Abstract
Introduction
Subject recruitment is critical for understanding fatal diseases like ALS, however linking patients with researchers can be challenging. The US population-based National ALS Registry allows recruitment of persons with ALS (PALS) for research opportunities.
Methods
The Registry’s Research Notification Mechanism was used to recruit PALS aged ≥21 years; participants completed a web-based epidemiologic survey. PALS (n=2,232) were sent an email describing the study, and 268 surveys were completed.
Results
The mean age (± SD) of eligible participants was 57.7 ± 9.3 years for men and 61.5 ± 8.9 for women. Most were men (63%) and Caucasian (92%). Of 256 potentially eligible participants, 37.5% (n=96) returned an authorization to disclose protected health information. ALS was confirmed for 94% (83/88) from physician responses.
Discussion
This analysis demonstrates the National ALS Registry’s usefulness in recruiting PALS for research. This recruitment source can potentially foster the discovery of better treatment options and therapies, and of prevention strategies.
Keywords: amyotrophic lateral sclerosis, ALS, National ALS Registry, recruitment, research
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neuromuscular disease that typically leads to death within 2–5 years of symptom onset 1. The uncertainty about the incidence and prevalence of ALS in the United States (US), as well as the lack of knowledge about the role of environmental exposures in its etiology, has created a need for structured data collection. In 2008, President George W. Bush signed the ALS Registry Act into law, allowing the federal Agency for Toxic Substances and Disease Registry (ATSDR) to create and maintain the population-based National ALS Registry. Launched in October, 2010, the primary objectives of the Registry are to quantify the incidence and prevalence of ALS in the US, describe the demographics of persons with ALS (PALS), and examine risk factors for the disease 2.
The National ALS Registry takes a two-pronged approach for tracking ALS cases in the US by using 1) existing national administrative databases (i.e., Medicare, Medicaid, Veterans Heath Administration, and Veterans Benefit Administration) and 2) a secure web portal that allows patients to self-enroll and take brief risk factor surveys 2. The Registry is the only congressionally-mandated ALS registry in the US and is one of the largest ALS registries in the world. To date, thousands of PALS, covering all 50 states, have entered the Registry, and new PALS enroll each week.
Another important purpose of the Registry is to enhance and facilitate ALS research. To achieve this, ATSDR modified the Registry in 2012 so that it can be used as a recruitment tool for researchers. As a result, the Registry’s new Research Notification Mechanism now electronically links Registry-enrolled PALS with external scientists who are conducting ALS research, such as epidemiologic studies or clinical trials. Linking PALS and scientists has the potential to accelerate the pace of research, which is important, as there is no current cure or effective treatment for ALS.
The main objective of this paper is to outline how researchers used the National ALS Registry’s Research Notification Mechanism to quickly and effectively recruit PALS for a case-control study investigating the association of environmental and occupational exposures (such as exposure to metals, pesticides, and solvents) and the development of ALS. Case-confirmation of PALS self-enrolled in the National ALS Registry was also evaluated. The demographics of those Registry-enrolled PALS identified through the Research Notification Mechanism for this analysis will also be presented. Results of the case-control study will be presented elsewhere.
MATERIALS AND METHODS
Research Notification Mechanism Process
The Registry’s Research Notification Mechanism consists of a concurrent two-stage process for both PALS and researchers. For PALS to take part in the Research Notification Mechanism, they must enroll initially electronically in the National ALS Registry. During the Registry enrollment process, PALS indicate via a check box whether they are interested in being notified about research opportunities. When PALS elect to participate in the Research Notification Mechanism, they undergo a one- time enrollment process. Nothing further needs to be done until individuals are notified about a potential research opportunity for which they are eligible. If PALS choose not to participate in the Notification Mechanism, it will not affect their entry into the National ALS Registry, because the Research Notification Mechanism is strictly voluntary.
For ALS researchers to use the Research Notification Mechanism for recruitment purposes, they must complete and upload an ATSDR pre-designed pdf application via the Registry web portal that briefly describes their research study and objectives. Once submitted, the application is evaluated for scientific merit and proper Institutional Review Board (IRB) clearance. If the application is approved (which typically takes 30 days or less), ATSDR then queries the Registry for PALS who meet the study’s specific eligibility criteria (e.g., age, gender, stage of disease), and then distributes the researcher’s study material and contact information to PALS via email. PALS have to directly contact the researcher to participate in any study.
Study Population
The National ALS Registry was used as the sole recruitment tool for a national epidemiologic study investigating risk factors for ALS through administration of a detailed epidemiologic web-based survey. Using the Registry’s Research Notification Mechanism, the Medical University of South Carolina (MUSC) submitted an online application to ATSDR stating the study goals and objectives. This was the first application received by ATSDR since the implementation of the Mechanism. After ensuring IRB approval, ATSDR approved the MUSC study proposal. On February 28, 2013, a single email advertising the study was then sent by ATSDR to Registry-enrolled PALS ≥ 21 years of age (n=2,232). The email contained contact information for study staff and an attached study flier with a URL (Universal Resource Locator) link to the survey. PALS completed the survey by 1 of 2 methods: 1) by using the URL link in the original ASTDR study email attachment or 2) by contacting MUSC study staff for more information, which resulted in resending of the survey URL link by email.
Measures
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic capture tools hosted at MUSC 3. The web-based survey obtained self-reported information related to lifetime occupational history and occupational exposure, lifetime residential history, hobbies, physical activity, military history, etc. The survey began with an overview of the survey and implied consent (written statement) by provision of an electronic signature or confirmation of agreement to participate in the web-based survey. This was followed by an electronic screening assessment to determine eligibility. Inclusion criteria included: physician diagnosis of ALS, knowledge of the English language, residence in the US for at least 10 years, and age ≥21 years. PALS were excluded if they had a family member with ALS or had been diagnosed previously with Parkinson disease, Parkinsonism, Alzheimer disease, dementia, or poliomyelitis/post-polio syndrome. After eligibility was determined, the survey took an average of 30–40 minutes to complete.
Case-Confirmation
In addition to completing the survey, PALS were asked to complete a short form authorizing disclosure of protected health information (PHI) for the purpose of confirmation of ALS diagnosis. The form required PALS to provide the name and contact information for the ALS physician who made the diagnosis, indicate their preferred method(s) for exchange of information with their physician (email, fax, and/or mail), sign, date, and return the form to study staff at MUSC by email, mail, or fax. Upon receipt of the authorization to disclose PHI form, study staff reviewed the form to ensure completion.
Next, the forms were forwarded to the diagnosing ALS physician for confirmation of ALS as definite, probable, or laboratory-supported probable according to the revised El Escorial criteria 4 included at the top of the form. Physicians were also requested to adhere to the patient’s preferred method of communication in returning the form to researchers. Matched population-based controls will later be identified to compare responses among cases and controls.
Statistical Analysis
Demographic characteristics were compared by descriptive statistics including proportions and t-tests. All analyses were conducted using SPSS, version 21 5.
RESULTS
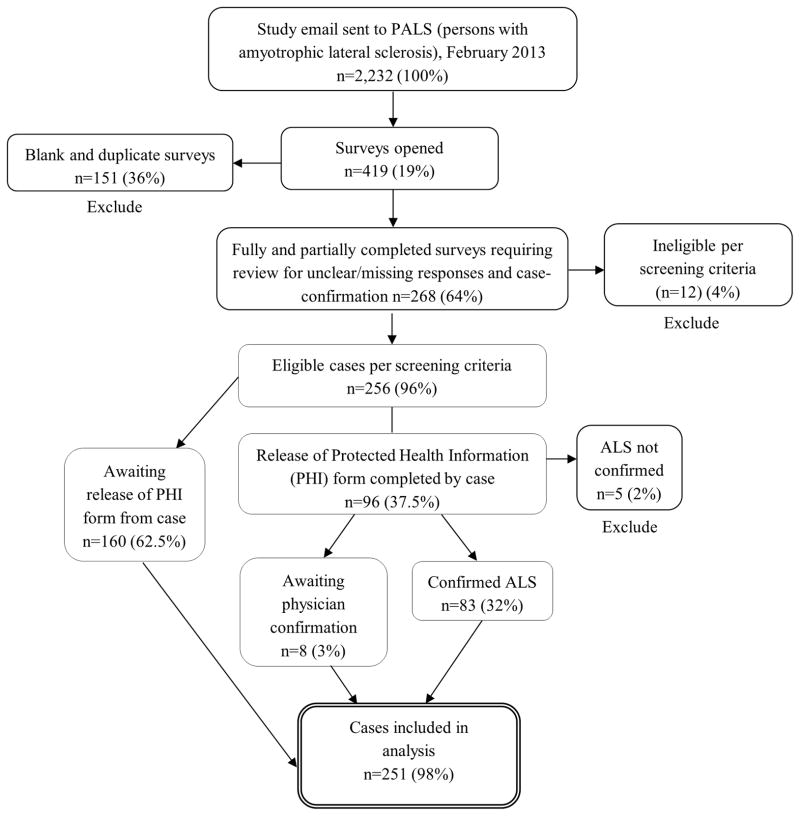
Of the 2,232 emails sent initially, 114 were undeliverable. ATSDR was informed about 9 deceased patients. Sixty-two surveys were completed on February 28, the day of the ASTDR email, and an additional 206 surveys were completed as of November 8, 2013 (n=268). The interest level in the survey was high with over 100 emails and phone calls received in the first 45 days of the ASTDR email. Eligibility was determined by 2 levels of screening criteria. The first level involved study screening criteria: family history of ALS (n=8); previous diagnosis of Parkinson disease, Parkinsonism, Alzheimer disease, dementia, or poliomyelitis/post-polio syndrome (n=3); or not self-reporting a diagnosis of ALS (n=1), by which 12 (4%) of the 268 were excluded, leaving 256 study subjects potentially eligible for inclusion in the analysis. The second level required physician confirmation of ALS. Completed disclosure of PHI forms were received from 96 subjects (37.5%). Of the 96 completed forms received from subjects, ALS diagnosis was confirmed for 83 (86%), could not be confirmed for five (5%), and eight (8%) await the physician’s response. Thus, of the 88 forms with physicians’ responses, ALS was confirmed for 94% (n=83) and could not be confirmed for 6% (n=5). After excluding the 5 subjects without ALS, a total of 251 were eligible for inclusion in the analysis (Figure 1). Study subjects ranged in age from 29–80 years, and men were significantly younger than women (P=0.002). Demographic characteristics of the cohort are listed in Table 1.
Figure 1.
Flowchart of eligible ALS patients for analysis
Table 1.
Demographic Characteristics of ALS study subjects
| Characteristic | Men (n=158) n (%) | Women (n=93) n (%) | Total (n=251) n (%) |
|---|---|---|---|
|
| |||
| Age, mean ± SD (years)* | 57.7 ± 9.3 | 61.5 ± 8.9 † | 58.9 ± 10.0 |
|
| |||
| Race/Ethnicity | |||
| Caucasian | 144 (91) | 88 (95) | 232 (92) |
| African-American | 3 (2) | 2 (2) | 5 (2) |
| Latin/Latino/Hispanic | 5 (3) | 2 (2) | 7 (3) |
| Asian/Pacific Islander | 3 (2) | 1 (1) | 4 (2) |
| Unknown/missing | 3 (2) | - | 3 (1) |
|
| |||
| US region ‡, § | |||
| Northeast | 19 (12) | 13 (14) | 32 (13) |
| South | 54 (34) | 41 (44) | 95 (38) |
| Midwest | 28 (18) | 16 (17) | 44 (18) |
| West | 56 (35) | 23 (25) | 79 (32) |
Abbreviations: sd, standard deviation; US, United States.
Age was missing for 2 men and 2 women.
Age was significantly different when compared by gender (P=0.002).
Northeast includes: CT, MA, ME, NH, NJ, NY, PA, RI, and VT. South includes: AL, AR, DE, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, VA, WV, and D.C. Midwest includes: ND, NE, KS, IA, IL, IN, MI, MN, MO, OH, SD, and WI. West includes: AK, AZ, CA, CO, ID, HI, MT, NM, NV, OR, UT, WA, and WY.
One man was a US citizen living abroad.
Rather than confirming diagnosis by signing the provided form, 14 physicians or medical records offices instead sent the participant’s medical records to MUSC, which were reviewed for confirmation or lack of confirmation of ALS by the study neurologist. Furthermore, physicians did not confirm ALS diagnosis for 5 patients. Of the 5 without ALS, 1 had possible ALS, 1 was diagnosed with facial onset sensorimotor neuropathy (FOSMN), 1 had probable primary lateral sclerosis (PLS), and 2 did not meet the diagnostic criteria.
DISCUSSION
Subject recruitment for research is critical for understanding fatal diseases like ALS; however, linking patients with researchers is not so straightforward. Researchers often face challenges such as timely recruitment and retention, obtaining heterogeneity, and enlisting patients who meet certain eligibility requirements. Additionally, the rarity of ALS makes obtaining sufficient sample sizes for research studies difficult, even in multidisciplinary ALS clinics 6. PALS are traditionally recruited for epidemiologic studies through clinician databases, mailings/advertisements, and ALS organizations. While these approaches are helpful, the recruitment can take substantial time and effort without necessarily yielding a large sample size. For example, an ongoing epidemiologic study conducted by 1 of the authors (DS) at MUSC has recruited 50 clinic-based PALS over a 12-month period, 35 of whom have completed the study’s survey fully. A 2013 case-control study carried out by Malek et al. recruited 49 PALS from 3 ALS clinics in Pennsylvania (2 in Pittsburgh and 1 in Philadelphia) over a 12-month period 7.
According to Bedlack et al., ALS Research Group (ALSRG) members reported a clinical trial enrollment rate of 25%, with substantial variability across clinics (0–75%) 8. Additionally, those authors mentioned lower enrollment rates in other studies: 9.9% (Duke University) and 2.2% (Massachusetts General Hospital). Similarly, patients face their own challenges, including identifying potential research opportunities available, meeting eligibility criteria, and fully understanding complex research descriptions and study designs. This analysis clearly demonstrates the effectiveness of the National ALS Registry’s Research Notification Mechanism in recruiting a relatively large number of PALS for research in a relatively short time. Moreover, our enrollment rate of 11.5% is well within the range reported by other studies.
The majority of PALS who responded to the survey were men and were Caucasian, with a mean age of 58.9 ± 10 years. These findings are consistent with prior epidemiologic studies of demographics of ALS in the US1. Twelve study subjects were excluded for not meeting the eligibility criteria. Spouses of deceased patients expressed interest in participating in the study, and 1 completed the survey.
Physician confirmation was required to help exclude any non-ALS subjects. Physicians who responded to subjects’ requests to disclose PHI have done an excellent job confirming the ALS diagnosis in a short period of time. We found that 86% of those who had completed a release of PHI form indeed were confirmed to have ALS, although 8 individuals still await physician confirmation. After exclusion of these 8, ALS was confirmed for 94% of subjects. This attests to the data quality of the National ALS Registry self-enrollment portion. During its online enrollment process, the Registry uses a set of 6 validation questions to screen for possible cases of ALS. The Veterans Administration used these questions for their own, now defunct, ALS Registry; among 1,290 self-referred patients, 98.7% passed screening, and 74.6% had ALS verified based on neurologist medical record review9.
Study limitations include selection bias that may have resulted in study participants differing from PALS who did not participate. In addition, PALS who were not enrolled in the Registry may have less severe disease. A relative sample bias exists toward Caucasian men. However, recent trials conducted by Zhao et al. (65% men ),8 Wills et al. (54% men ),9 and Cudkowicz et al. (64% men) 10 have enrolled a similar proportion of men, the large majority or all of whom have been Caucasian. The response rate of 11.5% was discussed previously, although it may have been improved with additional recruitment emails about the study. In our attempt to contact PALS regarding completion of the disclosure of PHI form for case-confirmation and for clarification of unclear survey responses, several PALS could not be reached due to a non-working email address, failure to provide a phone number or mailing address, or because they were deceased. PALS without confirmed diagnosis of ALS will not be included in future analyses. Thus, it is possible that eligible PALS lacking case-confirmation may be excluded.
Since the Research Notification Mechanism’s May 2012 deployment, approximately 96% of enrollees in the National ALS Registry have elected to be notified about ALS research opportunities. To date, ATSDR has approved 9 institutions to use the Registry for research recruitment, sending out more than 13,000 e-mail notifications to PALS on behalf of the recruiting institutions. More details of approved research studies and clinical trials can be found on the National ALS Registry website: http://wwwn.cdc.gov/ALS/ALSResearchNotificationClinicalTrialsStudies.aspx.
CONCLUSIONS
The National ALS Registry’s Research Notification Mechanism has proven to be an effective tool for linking PALS with ALS researchers. The mechanism benefits PALS by conveniently delivering timely and tailored research opportunities via email. Furthermore, the high percentage of PALS who are taking part in this mechanism (96%), along with the promptness with which they complete surveys, demonstrates their eagerness to connect with researchers. This mechanism also benefits researchers by speeding-up recruitment, increasing study sample size, achieving geographic diversity, and easily and efficiently identifying PALS who meet specific eligibility requirements. ATSDR will continue to use this Mechanism to link interested PALS with researchers in hopes of finding better treatment options and therapies, prevention strategies, and ultimately a cure for ALS.
Acknowledgments
Acknowledgement: The authors would like to acknowledge grant support from the South Carolina Clinical and Translational Research Institute (SCTR) – Office of Biomedical Informatics Services (NIH/NCATS UL1TR000062).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Agency for Toxic Substances and Disease Registry.
ABBREVIATIONS
- ALS
amyotrophic lateral sclerosis
- ASTDR
Agency for Toxic Substances and Disease Registry
- FOSMN
facial onset sensorimotor neuropathy
- SCTR
South Carolina Clinical and Translational Research Institute
- IRB
Institutional Review Board
- ICD-9
International Classification of Diseases, 9th Revision
- MUSC
Medical University of South Carolina
- PALS
persons with ALS
- PHI
protected health information
- PLS
probable primary lateral sclerosis
- SOD+
superoxide dismutase
- SCTR
South Carolina Clinical and Translational Research Institute
- HAPs
hazardous air pollutants
- US
United States
- URL
Universal Resource Locator
References
- 1.Mitsumoto H, Chad DA, Pioro EP, editors. Differential Diagnoses in Amyotrophic lateral sclerosis. Philadelphia, PA: FA Davis; 1998. [Google Scholar]
- 2.Antao VC, Horton DK. The National Amyotrophic Lateral Sclerosis (ALS) Registry. Journal of environmental health. 2012;75(1):28–30. [PMC free article] [PubMed] [Google Scholar]
- 3.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci. 1994;124 (Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 5.IBM Corp. IBM SPSS Statistics for Windows. 21.0. Armonk, NY: IBM Corp; 2012. [Google Scholar]
- 6.Berry JD, Cudkowicz ME. New considerations in the design of clinical trials for amyotrophic lateral sclerosis. Clinical investigation. 2011;1(10):1375–1389. doi: 10.4155/cli.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malek AM, Barchowsky A, Bowser R, Heiman-Patterson T, Lacomis D, Rana S, Youk A, Stickler D, Lackland DT, Talbott EO. Environmental and Occupational Risk Factors for Amyotrophic Lateral Sclerosis: A Case-Control Study. Neuro–degenerative diseases. 2013 doi: 10.1159/000355344. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Cudkowicz ME, Shefner JM, Krivickas L, David WS, Vriesendorp F, Pestronk A, Caress JB, Katz J, Simpson E, Rosenfeld J, Pascuzzi R, Glass J, Rezania K, Harmatz JS, Schoenfeld D, Greenblatt DJ. Systemic pharmacokinetics and cerebrospinal fluid uptake of intravenous ceftriaxone in patients with amyotrophic lateral sclerosis. Journal of clinical pharmacology. 2014 doi: 10.1002/jcph.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wills AM, Hubbard J, Macklin EA, Glass J, Tandan R, Simpson EP, Brooks B, Gelinas D, Mitsumoto H, Mozaffar T, Hanes GP, Ladha SS, Heiman-Patterson T, Katz J, Lou JS, Mahoney K, Grasso D, Lawson R, Yu H, Cudkowicz M for the MDACRN. Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cudkowicz ME, van den Berg LH, Shefner JM, Mitsumoto H, Mora JS, Ludolph A, Hardiman O, Bozik ME, Ingersoll EW, Archibald D, Meyers AL, Dong Y, Farwell WR, Kerr DA investigators E. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet neurology. 2013;12(11):1059–1067. doi: 10.1016/S1474-4422(13)70221-7. [DOI] [PubMed] [Google Scholar]