Abstract
Background
The objective of this study was to evaluate if peritumoral (PT) perfusion parameters obtained from dynamic susceptibility weighted contrast enhanced perfusion MRI can predict overall survival (OS) and progression free survival (PFS) in patients with newly diagnosed Glioblastoma multiforme (GBM).
Methods
Twenty-eight newly diagnosed GBM patients, who were treated with resection followed by concurrent chemoradiation and adjuvant chemotherapy, were included in this study. Evaluated perfusion parameters were pre- and post-treatment peritumoral relative cerebral blood volume (rCBV) and relative cerebral blood flow (rCBF). Proportional hazard analysis was used to assess the relationship OS, PFS and perfusion parameters. Kaplan-Meier survival estimates and log-rank test were used to characterize and compare the patient groups with high and low perfusion parameter values in terms of OS and PFS.
Results
Pretreatment PT rCBV and rCBF were not associated with OS and PFS whereas there was statistically significant association of both posttreatment PT rCBV and rCBF with OS and posttreatment rCBV with PFS (association of PFS and posttreatment rCBF was not statistically significant). Neither the Kaplan-Meier survival estimates nor the log-rank test demonstrated any differences in OS between high and low pretreatment PT rCBV values and rCBF values; however, high and low post-treatment PT rCBV and rCBF values did demonstrate statistically significant difference in OS and PFS.
Conclusions
Our study found posttreatment, not pretreatment, PT perfusion parameters can be used to predict OS and PFS in patients with newly diagnosed GBM.
Keywords: Peritumoral rCBV, rCBF, Perfusion MRI, GBM, Glioblastoma, Overall survival, Progression free survival
Introduction
Glioblastoma multiforme (GBM), the most common primary malignant brain tumor, represents a group of aggressive tumors that varies significantly in morphology, genetic, and clinical behavior. [1] Despite use of a standard aggressive treatment regime that includes tumor resection, followed by concurrent chemoradiation with temozolomide and radiation therapy, and subsequent adjuvant temozolomide, the post-diagnosis median survival of GBM patients is approximately 14 months. [2-4] Results of previous studies have demonstrated that physiologic imaging techniques, such as contrast enhanced dynamic susceptibility (DSC) weighted magnetic resonance imaging (MRI) can be used effectively in the evaluation of GBM. [5-10] Relative cerebral blood volume (rCBV), a perfusion parameter generated from DSC perfusion imaging, is most commonly used in clinical evaluation of GBM. [5, 11-14] It has been shown that tumoral rCBV correlates well with tumor grade, overall survival (OS), and progression-free survival (PFS). [15, 16]
Evaluation of enhancing component of previously treated GBM with DSC perfusion MRI sometimes can be difficult because of presence of blood products and radiation-induced changes. [17] Due to their inherent paramagnetic properties, blood products introduce magnetic inhomogeneity that severely affects the quality of DSC perfusion imaging, as the technique is exquisitely sensitive to any change in magnetic homogeneity. [18] Radiation-induced changes, including hemorrhage, are also more prevalent within the enhancing component of the tumor because of higher radiation dose to the area. Additionally, radiation-induced changes typically demonstrate lower rCBV, compared to GBM. [9, 19, 20] Assessment of the enhancing component of a treated GBM with DSC perfusion imaging may have spurious results if treatment-induced changes and tumor coexist. In the posttreatment setting, treatment-related changes are less common in the peritumoral area, a region that can also be evaluated with DSC-MRI for demonstration of tumor-related changes.
Glial neoplasms are highly infiltrating tumors. Infiltrating neoplastic glial cells can be present in areas of T2 prolongation, beyond the enhancing component, as evidenced by stereotactic biopsy of the enhancing and peri-enhancing areas. [21] The presence of tumor and abnormal tumoral vessels in the peritumoral region of GBM has been documented using diffusion tensor imaging, perfusion imaging, and MRI spectroscopy. [6, 22-25] In primary high-grade gliomas, the peritumoral area of abnormal T2 hyperintensity contains a mixture of tumor cells, abnormal tumoral blood vessel, and various degrees of interstitial edema. [24] Thus, evaluation of the peritumoral areas with perfusion imaging can also provide information about the tumor biology. In fact, multiple studies have previously described higher rCBV in the peritumoral region of GBM. [6, 25-27] Higher rCBV in the peri-enhancing area has also been shown to be a marker of potential tumor growth. [25] The role of peritumoral rCBV in prediction of survival in newly diagnosed GBM patients is not well documented in the literature. We hypothesize that peritumoral rCBV can be used to predict OS and PFS in GBM patients.
Relative cerebral blood flow (rCBF) is another perfusion parameter that estimates blood flow through a definite area of brain tissue per unit of time and can be calculated from perfusion DSC-MRI. Unlike rCBV, rCBF has been used less commonly to evaluate GBM, [28, 29] likely, because calculation of rCBF is difficult and rCBF is dependent on rCBV through the central volume principle. However, tumoral CBF has been shown to correlate with tumor grade, [30] blood vessel tortuosity, [31] and to differentiate high-grade gliomas from low-grade gliomas [28]. In addition, peritumoral rCBF has been shown to differentiate high-grade gliomas from metastasis [26].
The objective of this retrospective pilot study was to determine if peritumoral perfusion parameters can be used to predict OS and PFS in newly diagnosed GBM patients.
Materials and Methods
Patient selection
The local ethical committee approved this Health Insurance Portability and Accountability Act (HIPAA) compliant study. Medical records and MRI imaging studies of all patients with GBM that were diagnosed and treated at the University of Alabama at Birmingham Medical Center between January 2010 and April 2012 were retrospectively reviewed. Patients with newly diagnosed, histopathologically confirmed, primary GBM and treatment with current standard therapy (maximum safe resection, followed by concurrent chemoradiation, and adjuvant temozolomide) were included in the study. Diagnosis of primary GBM was based on clinico-radiological findings that meet the following three criteria: 1) neurological symptoms for less than 3 months with no known history of any CNS disease in the past, 2) brain imaging findings at presentation are suggestive of high-grade infiltrative tumor, and 3) histopathologically proven GBM. [32] We did not include any patient with secondary GBM, as the secondary GBM may have different perfusion parameters in the peritumoral area. Secondary GBM was defined as the presence of a known grade 2 tumor or tumor symptoms for more than 1 year in any given patient. Patients with a history of treatment with any investigational drugs were excluded from the analysis. Of the 102 patients with a diagnosis of GBM that were treated, 28 patients between 27-75 years-of-age (M=15, F=13) were eligible for inclusion during the study period. Seventy-four patients were excluded from the study due to lack of perfusion imaging (n=24), presence of poor perfusion technique/artifacts (n=10), treatment with investigational drugs (n=23), or continuation of treatment in another medical facility (n=17).
Treatment and Follow-up
All patients were treated with maximum safe resection, followed by combined temozolomide (75 mg/m2 per day) for 42 consecutive days during radiation therapy. For radiation treatment (RT), the treatment dose was 59-60 Gy in 2-Gy fractions to the residual tumor volume, plus 2-2.5-cm volumetric expansion. Following completion of concurrent chemoradiation, all patients were treated with a maintenance dose of temozolomide (150-200 mg/m2). All patients had an MRI within a month of completing chemoradiation. MRIs (including perfusion MRI) before the resection and after the completion of combined chemoradiation were analyzed in this study. In the study patient population, tumor recurrences were treated either with bevacizumab with– or without second-line chemotherapeutic agents (n=11) or repeat surgery (n=3). None of the patients was treated with second radiation (Table 1). Patients were followed at least 24 months after the initial diagnosis. Patients, who were still alive, were treated as censored cases. As has been suggested elsewhere, we chose to calculate OS as the primary endpoint because median post-progression survival of GBM patient is short. [33]
Table 1.
Patient disposition
| Variables | |||||
|---|---|---|---|---|---|
| Age (in years) | Treatment At Diagnosis | Treatment At Recurrence | |||
| Age, mean | 56 | Resection | 25 | Re-resection* | 3 |
| Age, median | 53 | Biopsy only | 3 | Avastin/second-line chemotherapy | 11 |
| Age, range | 27-75 | Chemoradiation | 28 | Re-radiation | 0 |
All the three patients were treated with resection at diagnosis.
Conventional MR Imaging
MRI imaging was performed on 1.5T magnets (Echospeed, GE Medical Systems, Milwaukee, Wisconsin and Achieva, Phillips Medical System, Netherlands). Conventional MRI sequences included a sagittal T1 spin-echo, axial fat suppressed fast/turbo spin-echo,T2, axial FLAIR, coronal GRE (or FFE in Phillips system), post contrast axial, and coronal T1 spin-echo sequences after administration of 0.05 mmol/kg of gadoteridol (ProHance, Bracco Diagnostic Inc, Princeton, NJ).
DSC Perfusion Imaging
Contrast enhanced dynamic susceptibility weighted perfusion imaging was performed using a single-shot T2* weighted echo-planar imaging sequence (TR/TE in ms: 1900/40 for GE system or 2500/40 in Phillips system; 192 × 128 acquisition matrix, flip angle of 72°) with another dose of 0.05 mmol/kg of gadoteridol bolus at a rate of 4 mL/s through an 18-20 G intravenous catheter followed by 20 mL of saline chase at the same injection rate after completion of the post contrast T1 weighted (T1WI) sequences. For the Phillips scanner, the slice thickness was 5 mm without any intersection gap, whereas 10 mm slice thickness with no intersection gap was used on the GE scanner. A second dose of 0.05 mmol/kg of gadoteridol (total: 0.1 mmol/kg: 0.05 mmol/kg for the contrast enhanced study and 0.05 mmol/kg for the DSC perfusion acquisition) was used to obtain the perfusion DSC-MRI in all patients in order to diminish the T1 effects that result from contrast extravasation and the intravascular-toextravascular gadolinium concentration gradient as suggested elsewhere. [34] A total of 60-image dynamics were obtained, including the first 15 dynamic acquisitions that were performed before contrast injection in order to obtain a steady-state pre-contrast signal-intensity baseline.
DSC Perfusion Imaging Data Postprocessing
The DSC perfusion imaging data sets were processed using FDA-approved software IBNeuro 1.0 (Imaging Biometrics LLC., Milwaukee, Wisconsin), a plugin to the Aycan Osirix Pro (http://www.aycan.com/). The principle of the post processing technique is based on correction for contrast extravasation as detailed elsewhere. [34] Pixel-by-pixel CBV maps were created with leakage correction. To calculate CBF maps, arterial input function was manually placed over one of the M2 branches of the contralateral middle cerebral artery. CBV and CBF maps were then overlaid on post contrast T1WI sequence, obtained in the same plane of the DSC perfusion imaging with similar slice thickness and inter-slice gap as well as on FLAIR sequence.
DSC Perfusion Data analysis
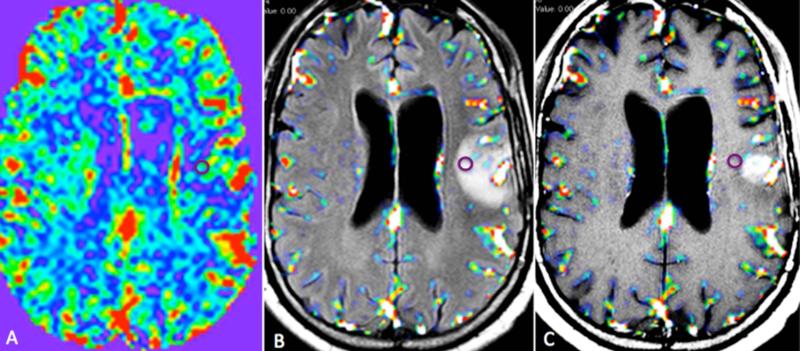
ROI placement: Tumoral area was defined as the enhancing component of the tumor. Peritumoral area was defined as the area of FLAIR hyperintensity beyond the enhancing component of the tumor. Regions-of-interest (ROI)-based analysis was performed on the overlaid CBV and CBF maps. An experienced neuroradiologist (AKB), blinded to the survival data, visually analyzed all of the slices through the peritumoral area on both the CBV map in RGB scale, and the overlaid CBV maps, and two ROIs were placed in the region of maximal abnormality as visually determined in the maps. If high CBV values were identified on multiple slices, ROI analysis was done for all of those slices and the highest two recorded values were included in the analysis. Two separate ROI measurements were made, as it has been shown that taking multiple measurements is a more reliable method of obtaining the highest CBV values in a lesion, compared to a single lesion measurement. [35] To minimize the confounding factors in ROI selection and placement, the ROIs were placed over the maximum area of peritumoral abnormalities and the size of the ROIs was kept constant (area=0.248 cm2 = 4/5 pixels) for all patients. We did not use a large-sized ROI covering the entire peritumoral volume to exclude either tumoral or normal large blood vessel from the ROI. Additionally, small ROI (radius=1.8 mm) has been successfully used in analysis of perfusion parameters in evaluation of GBM. [36, 37] For normalization of the CBV values, another ROI of similar size (0.248 cm2) was placed on the contralateral normal appearing white matter in the centrum semiovale. The average mean rCBV value of the two peritumoral ROIs was used for statistical analysis (Figure 1). Using calculated CBF maps, all of the above-mentioned steps were repeated to obtain average mean calculated rCBF value of the two peritumoral ROIs for analysis.
Fig. 1.
Pixel-by-pixel rCBV map (A), rCBV map overlaid on an axial FLAIR image (B) and axial post-contrast T1 weighted image (C) of a left temporal GBM obtained from the pretreatment perfusion scan. The ROI on the images includes the highest rCBV value within the peritumoral area.
Quality control of ROI: ROIs were carefully placed excluding any blood vessel and hemorrhage. Both the two ROIs were then copied to the exactly same slice of postcontrast axial T1WI and FLAIR image using copy ROI function of Osirix. This step was performed only for quality control as underlying structures can be obscured on the overlaid images. If any of the two ROIs included any enhancing component of the tumor or any vessel, it was meticulously repositioned. If the CBV value of the repositioned ROI was lower than an ROI from different slice, the ROI with higher CBV value was included in the analysis. If any ROI was outside the FLAIR-hyperintense areas, it was repositioned in a similar fashion.
Statistical Analysis
The primary objective of this study was to determine if peritumoral rCBV and rCBF could be used to predict OS and PFS in patients with newly diagnosed GBM. The analysis was separately performed for peritumoral rCBV and peritumoral rCBF from both the pretreatment and posttreatment perfusion MRIs. We used proportional hazard analysis to assess the relationship between patients’ OS, PFS, and perfusion parameters. Multivariate analysis was performed to determine the best predictor of survival. We used Kaplan-Meier survival estimates and log-rank test to characterize and compare the groups with high and low values of the perfusion parameters (rCBV and rCBF) in terms of OS defined as the period between time of diagnosis to the time of death. The median values were used to differentiate high versus low values in order to have equal number of patients in both patient groups with high and low values. We did not use the reference value from literature to establish a threshold because the rCBV values, even the relative values, are heavily influenced by different parameters and vary between studies. Data for the deceased patients were included uncensored, whereas the data from the live patients at the time of publication (at least 24 months) were right censored. Patients were classified in groups with low and high values by using the median rCBV value and median rCBF values. All statistical computations were performed by using software (SAS System for Windows, version 9.0, 2002; SAS Institute, Cary, NC), and results were declared significant with P < 0.05.
Results
The analysis is based on pretreatment perfusion MRI in 24 patients and posttreatment perfusion MRI in 28 patients. Pretreatment perfusion MRIs in 4 of the 28 patients were either not available (n=3) or limited by artifacts (n=1). The mean and median age at diagnosis was 56 and 53 years respectively. Values of the pretreatment and posttreatment perfusion parameters are shown in Figure 3.
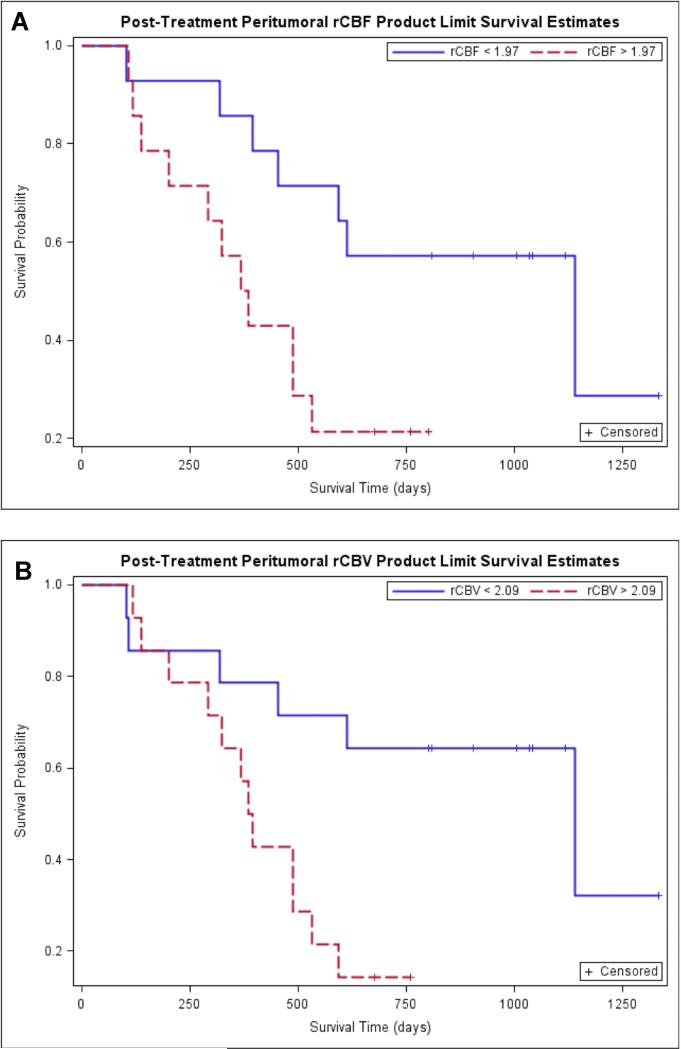
Fig. 3.
Kaplan-Meier survival curves for prediction of OS according to posttreatment peritumoral perfusion parameters. A. Kaplan-Meier survival curves for prediction of OS according to posttreatment peritumoral rCBF demonstrate poor OS in tumors with high rCBF (>1.97) (the red line) compared to tumors with low rCBF (<1.97) (the blue line). This difference in survival experience in the two groups was further confirmed by the three significant tests of equality. B. Kaplan-Meier survival curves for prediction of OS according to posttreatment peritumoral rCBV demonstrate poor OS in tumors with high rCBV (>2.09) (the red line) compared to tumors with low rCBV (<2.09) (the blue line). This difference in survival experience in the two groups was further confirmed by the three significant tests of equality.
Survival analysis
Follow-up period was at least 24 months. Of the 28 patients in the posttreatment group, 18 patients (64.28%) died and 10 patients (35.71%) were alive during the follow-up period. Of the 24 patients in the pretreatment group, 15 patients (62.5%) died and 9 patients (37.5%) were alive during the follow-up period. As the association of age with OS was not statistically significant in our patient population, the perfusion parameter was not age-adjusted. The OS was calculated from all the 28 patients. However, of the 28 patients, progression date is known for 20 patients. Four of the other 8 patients, in whom no progression date was recorded, died and the other 4 patients were still alive during the follow-up period with no progression. The progression-free survival analysis was performed based on 24 patients excluding the 4 patients who were already dead (two of them died due to unrelated disease and the cause of death is unknown in the other two patients).
From the univariate proportional hazard analysis, perfusion parameters that were significantly (p<0.05) associated with OS included post-treatment peritumoral rCBF (HR=2.02, 95% CI= 1.151-3.52, p=0.013) and post-treatment peritumoral rCBV (HR=2.192, 95% CI= 1.310-3.670, p=0.0028). The association of pretreatment perfusion parameters and OS was not statistically significant (Table 2). The multivariate analysis did not converge, most likely due to the low number of patients. From the univariate proportional hazard analysis, the posttreatment peritumoral perfusion parameter that was significantly (p<0.05) associated with PFS included rCBV (HR=1.830, 95% CI= 1.046-3.201, p=0.03). The association of posttreatment rCBF, pretreatment perfusion parameters, and PFS was not statistically significant (Table 3). The multivariate analysis did not converge, most likely due to the low number of patients.
Table 2.
Univariate proportional hazard analysis of peritumoral perfusion parameters and OS
| Pretreatment perfusion parameters | Posttreatment perfusion parameters |
|---|---|
| rCBF | rCBF |
| Hazard ratio: 1.126 | Hazard ratio: 2.02 |
| 95% Confidence interval: 0.755-1.68 | 95% Confidence interval: 1.151-3.52 |
| p Value: 0.56 | p Value: 0.013 |
| rCBV: | |
| rCBV: | Hazard ratio: 2.192 |
| Hazard ratio: 1.046 | 95% Confidence interval: 1.31-3.67 |
| 95% Confidence interval: 0.751-1.456 | p Value: 0.003 |
| p Value:0.789 | |
rCBV: Relative cerebral blood volume; rCBF: Relative cerebral blood flow
Table 3.
Univariate proportional hazard analysis of peritumoral perfusion parameters and PFS
| Pretreatment perfusion parameters | Posttreatment perfusion parameters |
|---|---|
| rCBF | rCBF |
| Hazard ratio, 1.196 | Hazard ratio, 1.446 |
| 95% CI [0.834, 1.714] | 95% CI [0.886,2.369 |
| P Value, 0.33 | p Value, 0.1426 |
| rCBV | rCBV |
| Hazard ratio, 1.088 | Hazard ratio, 1.830 |
| 95% CI [0.812,1.457] | 95% CI [1.046, 3.201] |
| p Value, 0.573 | p Value, 0.034 |
rCBV: Relative cerebral blood volume; rCBF: Relative cerebral blood flow
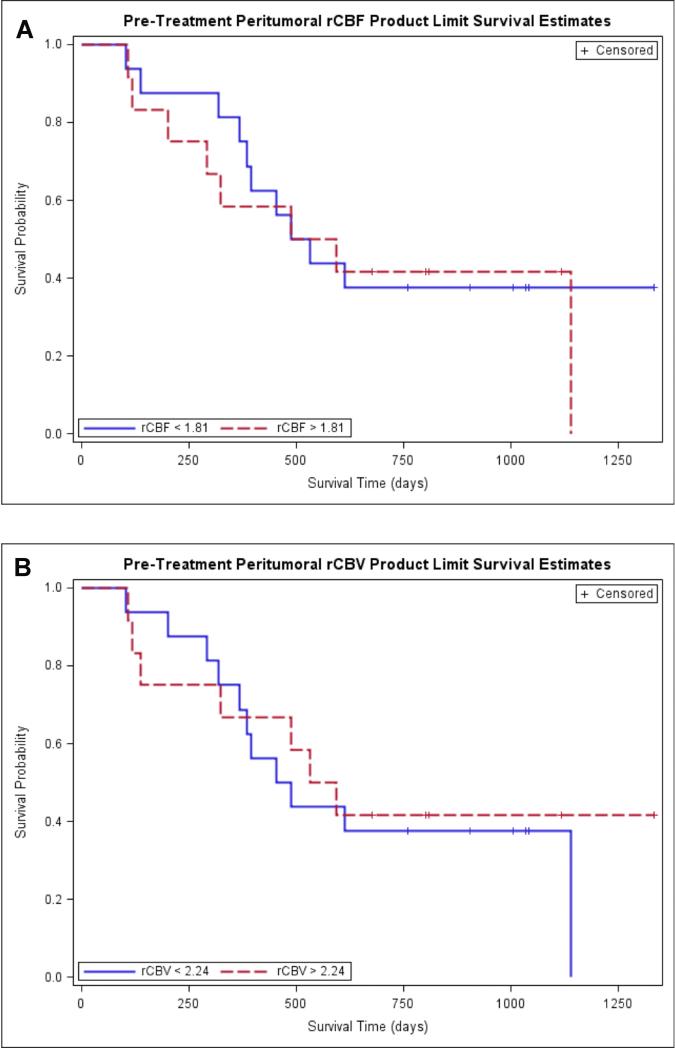
Kaplan-Meier survival curves for prediction of OS according to pretreatment peritumoral rCBF and peritumoral rCBV demonstrated no difference in OS in tumors with high rCBV (>2.24) and high rCBF (>1.81) compared to tumors with low rCBV (<2.24) and low rCBF (<1.81) (Figure 5) (Figure 2, table 4). When the Kaplan-Meier curves and long rank tests were used to evaluate the association of OS with posttreatment peritumoral rCBV and peritumoral rCBF, it was found that higher values of peritumoral rCBV (>2.09) and peritumoral rCBF (>1.97) were associated with lower survival. The binary representation (high versus low) of both peritumoral rCBV and rCBF was statistically significant with p values of 0.006 and 0.02 respectively. (Figure 3, table 4).
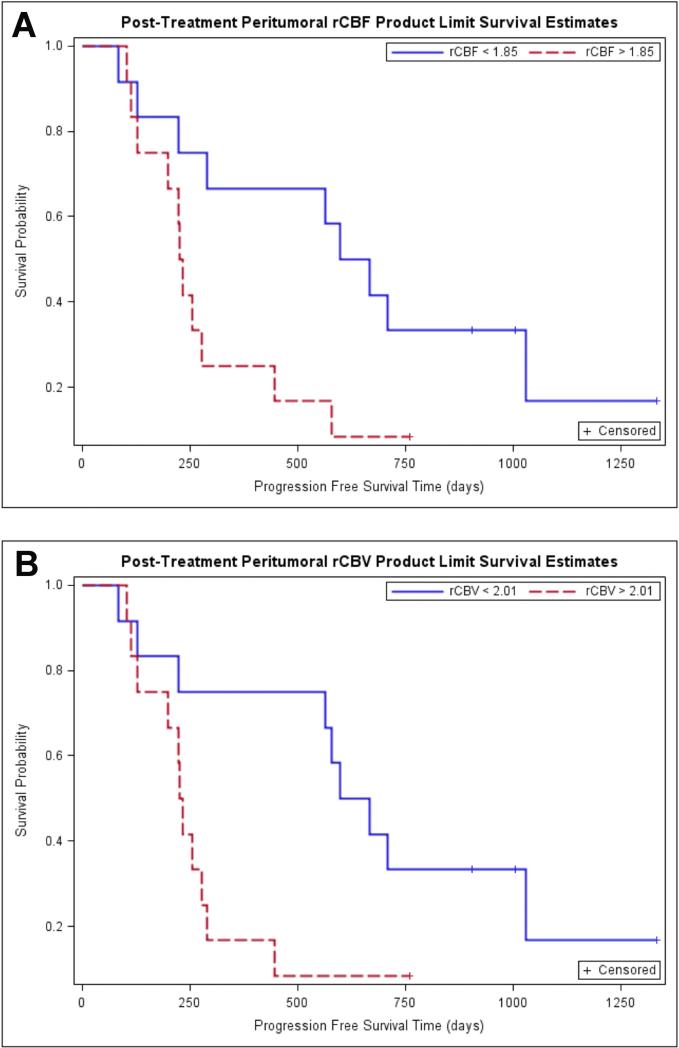
Fig. 5.
Kaplan-Meier survival curves for prediction of PFS according to posttreatment peritumoral perfusion parameters. A. Kaplan-Meier survival curves for prediction of PFS according to posttreatment peritumoral rCBF demonstrate poor PFS in tumors with high rCBF (>1.85) (the red line) compared to tumors with low rCBF (<1.85) (the blue line). This difference in survival experience in the two groups was further confirmed by the three significant tests of equality. B. Kaplan-Meier survival curves for prediction of OS according to posttreatment peritumoral rCBV demonstrate poor OS in tumors with high rCBV (>2.01) (the red line) compared to tumors with low rCBV (<2.01). This difference in survival experience in the two groups was further confirmed by the three significant tests of equality.
Fig. 2.
Kaplan-Meier survival curves for prediction of OS according to pretreatment peritumoral perfusion parameters. A. Kaplan-Meier survival curve of peritumoral rCBF demonstrates no difference in OS in tumors with high rCBF (>2.90) (the red line) compared to tumors with low rCBF (<2.90) (the blue line). B. Kaplan-Meier survival curves for prediction of OS according to pretreatment peritumoral rCBV demonstrate no difference in OS in tumors with high rCBV (>3.33) (the red line) compared to tumors with low rCBV (<3.33) (the blue line).
Table 4.
Median overall and Progression-Free Survival based on posttreatment peritumoral perfusion characteristics
| Post-Treatment Peritumoral (N=24) | ||||
|---|---|---|---|---|
| rCBF<1.85 | rCBF>1.85 | rCBV<2.09 | rCBV>2.09 | |
| Median Progression Free Survival (days) | 632.5 (128-1030) | 230.5 (112-447) | 632.5 (128-1030) | 230.5 (112-290) |
| PFS Survival | ||||
| At 6 months | 83.3 (48.2-95.6) | 75.0 (40.8-91.2) | 83.3 (48.2-95.6) | 75.0 (40.8-91.2) |
| At 12 months | 66.7 (33.7-86.0) | 25.0 (6.1-50.5) | 75.0 (40.8-91.2) | 16.7 (2.7-41.3) |
| At 18 months | 58.3 (27.0-80.1) | 16.7 (2.7-41.3) | 66.7 (33.7-86.0) | 8.3 (0.5-31.1) |
| At 24 months | 33.3 (10.3-58.8) | 8.3 (0.5-31.1) | 33.3 (10.3-58.8) | 8.3 (0.5-31.1) |
| Post-Treatment Peritumoral (N=28) | ||||
| Median Overall Survival (days) | 1141 (395-NE) | 376 (138-368) | 1141 (318-NE) | 389.5 (202-533) |
| Overall Survival (%) | ||||
| At 6 months | 92.9 (59.1-99.0) | 78.6 (47.2-92.5) | 85.7 (53.9-96.2) | 85.7 (53.9-96.2) |
| At 12 months | 85.7 (53.9-96.2) | 50.0 (22.9-72.2) | 78.6 (47.2-92.5) | 57.1 (28.4-78.0) |
| At 18 months | 71.4 (40.6-88.2) | 21.4 (5.2-44.8) | 71.4 (40.6-88.2) | 21.4 (5.2-44.8) |
| At 24 months | 57.1 (28.4-78.0) | 21.4 (5.2-44.8) | 64.3 (34.3-83.3) | 14.3 (2.3-36.6) |
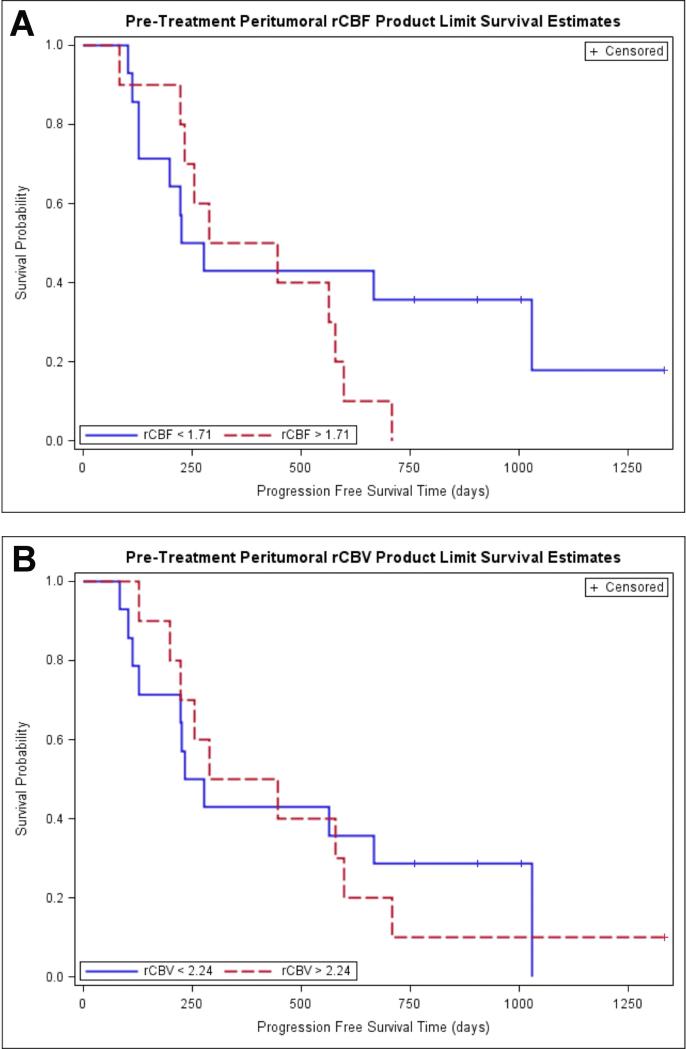
Kaplan-Meier survival curves for prediction of PFS according to pretreatment peritumoral rCBF and peritumoral rCBV demonstrated no difference in PFS in tumors with higher versus lower rCBV and rCBF (Figure 4, table 4). When the Kaplan-Meier curves and long rank tests were used to evaluate the association of PFS with posttreatment peritumoral rCBV and peritumoral rCBF, it was found that higher values of peritumoral rCBV (>2.01) and peritumoral rCBF (>1.85) were associated with lower PFS. The binary representation (high versus low) of both peritumoral rCBV and rCBF were statistically significant with p values of 0.019 and 0.0331 respectively (Figure 5, Table 4).
Figure 4.
Kaplan-Meier survival curves for prediction of PFS according to pretreatment peritumoral perfusion parameters. A. Kaplan-Meier survival curve of peritumoral rCBF demonstrates no difference in PFS in tumors with high rCBF (>1.71) (the red line) compared to tumors with low rCBF (<1.71) (the blue line). B. Kaplan-Meier survival curves for prediction of PFS according to pretreatment peritumoral rCBV demonstrate no difference in PFS in tumors with high rCBV (>2.24) (the red line) compared to tumors with low rCBV (<2.24) (the blue line).
Discussion
Relative CBV is the most robust perfusion parameter in evaluation of GBM. [7, 38] Prior studies have demonstrated good association of tumoral rCBV with OS. [39-41] There is no study in literature, to our knowledge, evaluating peritumoral rCBV in prediction of OS and PFS. The results of our study demonstrate strong association of posttreatment peritumoral rCBV with OS (Hazard ratio of 2.192; p=0.0028) and with (HR=1.830; p=0.034).
Several studies, both clinical and preclinical, have demonstrated that rCBV strongly correlates with the degree of the tumoral angiogenesis and microvessel density in patients with GBM. [42, 43] As the microvessel density increases several folds in GBM, rCBF also increases within the tumorous tissue, similar to rCBV. Prior studies have demonstrated correlation of tumoral CBV and CBF.[29] However, these studies have compared tumoral rCBV and rCBF, not peritumoral rCBV and rCBF, in grading of gliomas and did not compare the perfusion parameters to predict survival. Results of our study demonstrate statistically significant association of posttreatment peritumoral rCBF with OS (Hazard ration of 2.02; p=0.013); however, the association with PFS was not statistically significant (HR=1.446; p=0.142). This suggests that rCBF can also be used as a predictor of OS and PFS.
Kaplan-Meier analysis using high and low values for pretreatment peritumoral rCBV and rCBF cannot differentiate in OS and in PFS between higher and lower values. However, Kaplan-Meier analysis using high and low values for posttreatment peritumoral rCBV and rCBF was able to demonstrate statistically significant differences in overall survival and PFS between higher and lower values. This result may be compared with a study described by Law et al in which 189 glioma patients of different histologic grades demonstrated difference in OS between higher and lower value of tumoral rCBV, although the difference was not statistically significant in their cohort of patients. [16] Our results suggest that evaluation of posttreatment peritumoral perfusion parameters can be used to predict OS in patients with newly diagnosed GBM. Our study also demonstrates that posttreatment perfusion parameters are better in prediction of OS compared to the pretreatment peritumoral perfusion parameters.
As with any retrospective analysis, this study had inherent bias and many limitations. The main limitation of this study is the relatively small sample size that contributes to the statistical uncertainty and that could be the reason for the statistically non-significant results. We did not include any patient enrolled in an investigational agent clinical trial in order to control any difference in survival benefit. Second, this is a retrospective study where patients were scanned on different MRI machines with different imaging acquisition techniques that introduce heterogeneity to the perfusion parameters. Another potential limitation of this study is that the recurrent tumor treatment regimens were not identical in all study patients. This might have influenced the OS. However, we think that this effect is minimum, as RTOG 0825 study has shown that Avastin has no effect on OS in newly diagnosed GBM patients [44] and there is no known survival benefit of second line chemotherapeutic agents. Similarly, the amount of residual tumor tissue was not controlled. In some patients, there was gross total resection, whereas in others there was significant residual tumor compared to the pretreatment tumor volume. As the amount of residual tumor volume can theoretically influence the overall survival, this is also a potential limitation of our study. Finally, we analyzed the perfusion data using a region-of-interest-based technique that might have possible subjectivity and concern for reproducibility although in a study with 50 patients, Wetzel et al has demonstrated clinically acceptable inter- and intra-observer reproducibility. [35]
In conclusion, the results and findings of this study demonstrate that there is statistically significant association of posttreatment, not pretreatment, peritumoral rCBV and rCBF with OS and PFS in patients with newly diagnosed primary GBM. Higher posttreatment peritumoral rCBV and rCBF values are associated with poor survival compared to the lower values. The results should be validated in larger, multicenter prospective studies, to evaluate the role of peritumoral perfusion parameters in prediction of OS and PFS, as peritumoral perfusion parameters can be very useful if treatment-related changes limit use of tumoral perfusion parameters.
Acknowledgments
Funding: This study was not funded.
Footnotes
- Asim K. Bag is consultant to “Dotarem Advisory Board”, Guerbet, LLC
- Other authors declare no conflict of interest.
Contributor Information
Asim K Bag, Department of Radiology The University of Alabama at Birmingham JT N432, 619 19th Street South, Birmingham, AL 35249-6830.
Phillip C Cezayirli, Department of Neurology The University of Alabama at Birmingham FOT 1020, 510 20th Street South, Birmingham, AL 35294-3410.
Jake J Davenport, Department of Radiology The University of Alabama at Birmingham JT N432, 619 19th Street South, Birmingham, AL 35249-6830.
Santhosh Gaddikeri, Department of Radiology University of Washington 959 Pacific Street, SS-202, Seattle, WA 98195-7117.
Hassan M Fathallah-Shaykh, Department of Neurology The University of Alabama at Birmingham FOT 1020, 510 20th Street South, Birmingham, AL 35294-3410.
Alan Cantor, Department of Preventive Medicine The University of Alabama at Birmingham MT 627, 1717 11th Avenue South, Birmingham, AL 35294-4410.
Xiosi S Han, Department of Neurology The University of Alabama at Birmingham FOT 1020, 510 20th Street South, Birmingham, AL 35294-3410.
Louis B Nabors, Department of Neurology The University of Alabama at Birmingham FOT 1020, 510 20th Street South, Birmingham, AL 35294-3410.
References
- 1.Kleihues P Burger PC, Aldape KD, et al. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumors of the central nervous system. IARC Press; Lyon: 2007. pp. 33–49. [Google Scholar]
- 2.Mangla R, Singh G, Ziegelitz D, Milano MT, Korones DN, Zhong J, Ekholm SE. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256:575–584. doi: 10.1148/radiol.10091440. doi:10.1148/radiol.10091440. [DOI] [PubMed] [Google Scholar]
- 3.Choi YJ, Kim HS, Jahng GH, Kim SJ, Suh DC. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol. 2013;54:448–454. doi: 10.1177/0284185112474916. doi:10.1177/0284185112474916. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24:1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 6.Cha S, Lupo JM, Chen MH, Lamborn KR, McDermott MW, Berger MS, Nelson SJ, Dillon WP. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2007;28:1078–1084. doi: 10.3174/ajnr.A0484. doi:10.3174/ajnr.A0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha S. Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol. 2006;27:475–487. [PMC free article] [PubMed] [Google Scholar]
- 8.Bisdas S, Kirkpatrick M, Giglio P, Welsh C, Spampinato MV, Rumboldt Z. Cerebral blood volume measurements by perfusion-weighted MR imaging in gliomas: ready for prime time in predicting short-term outcome and recurrent disease? AJNR Am J Neuroradiol. 2009;30:681–688. doi: 10.3174/ajnr.A1465. doi:10.3174/ajnr.A1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, Coons SW, Nakaji P, Yeh RF, Debbins J, Heiserman JE. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30:552–558. doi: 10.3174/ajnr.A1377. doi:10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am. 2009;19:527–557. doi: 10.1016/j.nic.2009.08.007. doi:10.1016/j.nic.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Hirai T, Murakami R, Nakamura H, Kitajima M, Fukuoka H, Sasao A, Akter M, Hayashida Y, Toya R, Oya N, Awai K, Iyama K, Kuratsu JI, Yamashita Y. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol. 2008;29:1505–1510. doi: 10.3174/ajnr.A1121. doi:10.3174/ajnr.A1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollin N, Guyotat J, Streichenberger N, Honnorat J, Tran Minh VA, Cotton F. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology. 2006;48:150–159. doi: 10.1007/s00234-005-0030-7. doi:10.1007/s00234-005-0030-7. [DOI] [PubMed] [Google Scholar]
- 13.Schmainda KM, Rand SD, Joseph AM, Lund R, Ward BD, Pathak AP, Ulmer JL, Badruddoja MA, Krouwer HG. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol. 2004;25:1524–1532. [PMC free article] [PubMed] [Google Scholar]
- 14.Chaskis C, Stadnik T, Michotte A, Van Rompaey K, D'Haens J. Prognostic value of perfusion-weighted imaging in brain glioma: a prospective study. Acta neurochirurgica. 2006;148:277–285. doi: 10.1007/s00701-005-0718-9. doi:10.1007/s00701-005-0718-9. [DOI] [PubMed] [Google Scholar]
- 15.Law M, Yang S, Babb JS, Knopp EA, Golfinos JG, Zagzag D, Johnson G. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2004;25:746–755. [PMC free article] [PubMed] [Google Scholar]
- 16.Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, Miller DC, Golfinos JG, Zagzag D, Johnson G. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247:490–498. doi: 10.1148/radiol.2472070898. doi:10.1148/radiol.2472070898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiroishi MS, Booker MT, Agarwal M, Jain N, Naghi I, Lerner A, Law M. Posttreatment evaluation of central nervous system gliomas. Mag Reson Imaging Clin of N Am. 2013;21:241–268. doi: 10.1016/j.mric.2013.02.004. doi:10.1016/j.mric.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Chavhan GB, Babyn PS, Thomas B, Shroff MM, Haacke EM. Principles, techniques, and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–1449. doi: 10.1148/rg.295095034. doi:10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barajas RF, Jr., Chang JS, Segal MR, Parsa AT, McDermott MW, Berger MS, Cha S. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2009;253:486–496. doi: 10.1148/radiol.2532090007. doi:10.1148/radiol.2532090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparetto EL, Pawlak MA, Patel SH, Huse J, Woo JH, Krejza J, Rosenfeld MR, O'Rourke DM, Lustig R, Melhem ER, Wolf RL. Posttreatment recurrence of malignant brain neoplasm: accuracy of relative cerebral blood volume fraction in discriminating low from high malignant histologic volume fraction. Radiology. 2009;250:887–896. doi: 10.1148/radiol.2502071444. doi:10.1148/radiol.2502071444. [DOI] [PubMed] [Google Scholar]
- 21.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865–874. doi: 10.3171/jns.1987.66.6.0865. doi:10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 22.De Belder FE, Oot AR, Van Hecke W, Venstermans C, Menovsky T, Van Marck V, Van Goethem J, Van den Hauwe L, Vandekerckhove M, Parizel PM. Diffusion tensor imaging provides an insight into the microstructure of meningiomas, high-grade gliomas, and peritumoral edema. J Comput Assist Tomogr. 2012;36:577–582. doi: 10.1097/RCT.0b013e318261e913. doi:10.1097/RCT.0b013e318261e913. [DOI] [PubMed] [Google Scholar]
- 23.Stewart JG, Sawrie SM, Bag A, Han X, Fiveash JB. Management of brain metastases. Curr Treat Options Neurol. 2010;12:334–346. doi: 10.1007/s11940-010-0074-9. doi:10.1007/s11940-010-0074-9. [DOI] [PubMed] [Google Scholar]
- 24.Law M, Cha S, Knopp EA, Johnson G, Arnett J, Litt AW. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222:715–721. doi: 10.1148/radiol.2223010558. doi:10.1148/radiol.2223010558. [DOI] [PubMed] [Google Scholar]
- 25.Blasel S, Franz K, Ackermann H, Weidauer S, Zanella F, Hattingen E. Stripe-like increase of rCBV beyond the visible border of glioblastomas: site of tumor infiltration growing after neurosurgery. J Neurooncol. 2011;103:575–584. doi: 10.1007/s11060-010-0421-4. doi:10.1007/s11060-010-0421-4. [DOI] [PubMed] [Google Scholar]
- 26.Server A, Orheim TE, Graff BA, Josefsen R, Kumar T, Nakstad PH. Diagnostic examination performance by using microvascular leakage, cerebral blood volume, and blood flow derived from 3-T dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in the differentiation of glioblastoma multiforme and brain metastasis. Neuroradiology. 2011;53:319–330. doi: 10.1007/s00234-010-0740-3. doi:10.1007/s00234-010-0740-3. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann P, Saliou G, de Marco G, Monet P, Souraya SE, Bruniau A, Vallee JN, Ducreux D. Cerebral peritumoral oedema study: does a single dynamic MR sequence assessing perfusion and permeability can help to differentiate glioblastoma from metastasis? Eur J Radiol. 2012;81:522–527. doi: 10.1016/j.ejrad.2011.01.076. doi:10.1016/j.ejrad.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 28.Hakyemez B, Erdogan C, Ercan I, Ergin N, Uysal S, Atahan S. High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol. 2005;60:493–502. doi: 10.1016/j.crad.2004.09.009. doi:10.1016/j.crad.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Thomsen H, Steffensen E, Larsson EM. Perfusion MRI (dynamic susceptibility contrast imaging) with different measurement approaches for the evaluation of blood flow and blood volume in human gliomas. Acta Radiol. 2012;53:95–101. doi: 10.1258/ar.2011.110242. doi:10.1258/ar.2011.110242. [DOI] [PubMed] [Google Scholar]
- 30.Law M, Young R, Babb J, Rad M, Sasaki T, Zagzag D, Johnson G. Comparing perfusion metrics obtained from a single compartment versus pharmacokinetic modeling methods using dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2006;27:1975–1982. [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh AH, Smith JK, Ewend MG, Bullitt E. Correlation of MR perfusion imaging and vessel tortuosity parameters in assessment of intracranial neoplasms. Technol Cancer Res Treat. 2004;3:585–590. doi: 10.1177/153303460400300608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleihues P Burger PC, Aldape KD, et al. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumors of the central nervous system. IARC Press; Lyon: 2007. pp. 33–49. [Google Scholar]
- 33.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. doi:10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27:859–867. [PMC free article] [PubMed] [Google Scholar]
- 35.Wetzel SG, Cha S, Johnson G, Lee P, Law M, Kasow DL, Pierce SD, Xue X. Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology. 2002;224:797–803. doi: 10.1148/radiol.2243011014. [DOI] [PubMed] [Google Scholar]
- 36.Young R, Babb J, Law M, Pollack E, Johnson G. Comparison of region-of-interest analysis with three different histogram analysis methods in the determination of perfusion metrics in patients with brain gliomas. J Magn Reson Imaging. 2007;26:1053–1063. doi: 10.1002/jmri.21064. doi:10.1002/jmri.21064. [DOI] [PubMed] [Google Scholar]
- 37.Hu LS, Eschbacher JM, Heiserman JE, Dueck AC, Shapiro WR, Liu S, Karis JP, Smith KA, Coons SW, Nakaji P, Spetzler RF, Feuerstein BG, Debbins J, Baxter LC. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro-oncology. 2012;14:919–930. doi: 10.1093/neuonc/nos112. doi:10.1093/neuonc/nos112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223:11–29. doi: 10.1148/radiol.2231010594. doi:10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 39.Lev MH, Ozsunar Y, Henson JW, Rasheed AA, Barest GD, Harsh GRt, Fitzek MM, Chiocca EA, Rabinov JD, Csavoy AN, Rosen BR, Hochberg FH, Schaefer PW, Gonzalez RG. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol. 2004;25:214–221. [PMC free article] [PubMed] [Google Scholar]
- 40.Law M, Oh S, Babb JS, Wang E, Inglese M, Zagzag D, Knopp EA, Johnson G. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging--prediction of patient clinical response. Radiology. 2006;238:658–667. doi: 10.1148/radiol.2382042180. doi:10.1148/radiol.2382042180. [DOI] [PubMed] [Google Scholar]
- 41.Mills SJ, Patankar TA, Haroon HA, Baleriaux D, Swindell R, Jackson A. Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol. 2006;27:853–858. [PMC free article] [PubMed] [Google Scholar]
- 42.Haris M, Husain N, Singh A, Husain M, Srivastava S, Srivastava C, Behari S, Rathore RK, Saksena S, Gupta RK. Dynamic contrast-enhanced derived cerebral blood volume correlates better with leak correction than with no correction for vascular endothelial growth factor, microvascular density, and grading of astrocytoma. J Comput Assist Tomogr. 2008;32:955–965. doi: 10.1097/RCT.0b013e31816200d1. doi:10.1097/RCT.0b013e31816200d1. [DOI] [PubMed] [Google Scholar]
- 43.Majchrzak K, Kaspera W, Szymas J, Bobek-Billewicz B, Hebda A, Majchrzak H. Markers of angiogenesis (CD31, CD34, rCBV) and their prognostic value in low-grade gliomas. Neurol Neurochir Pol. 2013;47:325–331. doi: 10.5114/ninp.2013.36757. Doi: 10.5114/ninp.2013.36757. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Jr., Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. doi:10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]