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a department of veterans affairs cooperative study. J Am Coll Cardiol. 2004;44:2149–2156. doi: 10.1016/j.jacc.2004.08.064. [DOI] [PubMed] [Google Scholar]
- 12.Shah PJ, Gordon I, Fuller J, Seevanayagam S, Rosalion A, Tatoulis J, Raman JS, Buxton BF. Factors affecting saphenous vein graft patency: Clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg. 2003;126:1972–1977. doi: 10.1016/s0022-5223(03)01276-5. [DOI] [PubMed] [Google Scholar]
- 13.Paz MA, Lupon J, Bosch X, Pomar JL, Sanz G. Predictors of early saphenous vein aortocoronary bypass graft occlusion. Ann Thorac Surg. 1993;56:1101–1106. doi: 10.1016/0003-4975(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 14.Domanski MJ, Borkowf CB, Campeau L, Knatterud GL, White C, Hoogwerf B, Rosenberg Y, Geller NL. Prognostic factors for atherosclerosis progression in saphenous vein grafts: The postcoronary artery bypass graft (POST-CABG) trial. J Am Coll Cardiol. 2000;36:1877–1883. doi: 10.1016/s0735-1097(00)00973-6. [DOI] [PubMed] [Google Scholar]
- 15.Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: Pathophysiology, management, and future directions. Ann Surg. 2013;257:824–833. doi: 10.1097/SLA.0b013e318288c38d. [DOI] [PubMed] [Google Scholar]
- 16.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and safety of edifoligide, an e2f transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RH, Ferguson TB, Lopes RD, Hafley GE, Mack MJ, Kouchoukos NT, Gibson CM, Harrington RA, Califf RM, Peterson ED, Alexander JH. Saphenous vein grafts with multiple versus single distal targets in patients undergoing coronary artery bypass surgery: One-year graft failure and five-year outcomes from the project of ex-vivo vein graft engineering via transfection (PREVENT) IV trial. Circulation. 2011;124:280–288. doi: 10.1161/CIRCULATIONAHA.110.991299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boos DD, Stefanski LA, Wu Y. Fast FSR variable selection with applications to clinical trials. Biometrics. 2009;65:692–700. doi: 10.1111/j.1541-0420.2008.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabik JF, III, Lytle BW, Blackstone EH, Houghtaling PL, Cosgrove DM. Comparison of saphenous vein and internal thoracic artery graft patency by coronary system. Ann Thorac Surg. 2005;79:544–551. doi: 10.1016/j.athoracsur.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 20.Cameron A, Kemp HG, Jr, Green GE. Bypass surgery with the internal mammary artery graft: 15 year follow-up. Circulation. 1986;74:III30–36. [PubMed] [Google Scholar]
- 21.Bourassa MG, Campeau L, Lesperance J, Grondin CM. Changes in grafts and coronary arteries after saphenous vein aortocoronary bypass surgery: Results at repeat angiography. Circulation. 1982;65:90–97. doi: 10.1161/01.cir.65.7.90. [DOI] [PubMed] [Google Scholar]
- 22.Campeau L, Enjalbert M, Lesperance J, Vaislic C, Grondin CM, Bourassa MG. Atherosclerosis and late closure of aortocoronary saphenous vein grafts: Sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation. 1983;68:II1–7. [PubMed] [Google Scholar]
- 23.Grondin CM, Campeau L, Lesperance J, Enjalbert M, Bourassa MG. Comparison of late changes in internal mammary artery and saphenous vein grafts in two consecutive series of patients 10 years after operation. Circulation. 1984;70:I208–212. [PubMed] [Google Scholar]
- 24.Chesebro JH, Fuster V, Elveback LR, Clements IP, Smith HC, Holmes DR, Jr, Bardsley WT, Pluth JR, Wallace RB, Puga FJ. Effect of dipyridamole and aspirin on late vein-graft patency after coronary bypass operations. N Engl J Med. 1984;310:209–214. doi: 10.1056/NEJM198401263100401. [DOI] [PubMed] [Google Scholar]
- 25.Goldman S, Zadina K, Krasnicka B, Moritz T, Sethi G, Copeland J, Ovitt T, Henderson W. Predictors of graft patency 3 years after coronary artery bypass graft surgery. J Am Coll Cardiol. 1997;29:1563–1568. doi: 10.1016/s0735-1097(97)82539-9. [DOI] [PubMed] [Google Scholar]
- 26.Magee MJ, Alexander JH, Hafley G, Ferguson TB, Jr, Gibson CM, Harrington RA, Peterson ED, Califf RM, Kouchoukos NT, Herbert MA, Mack MJ. Coronary artery bypass graft failure after on-pump and off-pump coronary artery bypass: Findings from PREVENT IV. Ann Thorac Surg. 2008;85:494–499. doi: 10.1016/j.athoracsur.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Lopes RD, Hafley GE, Allen KB, Ferguson TB, Peterson ED, Harrington RA, Mehta RH, Gibson CM, Mack MJ, Kouchoukos NT, Califf RM, Alexander JH. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med. 2009;361:235–244. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 28.Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y. Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: Results of a Veterans Administration cooperative study. Circulation. 1988;77:1324–1332. doi: 10.1161/01.cir.77.6.1324. [DOI] [PubMed] [Google Scholar]
- 29.Gao G, Zheng Z, Pi Y, Lu B, Lu J, Hu S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery a single-center, randomized, controlled trial. J Am Coll Cardiol. 2010;56:1639–1643. doi: 10.1016/j.jacc.2010.03.104. [DOI] [PubMed] [Google Scholar]
- 30.Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Kern KB, Sethi G, Sharma GV, Khuri S. Long-term graft patency (3 years) after coronary artery surgery. Effects of aspirin: Results of a va cooperative study. Circulation. 1994;89:1138–1143. doi: 10.1161/01.cir.89.3.1138. [DOI] [PubMed] [Google Scholar]
- 31.Parang P, Arora R. Coronary vein graft disease: Pathogenesis and prevention. Can J Cardiol. 2009;25:e57–62. doi: 10.1016/s0828-282x(09)70486-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zenati MA, Shroyer AL, Collins JF, Hattler B, Ota T, Almassi GH, Amidi M, Novitzky D, Grover FL, Sonel AF. Impact of endoscopic versus open saphenous vein harvest technique on late coronary artery bypass grafting patient outcomes in the ROOBY (randomized on/off bypass) trial. J Thorac Cardiovasc Surg. 2011;141:338–344. doi: 10.1016/j.jtcvs.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Williams JB, Peterson ED, Brennan JM, Sedrakyan A, Tavris D, Alexander JH, Lopes RD, Dokholyan RS, Zhao Y, O'Brien SM, Michler RE, Thourani VH, Edwards FH, Duggirala H, Gross T, Marinac-Dabic D, Smith PK. Association between endoscopic vs open vein-graft harvesting and mortality, wound complications, and cardiovascular events in patients undergoing cabg surgery. JAMA. 2012;308:475–484. doi: 10.1001/jama.2012.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dacey LJ, Braxton JH, Jr, Kramer RS, Schmoker JD, Charlesworth DC, Helm RE, Frumiento C, Sardella GL, Clough RA, Jones SR, Malenka DJ, Olmstead EM, Ross CS, O'Connor GT, Likosky DS. Long-term outcomes of endoscopic vein harvesting after coronary artery bypass grafting. Circulation. 2011;123:147–153. doi: 10.1161/CIRCULATIONAHA.110.960765. [DOI] [PubMed] [Google Scholar]
- 35.Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y. Saphenous vein graft patency 1 year after coronary artery bypass surgery and effects of antiplatelet therapy. Results of a Veterans Administration cooperative study. Circulation. 1989;80:1190–1197. doi: 10.1161/01.cir.80.5.1190. [DOI] [PubMed] [Google Scholar]
- 36.Collaborative overview of randomised trials of antiplatelet therapy--III: Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. BMJ. 1994;308:235–246. [PMC free article] [PubMed] [Google Scholar]
- 37.Williams JB, Lopes RD, Hafley GE, Bruce Ferguson T, Jr, Mack MJ, Michael Gibson C, Harrington RA, Peterson ED, Smith PK, Mehta RH, Alexander JH. Relationship between postoperative clopidogrel use and subsequent angiographic and clinical outcomes following coronary artery bypass grafting. J Thromb Thrombolysis. 2013;36:384–393. doi: 10.1007/s11239-013-0904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurbuz AT, Zia AA, Vuran AC, Cui H, Aytac A. Postoperative clopidogrel improves mid-term outcome after off-pump coronary artery bypass graft surgery: A prospective study. Eur J Cardiothorac Surg. 2006;29:190–195. doi: 10.1016/j.ejcts.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 39.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr., Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-st-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 40.Lytle BW, Loop FD, Taylor PC, Simpfendorfer C, Kramer JR, Ratliff NB, Goormastic M, Cosgrove DM. Vein graft disease: The clinical impact of stenoses in saphenous vein bypass grafts to coronary arteries. J Thorac Cardiovasc Surg. 1992;103:831–840. [PubMed] [Google Scholar]
- 41.Lytle BW, Loop FD, Taylor PC, Goormastic M, Stewart RW, Novoa R, McCarthy P, Cosgrove DM. The effect of coronary reoperation on the survival of patients with stenoses in saphenous vein bypass grafts to coronary arteries. J Thorac Cardiovasc Surg. 1993;105:605–612. [PubMed] [Google Scholar]
- 42.Desai ND, Cohen EA, Naylor CD, Fremes SE. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med. 2004;351:2302–2309. doi: 10.1056/NEJMoa040982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.