Abstract
Rapid pathogen detection and antimicrobial susceptibility testing (AST) are required in diagnosis of acute bacterial infections to determine the appropriate antibiotic treatment. Molecular approaches for AST are often based on the detection of known antibiotic resistance genes. Phenotypic culture analysis requires several days from sample collection to result reporting. Toward rapid diagnosis of bacterial infection in nontraditional healthcare settings, we have developed a rapid AST approach that combines phenotypic culture of bacterial pathogens in physiological samples and electrochemical sensing of bacterial 16S rRNA. The assay determines the susceptibility of pathogens by detecting bacterial growth under various antibiotic conditions. AC electrokinetic fluid motion and Joule heating induced temperature elevation are optimized to enhance the sensor signal and minimize the matrix effect, which improve the overall sensitivity of the assay. The electrokinetics enhanced biosensor directly detects the bacterial pathogens in blood culture without prior purification. Rapid determination of the antibiotic resistance profile of Escherichia coli clinical isolates is demonstrated.
Introduction
Acute bacterial infections are a major cause of patient morbidity and represent a significant burden of the healthcare system.1, 2 Infections such as sepsis and wound infections may be severe and even life-threatening.3–7 Pathogen detection and antimicrobial susceptibility testing (AST) are performed on patient samples, such as blood and wound swabs, to detect pathogens and their resistances.8, 9 Conventional culture-based analysis, however, requires at least two to three days for bacterial growth and could be much longer for slow growing pathogens.10–12 Without microbiological diagnosis of the pathogens and their resistance mechanisms, antimicrobial therapy is often initiated empirically, which drives the overuse of broad-spectrum antibiotics.13 Rapid diagnosis of acute infection will facilitate proper clinical management of infection diseases and reduce the emergence of multidrug-resistant organisms.14, 15
Molecular techniques, including multiplexed PCR,10, 16 DNA microarrays,17, 18 and molecular probes,19, 20 offer alternatives to conventional culture-based analysis. Genotypic ASTs, which target known resistance genes, are widely used to identify specific pathogens such as methicillin-resistant Staphylococcus aureus.21 In clinical settings where there is a wide array of ‘bug-drug’ combinations and emergence of new resistance mechanisms are of concern, genotypic AST are less reliable and have more limited clinical applicability.22 The combination of phenotypic AST of the physiological sample and molecular biosensors for bacterial detection may represent a promising alternative to the conventional approach in infectious diseases diagnostics.17, 18 However, most genotypic biosensors, such as real-time PCR, requires purified samples and additional extraction steps.23 The bacteria and molecular separation steps can significantly increase the overall assay time and system complexity, which limits their applications in non-traditional healthcare settings.
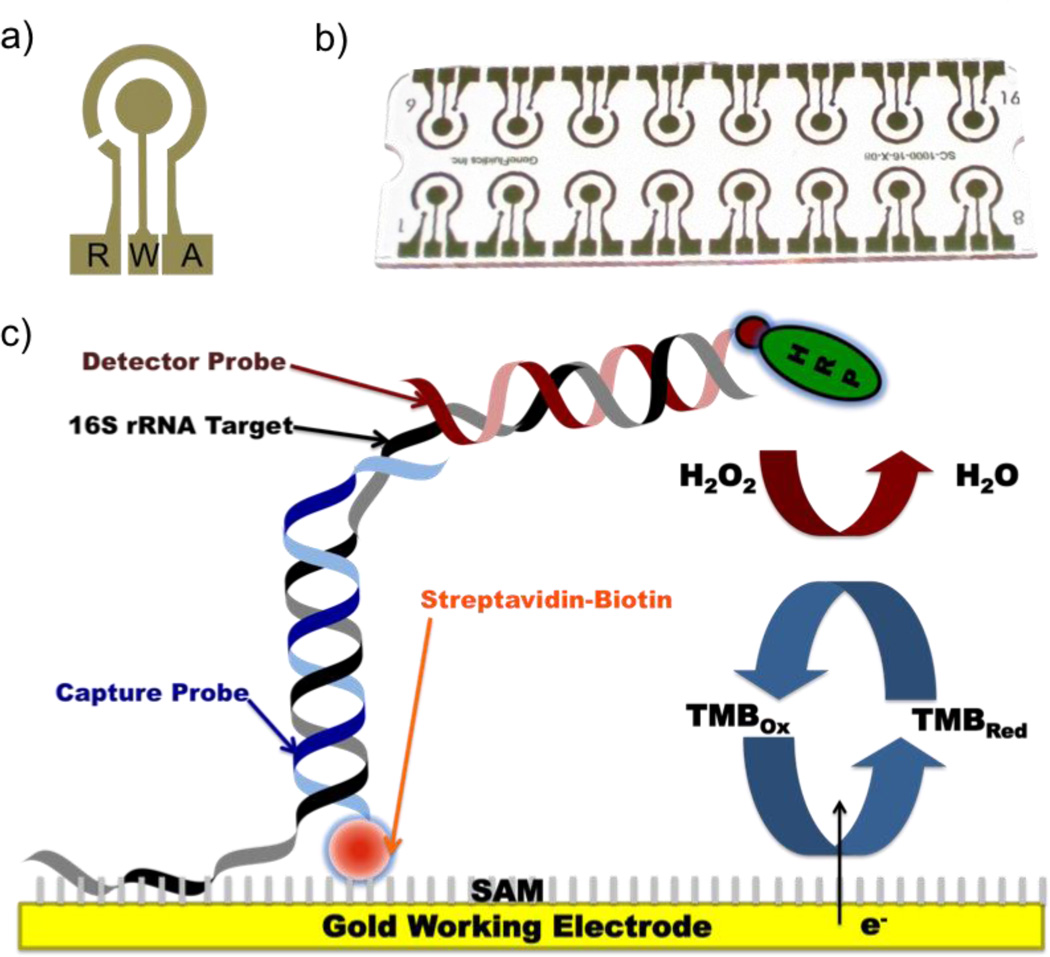
We have previously demonstrated that AC electrokinetic enhancement on an electrochemical biosensor platform can significantly increase the sensitivity and specificity of a molecular assay for detecting bacterial 16S rRNA.20, 24, 25 The self-assembled monolayer based electrochemical biosensor consists three electrodes including the working (W), reference (R) and auxiliary (A) electrodes. The detection strategy involves sandwich hybridization of the sequence-specific 16S rRNA to biotinylated or thiolated capture probes and fluorescein-labeled detector probes. The amperometric signal is generated by the electron transferring during horseradish peroxidase (HRP) catalyzed redox reaction (Figure 1). In the electrokinetics enhanced assay, a non-uniform electric field is applied to electrically conductive buffer, which results in Joule heating.26 Joule heating creates a local temperature distribution in the sensor, which induces gradients of conductivity, permittivity, and density depending on the electrode configuration. The interactions between the electric field and the gradients can lead to electrothermal forces and bulk fluid motion.27–30 The electrothermal fluid motion and the temperature rise can be optimized for enhancing molecular advection and binding kinetics of the hybridization assay. The temperature distribution at equilibrium can be estimated by the following equation:
| (1) |
where k is the thermal diffusivity, T is the temperature of the medium, σ is the conductivity of the medium, and E is the electric field. An analytical expression of the electrothermal force has been derived for parallel electrodes with a small gap: 28, 29
| (2) |
where M(ω,T) is a dimensionless factor describing the frequency dependence of the electrothermal force, r and θ are the polar coordinates, ε is the permittivity of the medium, and τ is the charge relaxation time. These equations characterize the temperature rise and the electrothermal force. With electrokinetic enhancement, the temperature rise will reduce non-specific binding on the electrode surface, and the bulk electrothermal fluid motion will continuously remove the sample matrix away from the working electrode (Figure 2a).
Figure 1.
(a–b) The electrode design for AC electrokinetics enhanced electrochemical biosensing. R, W and A represent reference, working and auxiliary electrodes, respectively. (c) The detection strategy for the electrochemical assay for bacterial 16S rRNA. The gold electrode is pre-coated with an alkanethoil self-assembled monolayer. The capture probe for hybridizing to the target molecular is immobilized on the electrode surface by biotin-streptavidin interaction. The target is also hybridized to a detector probe for signal generation.
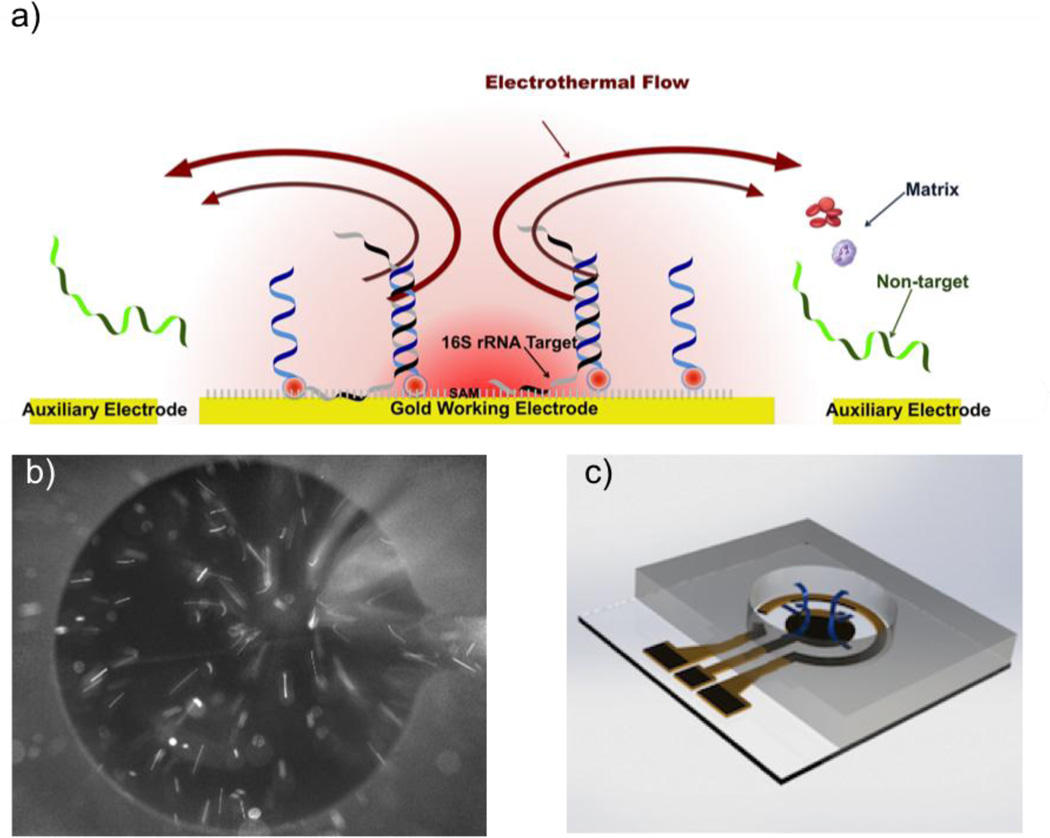
Figure 2.
(a) A schematic diagram of the AC electrokinetics enhanced hybridization process. (b) Visualization of the fluid motion by particle tracing. Tracer particles were seeded in hybridization buffer and a 200 kHz square wave with 5 volts peak to peak was applied. (c) 3D representation of electrothermal fluid motion in the sensor well.
In this study, we establish the electrochemical sensor platform and the AC electrokinetic process for rapid AST toward acute infection diagnosis. We analyze the effects of electrode thickness on the temperature distribution near the sensor surface and optimize the electrokinetic conditions for molecular detection in blood, which contains a broad spectrum of matrices including erythrocytes, leukocytes, platelets, and clotting factors. We evaluate the applicability of the AC electrokinetics enhanced electrochemical biosensor for rapid pathogen detection and AST without additional sample preparation and nucleic acid extraction steps. Antibiotic resistance profiles of Escherichia coli (E. coli) clinical isolates in blood are studied to demonstrate the applicability of the assay in diagnosis acute bacterial infections.
Material and Method
Reagents and samples
Oligonucleotide probes were obtained from Integrated DNA Technologies (Coralville, IA). The universal (UNI) probe pairs were designed to be complementary to the conserved region of all bacterial 16S rRNA gene sequences. The capture probe UNI798C (5’ TCG TTT ACR GCG TGG ACT ACC A 3’) and detector probe UNI776D (5’ GGG TAT CTA ATC CTG TTT GCT C 3’) were synthesized with 5’ biotin modification and 3’ fluorescein modification, respectively. Uropathogenic E. coli strain EC132 was obtained from the clinical microbiology laboratory at the Veterans Affairs Palo Alto Health Care System (VAPAHCS). EC132 is resistant to ampicillin (AMP) and ciprofloxacin (CIP), and is susceptible to trimethoprim-sulfamethoxazole (SXT).31, 32
Electrochemical sensor array
The electrochemical sensor electrode arrays were fabricated by sputtering 10 – 125 nm gold on plastic substrates. A laser machined plastic manifold containing 16 wells (Universal Laser System Inc. VLS2.30) was bonded to the sensor array for holding 50 µl hybridization buffer in each well. A multi-channel potentiostat (GeneFluidics) was used for amperometric reading of the sensor array. A negative control (i.e., no target) was performed in each chip. The signal-to-noise ratio, which normalizes the uncertainty due to senor preparation and reagent activity, is reported in this study.
Phenotypic Antibiotic Susceptibility Testing
Bacteria were cultured in Mueller Hinton (MH) broth (Becton Dickinson), grown to OD600 0.1 (1 × 108 CFU/ml) and then diluted to different concentrations with a 9-to-1 MH broth to blood ratio. AMP, CIP, and SXT were used in the experiments. Samples with or without antibiotic (AMP 100 µg/ml, CIP 4 µg/ml, and SXT 4/76 µg/ml) were incubated at 37°C in a shaking incubator for 3 hours. Then, 20 µl samples were immediately lysed in 12 µl of 10 mg/ml lysozyme containing 4 mM EDTA, 40 mM Tris-HCl of pH 8.0, and 0.2% Triton X-100. The samples were incubated at room temperature for 5 minutes. Then, 8 µl of 1 M NaOH was mixed with the sample and was followed by additional incubation for 5 minutes at room temperature. Detector probes (50 µl) in 2.5% bovine serum albumin (BSA) (Sigma) and 1 M phosphate buffer of pH 7.4 were added to the bacterial lysate and the mixture was incubated at 37°C for 10 minutes to allow hybridization between the bacterial 16S rRNA and the detector probe. The bacterial lysate-detector probe mixture (50 µl) was loaded on the sensor with 10 minutes incubation at room temperature with or without electrokinetic enhancement. Then, 6 µl of 0.5 U/ml anti-fluorescein HRP Fab fragments (Roche Diagnostics) in 1 M phosphate buffer saline containing 0.5% casein was added to the sensor and incubated at room temperature for 15 minutes. Substrate solution, 3,3′,5,5′-Tetramethylbenzidine (TMB), was added after washing and drying, and the amperometric signal was taken at 60s after applying -200mV in the potentiostat (GeneFluidics). Data are reported as mean ± standard deviation.
Results
Electrokinetic sensor enhancement
AC electrokinetics is implemented to create fluid motion near the sensor surface to facilitate molecular advection and generate Joule heating induced temperature elevation to enhance molecular binding (Figure 2a). Figure 2b shows the electrothermal fluid motion by observing the tracer particles near the working electrode. The fluid near the sensor electrode was moving toward the center of the working electrode and created 3D fluid circulations in the well (Figure 2c). This observation is consistent with our previous studies.20, 24, 25
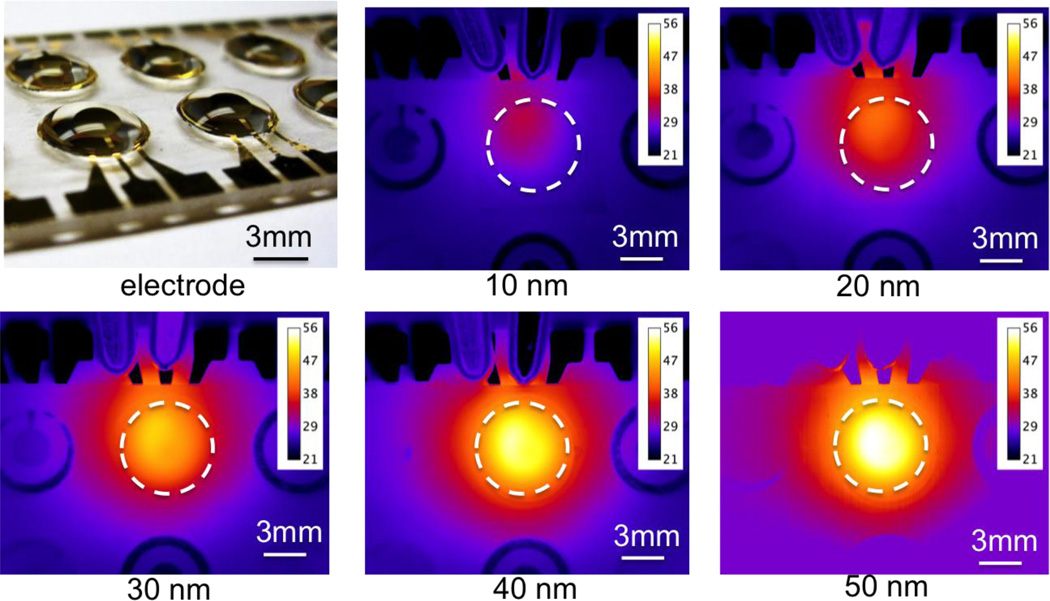
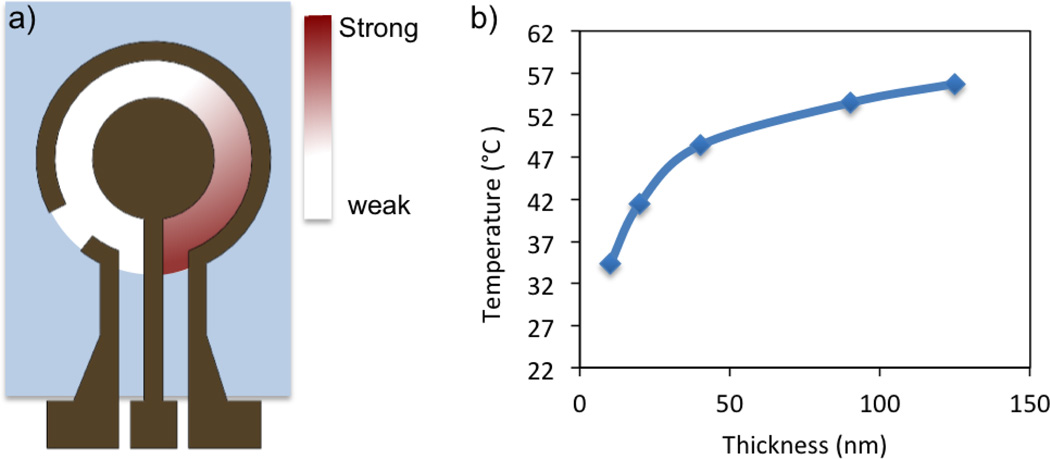
For clinical diagnostics, the resistance of the electrodes can be significant compared to the hybridization buffers and physiological samples, which have high conductivities (on the order of 1 S/m). For a given electrode design, the total resistance of the electrode is determined by the thickness of the electrode. To further optimize the electrokinetic process and improve the reliability of the assay, the local temperature distribution near the sensor surface was characterized by infrared thermometry. We examined the temperature distribution using electrodes with thicknesses of approximately 10 nm, 20 nm, 40 nm, 90 nm and 125 nm, and conductive hybridization buffers (2.5% BSA in 1M phosphate buffer). A square wave potential with a peak to peak magnitude of 6 volts and a frequency of 200 kHz was applied. The temperature of the solution increased with the thickness of electrode (Figure 3). The spatial temperature distribution was also influenced by the electrode thickness. The temperature rise in the 10 nm thick electrode was localized near the connection region of the working and auxiliary electrodes (Figure 3). With the 125 nm thick electrode, the temperature distribution was uniform in the well and maximized at the center of the working electrode. These results suggested the resistance of the electrodes was significant compared to the solution, resulting in non-uniform potential drop along the electrode gap and inhomogeneous heating. In particular, a portion of the energy was consumed for heating the electrode, causing a lower, non-uniform temperature distribution in thin electrodes (Figure 4). The non-uniform electric field distribution can be understood by considering a simple circuit model with a finite resistance in the electrode. Thick electrode layers (e.g., 125 nm or more) should, therefore, be used for electrokinetic sensor enhancement.
Figure 3.
The dependence of the electrode thickness on the temperature distribution in the sensor well. The experiments were performed in buffer solution containing 2.5% BSA and 1M phosphate. Data were collected using an infrared camera after the temperature reached equilibrium. A 200 kHz square wave with 6 volts peak to peak was applied.
Figure 4.
(a) A schematic diagram of the electric field distribution in the sensor well. Heating are concentrated near the connection region of the work and auxiliary electrodes (red color). (b) The maximum temperature in the sensor well as a function of the electrode thickness. The experiments were performed using Tris buffer with 2.5% BSA. A 200 kHz square wave with 6 volts peak to peak was applied.
Blood matrix effects
We then studied the protocol for phenotypic AST with blood culture using the electrochemical sensor. We previously demonstrated electrochemical detection of 16S rRNA for rapid biosensor based AST in clinical urine samples.33 AST was performed with 2.5 hours growth time at a 1:1 ratio of urine and MH broth. The effects of blood culture on biosensor-based AST, however, have not been evaluated. We therefore characterized the condition of rapid AST in blood culture. We observed additional culture time was required for AST in blood culture. For instance, a growth time of 5 hours was required at a 1:1 blood to MH broth ratio to obtain a similar signal level with 3 hours culture in MH broth (Figure 5). The additional culture time required was conceivably due to the matrix effect of blood that reduces the sensitivity of the sensor.34 Furthermore, the bacteria had a lower growth rate in blood compared to culture medium. To minimize the matrix effects on blood culture, we mixed blood sample with MH broth in a 1:9 ratio. AC electrokinetics was also incorporated to improve the sensitivity and decrease the total growth time required.
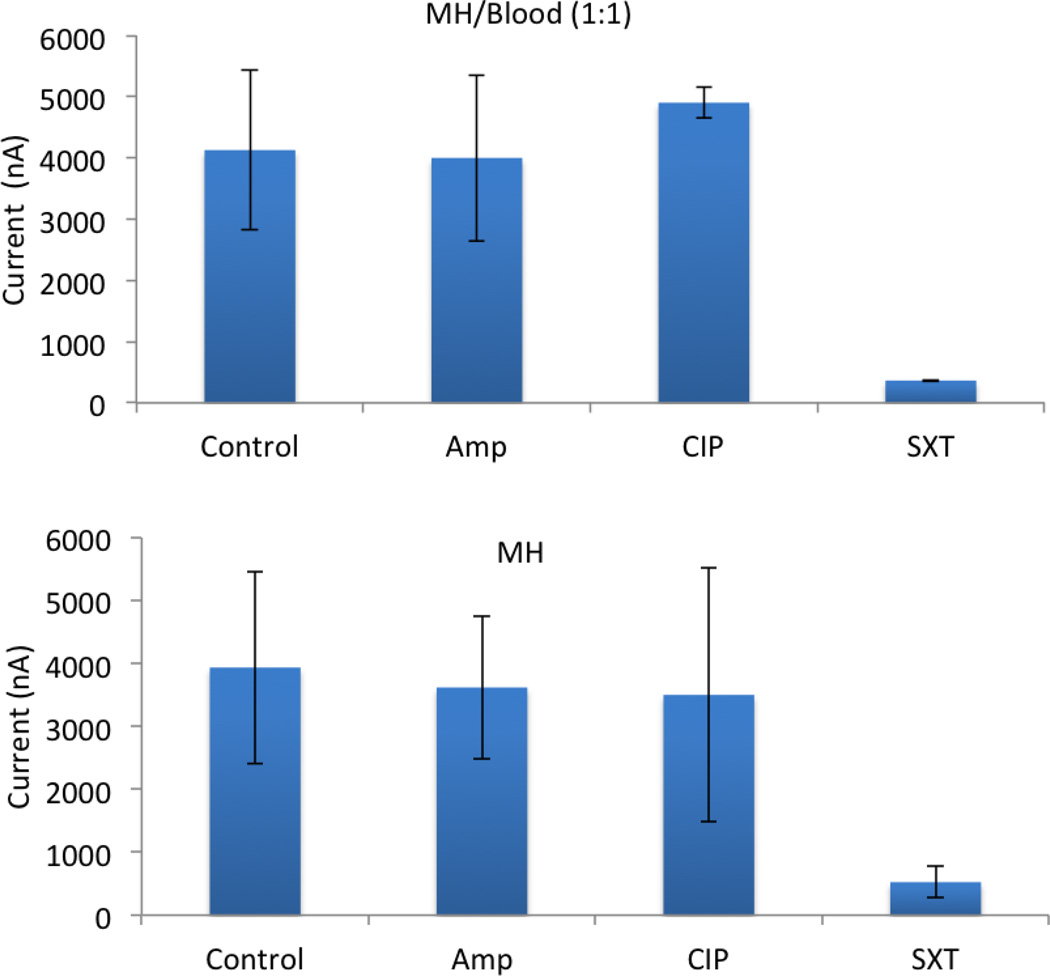
Figure 5.
Antimicrobial susceptibility testing in blood culture and MH broth. EC132 with a starting concentration of 5 × 106 CFU/ml were cultured in (a) blood-MH broth for 5 hours (1:1) and (b) MH broth for 3 hours. The antibiotic resistance profile was determined. X-axis represent different growth conditions: Control = no antibiotics; AMP = ampicillin; CIP = ciprofloxacin; and SXT (trimethoprim/sulfamethoxazole).
Electrokinetic facilitated antimicrobial susceptibility testing
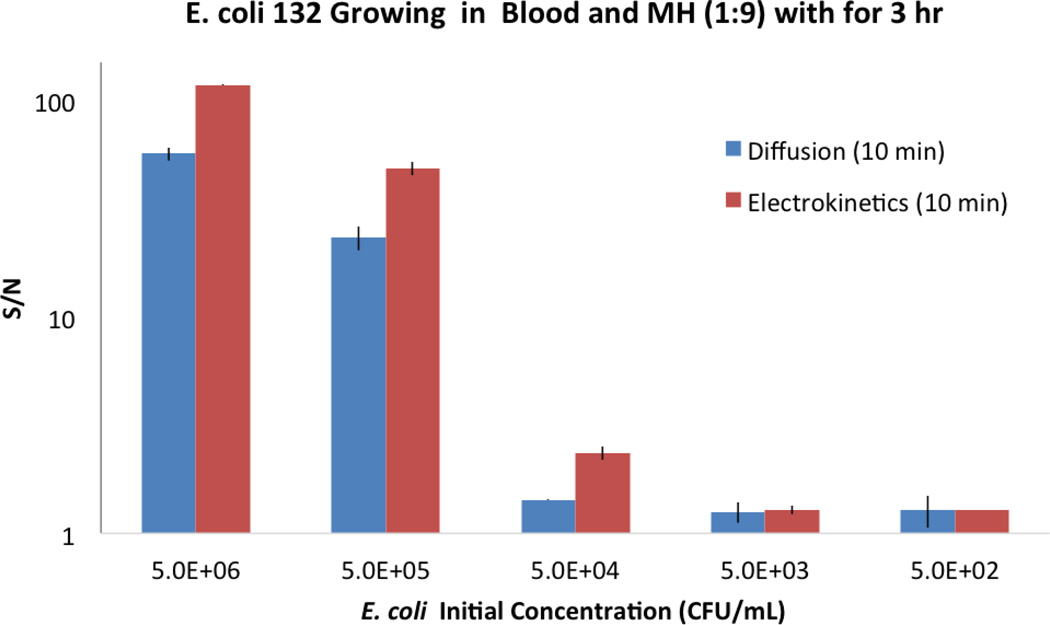
To reduce the matrix effect and improve the overall performance of the assay, we incorporated electrokinetic enhancement in the electrochemical sensor platform. We examined the effects of electrokinetic enhancement on sensor sensitivity with 3 hours of bacteria growth. EC132 was diluted into five different starting concentrations over a 5-log unit range in a mixture of blood and MH broth. The samples were cultured at 37°C. As shown in Fig. 6, electrokinetic enhancement increased the signal-to-noise ratio and improved the limit of the detection. Without electrokinetic enhancement, the limit of detection of the electrochemical assay with 3 hours inoculation time was approximately 105 CFU/ml. With electrokinetic enhancement, the signal-to-noise ratio of the assay was enhanced significantly. The limit of detection of the assay for direct detection in blood culture was improved for at least one order of magnitude to 104 CFU/ml.
Figure 6.
The signal-to-noise (S/N) ratios of the electrochemical assay with electrokinetic enhancement and by diffusion during the hybridization step. EC132 samples with different starting concentrations were cultured in blood-MH broth (1:9) for 3 hours.
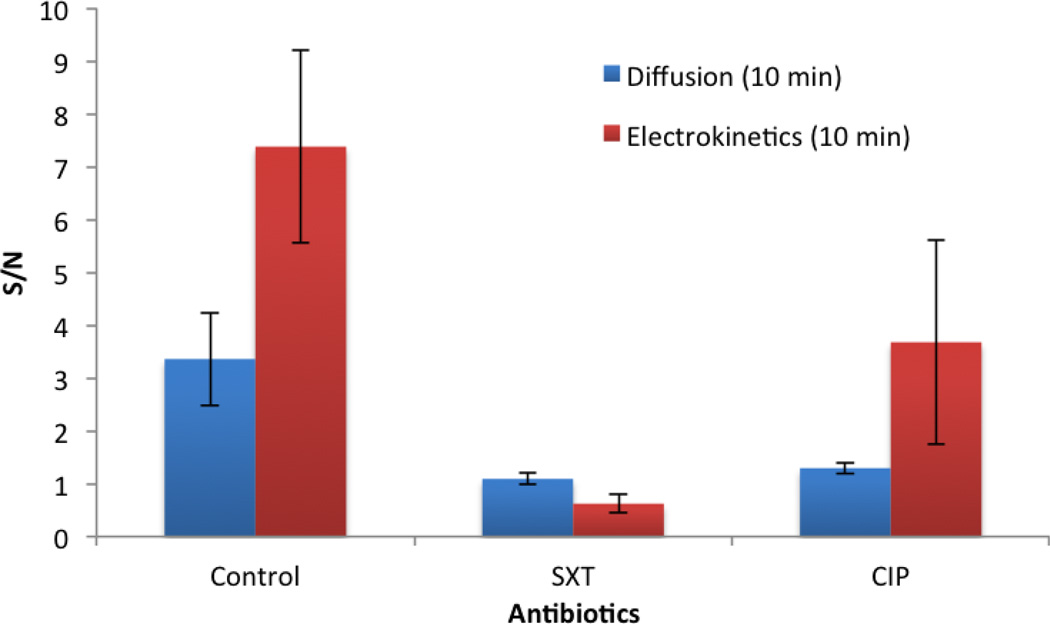
To demonstrate the applicability of the electrokinetics enhanced biosensor for rapid AST in blood culture, we examined the antibiotic resistance profile of EC132 with a starting concentration of 1 × 104 CFU/ml. EC132 is known to be resistant to CIP and susceptible to SXT. Without electrokinetic enhancement, the assay was not able to distinguish the bacterial growth with SXT and CIP due to the low signal-to-noise ratio. To determine the resistance profile of the bacteria, additional culture time or a higher bacteria concentration is required to reach a detectable signal-to-noise level. With AC electrokinetics, the matrix effect was reduced, as indicated by the improved signal-tonoise ratio of the assay. The antibiotic resistant profile was correctly identified after 3 hours of inoculation (Figure 7). These results indicate that AC electrokinetics can increase the limit of detection and decrease total assay time for AST using electrochemical biosensors.
Figure 7.
The signal-to-noise (S/N) ratios of the electrochemical assay with electrokinetic enhancement and by diffusion during the hybridization step. EC132 with the same starting concentration of 1 × 104 CFU/ml were cultured in blood-MH broth (1:9) for 3 hours with or without antibiotics (CIP and SXT).
Discussion
In this study, we investigate the electrokinetic and electrochemical sensing conditions for rapid AST in blood culture. A reproducible and controllable temperature distribution is required to efficiently enhance molecular advection and hybridization. Unregulated heating of the sample will not only reduce the signal from the specific target-probe hybridization but also denature the protein in the sample matrix, which cover the sensor surface. These effects will reduce the overall signal-to-noise ratio. We identified the electrode thickness was a key parameter to control the heating on the sensor surface. The sensing electrode layer was typically very thin (10 ~ 125 nm) and the sample had a high conductivity (on the order of 1 S/m). The resistance of the electrode was significant compared to the resistance of the hybridization buffer. For instance, the resistivities of the gold electrode and the hybridization buffer at 50°C were 2.69×10−8 Ωm and 0.17 Ωm, respectively. For a 10 nm thick electrode in the microwell, the resistance values per unit length of the electrode and the hybridization buffer were both on the order of 103 Ω/m. As a result, the potential dropped along the electrodes due to the finite resistance of the electrode and the heating was localized near the connection region. The heating uniformity was most sensitivity to the electrode thickness for thin electrodes (e.g., 10 nm). With thick electrodes (e.g., > 100 nm), the resistance of the electrode was relatively small (~102 Ω/m) and the thickness effect was minimized, which allowed reproducible and controllable heating for improving the sensor performance.
Blood has one of the most complex matrices, which include various serum proteins, electrolytes and a large number of blood cells, and displays a high viscosity.34 Some matrices, such as serum proteins, can adhere to the sensor surface or binds to the target nonspecifically, which decrease the sensitivity. The high viscosity of blood can also reduce molecular diffusion and the overall binding efficiency. To address this issue, we demonstrated that AC electrokinetic improved the limit of detection of the assay by reducing the matrix effect. In particular, the incorporation of AC electrokinetics created electrohydrodynamic fluid motion and temperature elevation to facilitate molecular advection and target hybridization. The Joule heating induced temperature elevation also reduces the effective viscosity and remove non-specific binding, which typically has a lower affinity. In our probe design, the melting temperature of the probe-target hybridization was approximately 60°C.35 The optimized electrokinetic condition, which resulted in a temperature of ~55°C near the sensor surface, was determined to achieve the maximum signal-to-noise ratio. This value was consistent with our previous study that the hybridization temperature for maximizing the signal to noise ratio was a few degree below the melting temperature.25
The universal probe pairs were applied in this study to measure the concentration of bacteria. The universal probes detected the conserved region of bacterial 16S rRNA and allowed phenotypic AST of bacterial pathogen to be performed without the knowledge of the bacterial species. By applying the appropriate electrokinetic conditions, the required starting concentration was reduced by one-order of magnitude. An average background of 8.45 nA was achieved in negative control represented no significant interspecies contamination. This improvement in sensitivity could also be translated into a reduction in culture time. For a typical E. coli strain, the doubling time is approximately 20 minutes.36, 37 A 10-fold improvement in sensitivity could be equivalent to over 1 hour of bacterial culture time. This electrokinetic enhancement could be more important for slow growing bacteria.
Molecular analysis techniques, such as real-time PCR, are typically faster than conventional phenotypic AST techniques based on culture. These techniques, however, require an additional bacterial isolation and molecular extraction steps to obtain high quality samples for molecular analysis. These sample preparation steps increase the assay time and limit their use in point-of-care diagnostics. The electrochemical sensor platform has a relative high tolerance to sample matrix and provides a promising approach for performing AST in physiological samples, such as blood culture. Another advantage of our approach is that only electronic interfaces are required to apply electrokinetic enhancement and measure the electrochemical sensor signal, which simplifies the system complexity and minimize the cost of the system. The electrochemical biosensor can also be integrated into microfluidic cartridge systems for point-of-care diagnostics.24, 38
Conclusion
In summary, we reported an electrokinetic enhanced biosensor platform for rapid AST. This AST approach combines phenotypic bacterial growth and genotypic molecular detection. The electrochemical sensor directly detects bacterial 16S rRNA in blood culture and AC electrokinetics is demonstrated for improving the sensitivity of the assay by reducing the matrix effect. With further development, the rapid AST approach could be broadly adopted for acute infection diagnosis in various point-of-care settings.
ACKNOWLEDGEMENTS
This work was supported by NIH Health Director's New Innovator Award (1DP2OD007161-01) and NIAID (1U01AI082457-01 and 2R44AI088756-03).
References
- 1.Pinner RW, Teutsch SM, Simonsen L, Klug LA, Graber JM, Clarke MJ, Berkelman RL. Trends in infectious diseases mortality in the united states. Jama-Journal of the American Medical Association. 1996;275:189–193. [PubMed] [Google Scholar]
- 2.Hawkey PM. Pre-clinical experience with daptomycin. J. Antimicrob. Chemother. 2008;62:7–14. doi: 10.1093/jac/dkn367. [DOI] [PubMed] [Google Scholar]
- 3.Daum RS, Ito T, Hiramatsu K, Hussain F, Mongkolrattanothai K, Jamklang M, Boyle-Wang S. A novel methicillin-resistance cassette in community-acquired methicillin-resistant staphylococcus aureus isolates of diverse genetic backgrounds. J. Infect. Dis. 2002;186:1344–1347. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 4.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. Community-acquired methicillinresistant staphylococcus aureus in children with no identified predisposing risk. Jama-Journal of the American Medical Association. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 5.Huttner B, Harbarth S. "Antibiotics are not automatic anymore" - the french national campaign to cut antibiotic overuse. Plos Medicine. 2009:6. doi: 10.1371/journal.pmed.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.An G, Nieman G, Vodovotz Y. Toward computational identification of multiscale "tipping points" in acute inflammation and multiple organ failure. Ann. Biomed. Eng. 2012;40:2414–2424. doi: 10.1007/s10439-012-0565-9. [DOI] [PubMed] [Google Scholar]
- 7.Ingber DE. From cellular mechanotransduction to biologically inspired engineering. Ann. Biomed. Eng. 2010;38:1148–1161. doi: 10.1007/s10439-010-9946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuper KM, Boles DM, Mohr JE, Wanger A. Antimicrobial susceptibility testing: A primer for clinicians. Pharmacotherapy. 2009;29:1326–1343. doi: 10.1592/phco.29.11.1326. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Jones RN, Coll Amer P. Performance accuracy of antibacterial and antifungal susceptibility test methods - report from the college of american pathologists microbiology surveys program (2001–2003) Arch. Pathol. Lab. Med. 2006;130:767–778. doi: 10.5858/2006-130-767-PAOAAA. [DOI] [PubMed] [Google Scholar]
- 10.Yanagihara K, Kitagawa Y, Tomonaga M, Tsukasaki K, Kohno S, Seki M, Sugimoto H, Shimazu T, Tasaki O, Matsushima A, Ikeda Y, Okamoto S, Aikawa N, Hori S, Obara H, Ishizaka A, Hasegawa N, Takeda J, Kamihira S, Sugahara K, Asari S, Murata M, Kobayashi Y, Ginba H, Sumiyama Y, Kitajima M. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Critical Care. 2010:14. doi: 10.1186/cc9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray PR. Determination of the optimum incubation period of blood culture broths for the detection of clinically significant septicemia. Journal of Clinical Microbiology. 1985;21:481–485. doi: 10.1128/jcm.21.4.481-485.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell J, Washington JA. Evaluation of the necessity for routine terminal subcultures of previously negative blood cultures. Journal of Clinical Microbiology. 1980;12:576–578. doi: 10.1128/jcm.12.4.576-578.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74:417. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy SB, Marshall B. Antibacterial resistance worldwide: Causes, challenges and responses. Nature Medicine. 2004;10:S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 15.Mach KE, Wong PK, Liao JC. Biosensor diagnosis of urinary tract infections: A path to better treatment? Trends Pharmacol. Sci. 2011;32:330–336. doi: 10.1016/j.tips.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Lilienfeld-Toal M, Lehmann LE, Raadts AD, Hahn-Ast C, Orlopp KS, Marklein G, Purr I, Cook G, Hoeft A, Glasmacher A, Stuber F. Utility of a commercially available multiplex real-time pcr assay to detect bacterial and fungal pathogens in febrile neutropenia. Journal of Clinical Microbiology. 2009;47:2405–2410. doi: 10.1128/JCM.00491-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldeisen JR, Wang T, Mitra D, Lee LP. A real-time pcr antibiogram for drug-resistant sepsis. Plos One. 2011:6. doi: 10.1371/journal.pone.0028528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen WL, Beuving J, Verbon A, Wolffs PF. One-day workflow scheme for bacterial pathogen detection and antimicrobial resistance testing from blood cultures. J Vis Exp. 2012 doi: 10.3791/3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riahi R, Mach KE, Mohan R, Liao JC, Wong PK. Molecular detection of bacterial pathogens using microparticle enhanced double-stranded DNA probes. Anal. Chem. 2011;83:6349–6354. doi: 10.1021/ac2012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sin MLY, Liu TT, Pyne JD, Gau V, Liao JC, Wong PK. In situ electrokinetic enhancement for self-assembled-monolayer-based electrochemical biosensing. Anal. Chem. 2012;84:2702–2707. doi: 10.1021/ac203245j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tondo EC, Guimarpes MCM, Henriques JAP, Ayub MAZ. Assessing and analysing contamination of a dairy products processing plant by staphylococcus aureus using antibiotic resistance and pfge. Can. J. Microbiol. 2000;46:1108–1114. doi: 10.1139/w00-111. [DOI] [PubMed] [Google Scholar]
- 22.Louie M, Cockerill FR. Susceptibility testing - phenotypic and genotypic tests for bacteria and mycobacteria. Infectious Disease Clinics of North America. 2001;15:1205. doi: 10.1016/s0891-5520(05)70191-4. [DOI] [PubMed] [Google Scholar]
- 23.Sin ML, Mach KE, Wong PK, Liao JC. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Review of Molecular Diagnostics. 2014;14:225–244. doi: 10.1586/14737159.2014.888313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang MX, Mohan R, Lu Y, Liu TT, Mach KE, Sin MLY, McComb M, Joshi J, Gau V, Wong PK, Liao JC. An ac electrokinetics facilitated biosensor cassette for rapid pathogen identification. Analyst. 2013;138:3660–3666. doi: 10.1039/c3an00259d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu T, Sin MLY, Pyne JD, Gau V, Liao JC, Wong PK. Electrokinetic stringency control in self-assembled monolayer-based biosensors for multiplex urinary tract infection diagnosis. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:159–166. doi: 10.1016/j.nano.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson D, Liu XZ, Venditti R, Li DQ, Krull UJ. Electrokinetically based approach for single-nucleotide polymorphism discrimination using a microfluidic device. Anal. Chem. 2005;77:4000–4007. doi: 10.1021/ac050236r. [DOI] [PubMed] [Google Scholar]
- 27.Green NG, Ramos A, Gonzalez A, Castellanos A, Morgan H. Electrothermally induced fluid flow on microelectrodes. Journal of Electrostatics. 2001;53:71–87. [Google Scholar]
- 28.Castellanos A, Ramos A, Gonzalez A, Green NG, Morgan H. Electrohydrodynamics and dielectrophoresis in microsystems: Scaling laws. Journal of Physics D-Applied Physics. 2003;36:2584–2597. [Google Scholar]
- 29.Ramos A, Morgan H, Green NG, Castellanos A. Ac electrokinetics: A review of forces in microelectrode structures. Journal of Physics D-Applied Physics. 1998;31:2338–2353. [Google Scholar]
- 30.Sin MLY, Gau V, Liao JC, Wong PK. Electrothermal fluid manipulation of high-conductivity samples for laboratory automation applications. Jala. 2010;15:426–432. doi: 10.1016/j.jala.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CH, Lu Y, Sin MLY, Mach KE, Zhang DD, Gau V, Liao JC, Wong PK. Antimicrobial susceptibility testing using high surface-tovolume ratio microchannels. Anal. Chem. 2010;82:1012–1019. doi: 10.1021/ac9022764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Gao J, Zhang DD, Gau V, Liao JC, Wong PK. Single cell antimicrobial susceptibility testing by confined microchannels and electrokinetic loading. Anal. Chem. 2013;85:3971–3976. doi: 10.1021/ac4004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mach KE, Mohan R, Baron EJ, Shih MC, Gau V, Wong PK, Liao JC. A biosensor platform for rapid antimicrobial susceptibility testing directly from clinical samples. J. Urol. 2011;185:148–153. doi: 10.1016/j.juro.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu ML, Lawi W, Snyder ST, Wong PK, Liao JC, Gau V. Matrix effects-a challenge toward automation of molecular analysis. Jala. 2010;15:233–242. [Google Scholar]
- 35.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sezonov G, Joseleau-Petit D, D'Ari R. Escherichia coli physiology in luriabertani broth. J. Bacteriol. 2007;189:8746–8749. doi: 10.1128/JB.01368-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingham CJ, van den Ende M, Pijnenburg D, Wever PC, Schneeberger PM. Growth and multiplexed analysis of microorganisms on a subdivided, highly porous, inorganic chip manufactured from anopore. Appl. Environ. Microbiol. 2005;71:8978–8981. doi: 10.1128/AEM.71.12.8978-8981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawi W, Wiita C, Snyder ST, Wei F, Wong D, Wong PK, Liao JC, Haake DA, Gau V. A microfluidic cartridge system for multiplexed clinical analysis. Journal of Association for Laboratory Automation. 2009;14:407–412. doi: 10.1016/j.jala.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]