Abstract
A number of novel oral agents are now approved for use in relapsing multiple sclerosis (MS). Among these agents, teriflunomide has shown promise with respect to clinical efficacy and safety in relapsing MS patients. In this review we aim to clarify the role of teriflunomide in the context of current and emerging MS treatment options by summarizing relevant points on the use of teriflunomide in MS, with a discussion of teriflunomide’s pharmacologic properties, pivotal clinical trials, and safety and tolerability.
Keywords: clinical trials, multiple sclerosis, review, teriflunomide
Introduction
The treatment landscape of relapsing multiple sclerosis (MS) has been changing rapidly in recent years and continues to evolve as novel oral and parenteral therapies become available. A number of oral disease-modifying agents (DMAs) have now been approved for widespread use in relapsing MS [Marriott and O’Connor, 2010]. Among the novel oral DMAs, teriflunomide has demonstrated clinical efficacy and safety in relapsing MS patients. In this review we aim to clarify the role of teriflunomide in the context of current and emerging MS treatment options by summarizing relevant points on the use of teriflunomide in MS, with a discussion of teriflunomide’s pharmacologic properties, clinical trials, and safety and tolerability.
Mechanism of action in MS
Teriflunomide is the active metabolite of the parent drug, leflunomide, which has been approved by the US Food and Drug Administration (FDA) for use in the treatment of rheumatoid arthritis (RA) since 1998 (Figure 1) [Osiri et al. 2003]. Leflunomide is rapidly converted almost entirely into teriflunomide following oral ingestion and thus teriflunomide has become the focus of development for use in patients with MS.
Figure 1.
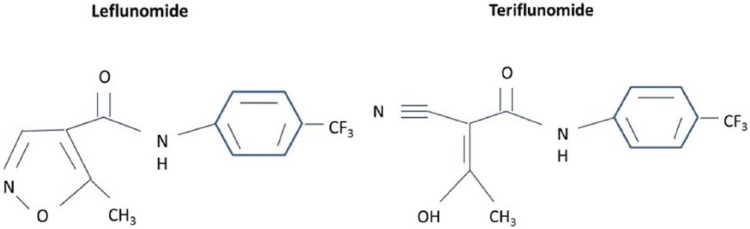
Related chemical structures of leflunomide and teriflunomide.
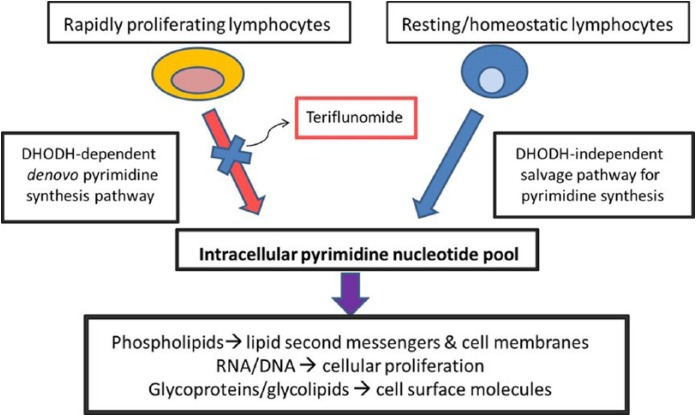
The precise mechanisms by which teriflunomide exerts its beneficial effects in MS are incompletely understood. Teriflunomide acts primarily as an inhibitor of dihydroorotate-dehydrogenase (DHODH), a key mitochondrial enzyme involved in the de novo synthesis of pyrimidines in rapidly proliferating cells such as T lymphocytes and B lymphocytes, thereby diminishing the inflammatory response to auto-antigens. Noteworthy is that blockade of this pathway does not affect homeostatically proliferating or resting hematopoietic cell lines since an alternate ‘salvage pathway’ that is independent of the DHODH exists [Bruneau et al. 1998; Cherwinski et al. 1995; Fox et al. 1999; Ruckemann et al. 1998]. In addition to DNA and RNA synthesis, pyrimidines are involved in numerous other cellular functions including phospholipid synthesis, protein and lipid glycosylation [Herrmann et al. 2000], and DNA strand repair [Fairbanks et al. 1995], which together lead to a variety of downstream immunomodulatory effects (Figure 2).
Figure 2.
Teriflunomide: mechanism of action.
Modified from Tallantyre et al. (2008), Gold and Wolinsky (2011).
DHODH, dihydroorotate-dehydrogenase.
Teriflunomide may have additional effects independent of DHODH inhibition, including inhibition of protein tyrosine kinases [Siemasko et al. 1998; Mattar et al. 1993] and cyclo-oxygenase-2 [Hamilton et al. 1999], which may contribute to its observed immunomodulatory effects in MS. In vitro studies have demonstrated that, in the presence of teriflunomide, exogenous reconstitution of pyrimidines will restore immune cell proliferation [Elder et al. 1997] but other cellular functions such as cell surface molecular expression, cytokine production and cellular migration remain impaired. These observations suggest that teriflunomide has immunological effects outside of its ability to inhibit pyrimidine synthesis in rapidly proliferating cells [Korn et al. 2001, 2004].
Pharmacokinetics
The oral bioavailability of teriflunomide is 100% and peak plasma levels are achieved within 1–2 hours of intake. Food intake, age, sex or hepatic impairment do not affect teriflunomide’s pharmacokinetics, although concomitant food ingestion can impair initial absorption [Limsakun, 2010]. Teriflunomide is almost entirely protein-bound in plasma (>99%) and demonstrates linear pharmacokinetics at a dose range of 5-25 mg/day, with a mean plasma half-life ranging between 10-18 days, with steady state levels attained within 20 weeks. Drug clearance is through a combination of biliary and renal mechanisms. Wash-out procedures using activated charcoal or cholestyramine can aid in the clearance of teriflunomide [Limsakun and Menguy-Vacheron, 2010; Tallantyre et al. 2008].
Clinical trials of teriflunomide: phase II trials
Teriflunomide was first shown to be effective in MS in a phase II ‘proof of concept’ study that was multicenter, randomized, placebo-controlled and double-blind. This trial included 179 relapsing MS patients (relapsing-remitting MS or progressive MS with superimposed relapses). Inclusion criteria consisted of patients between 18 and 65 years of age, Expanded Disability Status Scale (EDSS) ≤6.0 and presence of two relapses in the past 3 years, one which had taken place in the year prior to study enrollment. Patients were randomized to one of three study arms: placebo; teriflunomide 7 mg daily; or teriflunomide 14 mg daily. The primary outcome was the mean number of combined unique active lesions (CUALs) on magnetic resonance imaging (MRI) defined as either newly/persistently gadolinium-enhancing T1 lesions (T1-Gd) or new/enlarging T2 hyperintense lesions. Secondary endpoints included a number of additional MRI measures and clinical measures of disease activity including relapse frequency and disability progression. The treatment took place over a total of 36 weeks and participants underwent MRI scans every 6 weeks.
Both doses of teriflunomide demonstrated a significant relative decrease in MRI activity compared with placebo, including fewer CUALs, T1-Gd lesions and new/enlarging T2 lesions. Specifically, the median number of CUALs per scan compared with placebo over the study period was 0.5 versus 0.2 versus 0.3 in the placebo, teriflunomide 7 mg (p < 0.03 versus placebo) and teriflunomide 14 mg groups (p < 0.01 versus placebo), respectively. The treatment effect on the primary endpoint was seen as early as 6 weeks, reached significance by 12 weeks, and was sustained throughout the study duration [O’Connor et al. 2006].
Although this study was not powered to assess clinical outcomes, there was a trend towards a greater proportion of patients remaining relapse-free in the high-dose teriflunomide group compared with placebo (77% versus 62%, p = 0.098). Annualized relapse rates (ARR) in both teriflunomide treatment groups were numerically lower compared with placebo, but the difference did not reach statistical significance [O’Connor et al. 2006]. Furthermore, the proportion of patients with an increase in EDSS compared with baseline was significantly lower in the high-dose teriflunomide group compared with placebo, with a relative risk reduction of 69% (p < 0.04).
An open-label extension of the phase II teriflunomide trial is ongoing. An interim analysis followed 147 patients for a median duration of 7.1 years, with a maximum follow up of 8.5 years. Patients previously enrolled in one of the teriflunomide treatment arms continued on their original assigned dose (7 or 14 mg), while those in the placebo arm were re-allocated to one of the two doses of teriflunomide. The primary objective was to evaluate the long-term safety of teriflunomide in relapsing MS patients, while the secondary objective was to assess long-term clinical efficacy. An interim analysis of this open-label extension found that teriflunomide showed a favorable safety and tolerability profile and sustained clinical efficacy. The ARR in the study population remained low and there was minimal disability progression. In addition, there was suggestion of a dose-dependent benefit on a spectrum of MRI measures including T2 burden of disease, cerebral volume, new/enlarging T2 lesions and newly active lesions. Taken together, this study demonstrated that the beneficial clinical and radiological effects of teriflunomide observed in the phase II trial are maintained on a long-term basis. To date, this study provides the longest follow-up data of any existing oral DMA in MS. The long-term extension phase of this study is ongoing [Confavreux et al. 2012].
Two phase II studies assessing the value of teriflunomide as add-on therapy to first-line injectable DMAs have also been conducted [Freedman et al. 2010, 2012].
Teriflunomide as add-on therapy to interferon-β (IFN-β) was assessed in a multicenter, randomized, placebo-controlled, double-blind clinical trial. A total of 118 patients on a stable dose (>26 weeks) of any of the available formulations of IFN-β (IFN-β-1a, IFN-β-1b) with relapsing MS (relapsing–remitting MS and relapsing forms of progressive MS) were assigned in a 1:1:1 ratio to placebo, teriflunomide 7 mg, or teriflunomide 14 mg in addition to IFN-β. The study treatment period was 24 weeks, with an optional 24 week extension phase [Freedman et al. 2012]. Both doses of teriflunomide as add-on treatment demonstrated reduced MRI activity compared with IFN-β alone, with relative risk reductions of 84.6% (p = 0.0005) and 82.8% (p < 0.0001) in the number of T1-Gd lesions in the 7 mg and 14 mg groups, respectively. There was a corresponding relative reduction in T1-Gd lesion volume of 72.1% (p = 0.11) and 70.6% (p = 0.02) in the 7 mg and 14 mg treatment arms compared with IFN-β alone. There was a trend towards a reduction in ARR of 32.6% (p = 0.10) when comparing the 14 mg treatment arm with placebo. Finally, a post hoc subgroup analysis suggested that in patients with more active disease at baseline (those who had at least one relapse in the previous year or T1-Gd lesions at baseline) had a more pronounced treatment effect with teriflunomide add-on treatment compared with those with less active disease at baseline. This finding is of interest as it implies that there may be a subgroup of patients that would benefit more substantially from teriflunomide add-on therapy than others [Freedman et al. 2012]. A phase III clinical trial (TERACLES) was initiated to address this question more definitively, but was subsequently discontinued due to recruitment challenges and the changing landscape of MS therapies, which made it unlikely that patients would prefer combination therapy consisting of an oral and injectable DMA. In addition, combining two DMAs would likely be cost prohibitive in the real-world setting [ClinicalTrials.gov identifier: NCT01252355].
The utility of teriflunomide as add-on therapy to glatiramer acetate (GA) has been assessed in a multicenter, randomized, placebo-controlled, double-blind clinical trial of 123 relapsing MS patients on a stable dose of GA (>26 weeks). The primary objective was to evaluate the safety of teriflunomide as add-on therapy to GA, and the secondary objectives were to evaluate treatment effects based on MRI measures and clinical activity. Patients were randomized in a 1:1:1 fashion to placebo, teriflunomide 7 mg daily, or teriflunomide 14 mg daily in addition to GA for a treatment period of 24 weeks, with an optional 24 week extension phase. Teriflunomide as add-on therapy to GA showed acceptable safety and tolerability, and possibly improved disease control based on MRI measures. Specifically, compared with GA alone, there was an observed decrease in the number of T1-Gd lesions (p = 0.03) in the 7 mg teriflunomide add-on treatment group and a decrease in the volume of T1-Gd lesions in the 14 mg teriflunomide add-on treatment group (p = 0.04). However, further study is necessary to more definitely assess the safety and clinical benefit of teriflunomide as add-on therapy to GA [Freedman et al. 2010].
A 24-week extension of both phase II add-on trials of teriflunomide to either IFN-β or GA has been completed, with results pending [ClinicalTrials.gov identifier: NCT00811395].
Table 1 presents a summary of phase II clinical trials of teriflunomide.
Table 1.
Phase II clinical trials of teriflunomide in multiple sclerosis.
| Clinical trial name (ClinicalTrials.gov identifier) | Study phase/ design | Study participants | Study arms | Treatment period | Primary/key secondary outcomes | Results |
|---|---|---|---|---|---|---|
| Safety and efficacy of teriflunomide in MS with relapses (NCT01487096) |
Phase IIMulticenter, randomized, placebo-controlled, double-blind, parallel group | Relapsing MSn = 179 | - Placebo - Teriflunomide 7 mg - Teriflunomide 14 mg |
36 weeks | Primary: CUALs per MRISecondary: other MRI outcomes, relapse frequency, disability progression, safety and tolerability | - Decrease in CUALs in both Rx groups - Decrease in other MRI outcomes in both Rx groups - Decrease in disability progression in 14 mg Rx group, trend towards a decrease in 7 mg group - Well-tolerated |
| Pilot study of teriflunomide as adjunctive therapy to IFN-β in subjects with MS (NCT00489489) |
Phase IIMulticenter,randomized, placebo-controlled, double-blind, parallel group | Relapsing MS on stable dose of IFN-β (>26 weeks)n = 118 | - IFN-β + placebo - IFN-β + teriflunomide 7 mg - IFN-β + teriflunomide 14 mg |
24 weeks | Primary: number of patients with adverse events, clinically significant abnormalities Secondary: ARR, MRI outcomes |
- Well-tolerated - Reduced number of T1-Gd lesions both Rx groups - T1-Gd lesion volume reduced in 14 mg Rx groups - Trend towards reduced ARR in high-dose Rx group |
| Pilot study of teriflunomide as adjunctive therapy to GA in subjects with MS (NCT00475865) |
Phase IIMulticenterrandomized, placebo-controlled, double-blind, parallel group | Relapsing MS on stable dose of GA (>26 weeks)n = 123 | - GA + placebo - GA + teriflunomide 7 mg - GA + teriflunomide 14 mg |
24 weeks | Primary: number of patients with adverse eventsSecondary: ARR, MRI outcomes, fatigue | - Acceptable safety - Decrease in T1-Gd lesion count in 7 mg Rx group - Decrease in T1-Gd volume in 14 mg Rx group |
| Long-term safety of teriflunomide when added to IFN-β or GA in patients with MS (NCT00811395) |
Phase IIMulticenterrandomized, placebo-controlled, double-blind, parallel group | Relapsing MS with completion of phase II IFN-β or GA add-on studiesn = 182 | - IFN-β + placebo - IFN-β + teriflunomide 7 mg - IFN-β + teriflunomide 14 mg - GA + placebo - GA + teriflunomide 7 mg - GA + teriflunomide 14 mg |
24 weeks | Primary: number of patients with adverse eventsSecondary: ARR, disability progression, MRI outcomes | - Pending |
| Long-term safety and efficacy of teriflunomide (HMR1726) in MS with relapses (NCT00228163) |
Phase IIMulticenter, randomized,open-labelparallel group | Relapsing MS with completion of phase II monotherapy study n = 180 (estimated); n = 147 (interim) |
- Teriflunomide 7 mg daily - Teriflunomide 14 mg daily |
528 weeks | Primary: number of patients with adverse eventsSecondary: ARR, disability accumulation (EDSS, MSFC), MRI outcomes, QOL, fatigue | Interim results: Favorablesafety profile - low annualized relapse rates- minimal disability progression - dose-dependent benefit with high-dose Rx for several MRI outcomes |
| Study to investigate the immune response to influenza vaccine in patients with MS on teriflunomide (TERIVA) (NCT01403376) |
Phase IIMulticenter, multinational, parallel-group | Relapsing MS treated for ≥6 months with: teriflunomide 7 mg or 14 mg, stable dose of IFN-β | - Teriflunomide 7 mg + influenza vaccine - Teriflunomide 14 mg + influenza vaccine - IFNβ + influenza vaccine |
28 days | Primary: proportion of patients who achieved seroprotection to influenza vaccine strains H1N1, H3N2 and B at 28 days postvaccination | - MS patients treated with teriflunomide mounted effective immune responses to the seasonal influenza vaccination - As expected, MS patients in the reference IFN-β group mounted an effective immune response to influenza vaccine - No new safety concerns identified in patients treated with teriflunomide following influenza vaccination |
ARR, annualized relapse rate; CUAL, combined unique active lesions; EDSS, Expanded Disability Status Scale; GA, glatiramer acetate; IFN-β, interferon-beta; MRI, magnetic resonance imaging; MS, multiple sclerosis; MSFC, Multiple Sclerosis Functional Composite; QOL, quality of life; Rx, treatment; T1-Gad, gadolinium-enhancing lesions on T1 weighted sequence on MRI.
Clinical trials of teriflunomide: phase III trials
TEMSO (Teriflunomide Multiple Sclerosis Oral trial) was the first phase III clinical trial to assess the efficacy of teriflunomide in MS patients. TEMSO was a multicenter, randomized, placebo-controlled, double-blind study with the primary objective of assessing the clinical efficacy of teriflunomide in relapsing MS patients. Enrolled patients had relapsing MS, were between the ages of 18 and 55 years old, had EDSS scores ≤5.5, and at least 2 clinical relapses in the preceding 2 years or at least 1 relapse in the previous year. A total of 1088 patients were randomly assigned in a 1:1:1 ratio to placebo, teriflunomide 7 mg daily or teriflunomide 14 mg daily. The study treatment period was 108 weeks. The primary endpoint of the trial was the ARR. Secondary endpoints consisted of: sustained disability progression; various MRI measures of disease activity including total lesion volume, number of unique active lesions, T1-Gd lesions, T1-hypointense lesions and brain atrophy; and fatigue [O’Connor et al. 2011].
Patients in either teriflunomide treatment group had diminished ARR compared with placebo (relative risk reductions of 31.2% and 31.5% in the 7 mg and 14 mg groups, respectively, p < 0.001). In addition, both treatment arms had diminished proportions of patients with 12-week confirmed disability progression compared with the placebo arm (27.3%, 21.7%, 20.2% for placebo; log-rank p-value 7 mg p = 0.08, 14 mg p = 0.03). Both teriflunomide treatment arms had improved MRI-related measures of disease activity compared with placebo. Specifically, in comparisons of the 7 mg and 14 mg teriflunomide treatment groups with placebo, the change in total lesion volume was significantly lower (p = 0.03 and p < 0.001, respectively); there were fewer T1-Gd lesions per MRI scan (p < 0.001 for both comparisons) and there were fewer unique active lesions per scan (p < 0.001 for both comparisons). Changes in brain atrophy were not significantly different among the three study arms. There were no differences among study arms in change in fatigue compared with baseline (as measured by the fatigue impact scale) [O’Connor et al. 2011].
TEMSO confirmed clinical findings from the previous phase II trial, in addition to the safety and tolerability of teriflunomide. There is an on-going open-label extension of TEMSO, which will continue for a duration of 108 weeks or longer [ClinicalTrials.gov identifier: NCT00803049]. A recent analysis of patients who had received teriflunomide for up to 9 years during TEMSO and the extension period found that clinical and MRI disease activity remained low during the extension period, suggesting that the efficacy of teriflunomide is maintained for up to 9 years [Freedman et al. 2013].
A recent post hoc analysis of TEMSO demonstrated that teriflunomide reduced relapses leading to hospitalization [by 36% in the 7 mg group (p = 0.015) and 59% in the 14 mg group (p < 0.0001)] and intravenous corticosteroid use versus placebo [29 % (p = 0.001); 34 % (p = 0.0003] and that teriflunomide-treated patients spent fewer nights in hospital for relapses (p < 0.01). Furthermore, teriflunomide reduced the annualized rate of all hospitalizations (p = 0.01) and emergency room visits (p = 0.004), suggesting that teriflunomide has an impact on reducing healthcare costs [O’Connor et al. 2013].
TOWER (Teriflunomide Oral in People With Relapsing Remitting Multiple Sclerosis) was the second phase III trial which evaluated the efficacy and safety of teriflunomide in patients with relapsing MS. TOWER was a multicenter, randomized, placebo-controlled, double-blind study that enrolled patients had relapsing MS, were between the ages of 18 and 55 years, had EDSS scores ≤5.5, and at least 2 clinical relapses in the preceding 2 years or at least one relapse in the previous year. A total of 1169 patients were randomly assigned in a 1:1:1 ratio to placebo, teriflunomide 7 mg daily or teriflunomide 14 mg daily. Treatment duration was variable and ended 48 weeks after the last patient was recruited. The primary endpoint was ARR, while the key secondary endpoint was time to sustained accumulation of disability. There were no MRI endpoints.
TOWER showed that in 1169 randomized patients with relapsing MS there was a significant decrease in ARR in both the 7 mg and 14 mg teriflunomide treatment arms [22.3% (p = 0.02) and 36.3% (p < 0.0001), respectively). In the 14 mg treatment arm, there was a 31.5% reduction in the risk of 12-week sustained disability accumulation compared with placebo (p = 0.04), while there was no statistically significant difference in the 7 mg treatment arm [Confavreux et al. 2014].
A recent pooled analysis of the clinical efficacy of teriflunomide in the pivotal clinical trials (the phase II clinical trial, plus TEMSO and TOWER), which collectively amounts to over 2500 patient-years of teriflunomide exposure, was consistent with results observed in the individual studies. Furthermore there was no safety signal of concern. These results provide further evidence for teriflunomide’s efficacy and safety [Leist et al. 2013].
TENERE (Teriflunomide and Interferon Beta-1a in Patients with Relapsing Multiple Sclerosis) is a phase III trial that aimed to compare the efficacy of two doses of teriflunomide against IFNβ-1a (Rebif) in relapsing MS patients. A composite outcome of ‘treatment failure’ was utilized as the primary outcome measure, which was defined as the first occurrence of confirmed relapse or permanent treatment discontinuation for any cause. At 48 weeks, there was no statistical superiority of either dose of teriflunomide over Rebif in the primary endpoint of treatment failure (33%, 36%, 37% in the teriflunomide 7 mg, 14 mg, and Rebif groups). In addition, the 14 mg teriflunomide group and IFNβ-1a treatment group were numerically similar with respect to ARR (0.259 versus 0.216, respectively, p = 0.60), while the 7 mg teriflunomide group had a higher ARR (0.410, p = 0.03), suggesting that high-dose teriflunomide treatment may be similar to IFNβ-1a with respect to clinical efficacy measures in MS. One important issue that needs to be taken into account when considering the results of this study is that a higher proportion of patients in the Rebif arm were required to permanently discontinue the study (as per protocol) due to adverse events (AEs) compared with the teriflunomide treatment arms. These discontinuations were partly related to elevation in liver enzymes greater than three times the upper limit of normal. The study protocol was thus quite different from what is typically done in the clinical setting with patients on Rebif and liver enzyme elevations where most patients would have continued on the medication, since most of these observed laboratory abnormalities resolve spontaneously or with dose reduction. As a result, the real-world validity of the results observed in TENERE are unclear [Vermersch et al. 2014].
TOPIC (Teriflunomide Versus Placebo in Patients With First Clinical Symptom of Multiple Sclerosis) is a recently completed phase III clinical trial that evaluated the efficacy of teriflunomide in preventing conversion to clinically definite MS in patients with first demyelinating events suggestive of MS (clinically isolated syndrome, CIS). TOPIC was a multicenter, randomized, placebo-controlled, double-blind study that enrolled 618 patients with CIS who were randomly assigned to placebo, teriflunomide 7 mg daily or teriflunomide 14 mg daily. TOPIC ended early as the 2010 revised diagnostic criteria for MS allowed an earlier diagnosis of clinically definite MS (CDMS) in the study population. At 108 weeks, teriflunomide at the 14 mg dose decreased the risk of conversion to CDMS by 42.6% versus placebo (p = 0.0087) based on the hazard ratio calculated from a Cox proportional hazards model. Furthermore, teriflunomide 14 mg also reduced the risk of another relapse or new MRI lesion by 34.9% (p = 0.0003) compared with placebo. Similarly, teriflunomide at 7 mg decreased conversion to CDMS by 37.2% versus placebo (p = 0.0271) and the risk of recurrent relapse or new MRI lesion formation by 31.4% versus placebo (p = 0.0020) [Miller et al. 2013].
Table 2 presents a summary of phase III clinical trials of teriflunomide.
Table 2.
Phase III clinical trials of teriflunomide in multiple sclerosis.
| Clinical trial name (ClinicalTrials.gov identifier) | Study phase/ design | Study participants | Study arms | Treatment period | Primary/secondary outcomes | Results |
|---|---|---|---|---|---|---|
| Study of teriflunomide in reducing the frequency of relapses and accumulation of disability in patients with MS (TEMSO) (NCT00134563) |
Phase IIIMulticenter,randomized, placebo-controlled, double-blind, parallel group | Relapsing MSn = 1088 | - Placebo - Teriflunomide 7 mg - Teriflunomide 14 mg |
108 weeks | Primary: ARRSecondary: disability progression, fatigue, MRI outcomes | - Decreased ARR in both Rx groups - Trend towards decreased disability progression in 7 mg Rx group - Decreased disability progression in 14 mg Rx group - Improved MRI outcomes in both Rx groups (possible dose-effect) |
| An efficacy study of teriflunomide in patients with relapsing MS (TOWER) (NCT00751881) |
Phase IIIMulti-center, randomized, placebo-controlled, double-blind,parallel group | Relapsing MSn = 1110 (estimated) | - Placebo - Teriflunomide 7 mg - Teriflunomide 14 mg |
48–202 weeks | Primary: ARRSecondary: time to disability progression, change in fatigue, change in health status | - Decreased ARR in both Rx groups - Decreased disability progression in 14 mg Rx group versus placebo - No effect on disability progression in 7 mg Rx group |
| A study comparing the effectiveness and safety of teriflunomide and IFN-β-1a in patients with relapsing MS (TENERE) (NCT00883337) |
Phase IIIMulticenter, randomized,rater-blinded,parallel group | Relapsing MSn = 300 (estimated) | - IFNβ-1a 44 µg subcutaneous TIW - Teriflunomide 7 mg - Teriflunomide 14 mg |
48–174 weeks | Primary: time to failure (relapse or treatment discontinuation)Secondary: ARR, fatigue, subject satisfaction | Preliminary results: - No statistical superiority of Rebif over either teriflunomide group in time to Rx failure - ARR not different in 14 mg teriflunomide group and IFNβ-1a group |
| Long term safety and efficacy study of teriflunomide 7 mg or 14 mg in patients with relapsing-remitting MS(TEMSO) (NCT00803049) |
Phase IIIMulticenterrandomized,double-blind, parallel group | Relapsing MS with completion of TEMSOn = 1080 (estimated) | - Teriflunomide 7 mg - Teriflunomide 14 mg |
288 weeks | Primary: number of patients with adverse eventsSecondary: disability progression, ARR, MRI outcomes | Interim results: - Clinical and MRI disease activity remained low for up to 9 years |
| Efficacy and safety of teriflunomide in patients with relapsing MS and treated with IFN-β (TERACLES) (NCT01252355) |
Phase IIIMulticenter, randomized, placebo-controlled, double-blind,parallel group | Relapsing MS on stable dose of IFN-β (>6 months), with disease activityn = 1455 (estimated) | - IFN-β + placebo - IFN-β + teriflunomide 7 mg - IFN-β + teriflunomide 14 mg |
48–152 weeks | Primary: ARRSecondary: MRI-outcomes, disability progression, time to relapse, proportion relapse-free, fatigue, health status, hospitalization due to relapse | Terminated |
| Phase III study with teriflunomide versus placebo in patients with first clinical symptom of MS (TOPIC) (NCT00622700) |
Phase IIIMulticenter, randomized, placebo-controlled, double-blind, parallel group | First clinical episode suggestive of MS within 90 days of randomization and MRI with >2 lesions characteristic of MSn = 618 | - Placebo - Teriflunomide 7 mg - Teriflunomide 14 mg |
108–300 weeks | Primary: conversion to CDMS by relapseSecondary: conversion to CDMS by MRI criteria, ARR, disability progression, MRI outcomes, fatigue | - Decreased risk of conversion to CDMS in both Rx groups - Decreased risk of recurrent relapse, formation of MRI lesions in both Rx groups |
ARR, annualized relapse rate; CDMS, clinically definite multiple sclerosis; IFN-β, interferon-beta; MRI, magnetic resonance imaging; MS, multiple sclerosis; Rx, treatment; TBA, to be announced; TIW, three times a week.
Safety and tolerability
Teriflunomide is generally a well-tolerated drug, with predominately mild to moderate AEs and only rare serious adverse events (SAEs).
In the phase II clinical trial, there was a similar incidence of AEs across the placebo and teriflunomide arms. AEs more commonly reported in teriflunomide-treated patients included: alopecia, nausea, alanine aminotransferase (ALT) increase, paresthesias, back and limb pain, diarrhea and arthralgia. Observed SAEs included elevated liver function tests (LFTs), hepatic dysfunction, neutropenia, rhabdomyolysis and trigeminal neuralgia; and the incidence of these events was similar across the placebo and treatment arms. No significant differences were observed in numbers of patients with significantly abnormal laboratory tests across treatment arms, but there was a higher frequency of AEs leading to study withdrawal in the high-dose teriflunomide treatment arm [O’Connor et al. 2006].
The subsequent open-label extension (with up to 8.5 years follow up) of the phase II clinical trial confirmed the safety and tolerability profile observed in the double-blind phase of the study. The incidence of AEs was similar across teriflunomide treatment arms, with the exception of oral herpes infection, which was more common in the high-dose teriflunomide arm. Furthermore, the number of SAEs was similar across both doses of teriflunomide. Increases in ALT (<3 times the upper limit of normal) were commonly observed at both doses of teriflunomide (64.2% and 62.1% for 7 mg and 14 mg, respectively), but none of these increases in ALT were symptomatic, and in those patients with significant increases, laboratory value normalization occurred within 2 months of treatment discontinuation in the majority of cases. There was a higher incidence of leukopenia observed in the high-dose teriflunomide treatment arm (3.7% and 18.2% seen in the 7 mg and 14 mg groups, respectively), but the magnitude of the decrease in white blood cell (WBC) count was low and did not lead to treatment discontinuation in any of the cases [Confavreux et al. 2012].
In the reported open-label extension of the phase II clinical trial [Confavreux et al. 2012], there have been no reports of serious opportunistic infections or hypersensitivity reactions with teriflunomide use. The incidence of malignancy was similar to population-based estimates, with no pattern suggestive of malignancy due to immunosuppression. There was a single death in the reported open-label extension; however, an extensive safety evaluation determined that this death was difficult to attribute solely to teriflunomide, and that concurrent medical conditions and medications likely contributed [Confavreux et al. 2012].
The safety and tolerability of teriflunomide in TEMSO was reassuringly similar to what was observed in the phase II clinical trial and open-label extension, without the emergence of any new safety concerns [O’Connor et al. 2011]. Specifically, the incidence of AEs (87.5%, 89.1% and 90.8% in placebo, 7 mg teriflunomide and 14 mg teriflunomide groups, respectively) and SAEs (12.8%, 14.1% and 15.9% in placebo, 7 mg teriflunomide and 14 mg teriflunomide groups, respectively), were similar across treatment arms. AEs leading to study discontinuation (8.1%, 9.8%, and 10.9%) were also similar across treatment arms. AEs seen more frequently with teriflunomide treatment versus placebo, and associated with a dose effect included hair thinning/decreased hair density, nausea, diarrhea and elevated ALT levels (elevated ALT levels seen in 54.0% of teriflunomide 7 mg patients, 57.3% of teriflunomide 14 mg patients and 35.9% of placebo-treated patients). Reductions in neutrophil and lymphocyte counts were generally small in magnitude, but were slightly more marked in the teriflunomide 14 mg arm compared with the teriflunomide 7 mg and placebo arms but showed stabilization within 3 months. In rare individuals with moderate neutropenia (n = 3), there was spontaneous resolution despite continued treatment with teriflunomide in two, while one patient showed resolution with teriflunomide discontinuation. Small increases in blood pressure were seen more commonly in both teriflunomide treatment arms compared with placebo (+3 mmHg systolic in teriflunomide arms versus -1 mmHg in placebo). No deaths or serious opportunistic infections were observed [O’Connor et al. 2011].
Likewise, in the second phase III trial, TOWER [Confavreux et al. 2014], the safety profile of teriflunomide was similar, with the most common AEs reported being headache, elevated ALT level and alopecia. The incidence of AEs and SAEs were similar across study groups, while the incidence of AEs leading to treatment discontinuation was increased in teriflunomide treatment arms versus placebo (6.2%, 13.0%, 15.6% in placebo, teriflunomide 7 mg and teriflunomide14 mg groups, respectively). There were two noteworthy opportunistic infections reported during the study: one in the placebo group (hepatitis C concomitant with cytomegalovirus) and one in the 14 mg teriflunomide group (one intestinal tuberculosis). This patient was given standard antituberculosis medications and recovered. Four deaths occurred during the study, but none of these were suspected to be related to teriflunomide. One death in the placebo group was from a respiratory infection, one death in the teriflunomide 7 mg group due to a motor vehicle accident, and two deaths in the teriflunomide 14 mg group – one from suicide and one from a gram-negative infection complicated by disseminated intravascular coagulopathy. Further long-term follow up will be informative to more definitely assess if teriflunomide increases the risk of opportunistic infections.
Recently reported observations from the open-label extension of TEMSO where patients had received teriflunomide for up to 9 years found that the observed safety of teriflunomide was consistent with the 2 year core trial, with no new or unexpected AE signals, which is reassuring, as it suggests that the safety profile does not change in the long-term [Freedman et al. 2013].
Since teriflunomide is the active metabolite of the parent drug, leflunomide, the available long-term safety data on leflunomide provide indirect long-term safety data for teriflunomide. Overall, leflunomide appears to be well tolerated and has an acceptable safety profile. However, rarely, leflunomide can cause serious AEs including hepatotoxicity, hypertension, peripheral neuropathy, pneumonitis and cytopenia [White, 1980]. Two cases of progressive multifocal leukoencephalopathy (PML) have been reported in the context of approximately 2.1 million patient-years of leflunomide use. One of these cases was reported in a patient who had previously been on five other immunosuppressants (azathioprine, chloroquine, danazol, ciclosporin A and methotrexate) [Warnatz et al. 2003]. The second case was observed in a patient who had previously been on azathioprine [Rahmlow et al. 2008]. So although teriflunomide’s safety profile in clinical trials has been relatively benign, in light of the rare but serious AEs seen with long-term clinical data from leflunomide, a high degree of vigilance should be practiced when monitoring patients on this novel drug.
FDA safety monitoring guidelines for teriflunomide are presented in Table 3.
Table 3.
Safety monitoring guidelines for teriflunomide.
| Timeframe | Suggested parameters to monitor |
|---|---|
| Prior to initiation | CBC and LFTs (within 6 months prior to initiation) |
| Measure blood pressure | |
| Screen for latent tuberculosis | |
| After initiation | Monthly LFTs for the first 6 months, then every 6 months thereafter |
| CBC should be assessed if signs/symptoms of hematologic toxicity | |
| Monitor blood pressure periodically |
CBC, complete blood count; LFT, liver function test.
Considerations in women of child-bearing age
The teratogenic potential of leflunomide and teriflunomide has been demonstrated in animals [Brent, 2001; Fukushima et al. 2007; Sanofi, 2012], although the teratogenic risk of teriflunomide in humans has not been well characterized due to limited experience. The FDA categorizes teriflunomide into pregnancy risk category X.
Due to teriflunomide’s known teratogenic effects in animals, strict contraception is recommended for all females of reproductive age, and males are cautioned not to father a child while on therapy as teriflunomide may be transmitted in semen and the degree of transvaginal absorption is not well characterized. Preclinical studies have demonstrated that teriflunomide does not appear to damage sperm DNA, even at doses six times higher than the mean human steady-state exposure. In addition, there appears to be no effect of teriflunomide on fertility or reproductive performance in teriflunomide-treated rats, though there was a small effect on sperm count at the highest dose [Davenport et al. 2013].
In the pivotal clinical trials, women who became pregnant while on teriflunomide were required to undergo a cholestyramine or activated charcoal-based washout procedure after treatment discontinuation. After the washout procedure, it was necessary to confirm that the teriflunomide plasma level was <0.02 mg/l, which is thought to be a level representing minimal risk in pregnancy. Without the washout procedure, it can take up to two years to achieve systemic clearance of teriflunomide to an acceptable level [O’Connor et al. 2006].
In the phase II clinical trial, six pregnancies were documented in the 7 mg teriflunomide treatment group. Four patients chose to terminate the pregnancy, while two underwent the washout procedure as soon as they became aware of the pregnancy. In both cases, healthy infants were subsequently delivered with no structural deficits or other health concerns [O’Connor et al. 2006]. Similarly, in TEMSO, 11 pregnancies were reported, with four spontaneous abortions and six induced abortions. One patient in the 14 mg teriflunomide treatment arm who had been on treatment for 31 days of her pregnancy underwent the washout procedure and delivered a child with no significant health concerns [O’Connor et al. 2011]. In TOWER, there were 18 pregnancies reported – 14 in female patients taking teriflunomide and four in female partners of male patients. Of the 14 female patients, 10 elected to have induced abortions, while four pregnancies (one in the placebo group, two in the 7 mg group, one in the 14 mg group) resulted in healthy babies. Of the four reported pregnancies of female partners of male patients, one elected to have an induced abortion, while three pregnancies resulted in healthy babies (all in the 7 mg teriflunomide group) [Confavreux et al. 2014].
A recent retrospective analysis of all reported pregnancies in the teriflunomide clinical trial development program identified 69 pregnancies in women exposed to teriflunomide and 12 in partners of men exposed to teriflunomide. Teriflunomide exposure was up to 11 weeks in women and nearly all patients underwent an accelerated elimination procedure. There were no structural defects or functional deficits reported in newborns with potential prenatal teriflunomide exposure. The spontaneous abortion rate among female patients was similar to known rates in comparable populations of patients with MS, and the mean birth weight and gestational age of newborns was within typical ranges for the general population. A new teriflunomide pregnancy registry has been established, which will continue to collect prospective data on this important issue [Kieseier et al. 2013].
The safety of leflunomide has been evaluated in a number of studies. In a cohort of 64 RA patients with leflunomide exposure during pregnancy, there was no observed increase in structural AEs in infants [Chambers et al. 2010]. Another study evaluating 45 patients exposed to leflunomide during pregnancy or preconception resulted in two children with structural anomalies; however, there was a potential alternate etiology identified for at least a portion of the observed defects [Cassina et al. 2012]. A recent analysis of 65 (43 in patients exposed to teriflunomide) pregnancies reported in all clinical trials of teriflunomide to date found no structural or functional deficits in any newborns with prenatal teriflunomide exposure following elimination. Furthermore, the proportion of spontaneous abortions was within population-based norms [Kieseier et al. 2013]. Although these results are somewhat reassuring, in the absence of any definitive clinical data, teriflunomide’s teratogenic potential remains a significant concern. Thus, for the time being, individuals taking teriflunomide should practice strict contraception.
Other safety considerations
Teriflunomide’s known immunomodulatory effects naturally raise the concern of whether relapsing MS patients on teriflunomide have the ability to mount an appropriate immune response to vaccines. TERIVA (Study to Investigate the Immune Response to Influenza Vaccine in Patients With Multiple Sclerosis on Teriflunomide) was a multicenter, multinational, parallel-group study of 128 individuals on either dose of teriflunomide or an IFN-β drug designed to address this concern. The primary endpoint of TERIVA was the proportion of patients who achieved seroprotection to influenza vaccine strains H1N1, H3N2 and B at 28 days postvaccination. The safety of the influenza vaccine in teriflunomide-treated patients was also assessed. After screening, all enrolled study patients received the influenza vaccine and antibody titers were assessed at day 28.
After 28 days, MS patients treated with teriflunomide at either dose mounted effective immune responses to the seasonal influenza vaccination. Patients in the IFN-β reference group also mounted an effective immune response to influenza vaccine, as expected. Reassuringly, there were no new safety concerns identified in patients treated with teriflunomide following influenza vaccination [Bar-Or et al. 2013a].
Another recent study in 46 subjects demonstrated that teriflunomide does not impair the ability to achieve seroprotection against rabies (considered a neo-antigen). Although mean titers of rabies antibodies were lower in teriflunomide-treated subjects in comparison with the placebo-treated subjects, the titers were nonetheless well above the threshold necessary for seroprotection against rabies [Bar-Or et al. 2013b].
Conclusion
Teriflunomide has demonstrated efficacy and safety in a number of phase III trials in the treatment of relapsing MS, both as monotherapy or as an add-on agent. The convenience of administration and tolerability of teriflunomide makes it an attractive agent to add to the current available treatment armamentarium of relapsing MS. Existing experience with its parent drug, leflunomide, gives teriflunomide the added benefit of long-term safety data, which is very useful, since long-term safety will ultimately determine the real world longevity of a DMA in the treatment of MS.
With the growing options of available oral and parenteral treatments for relapsing MS, the specific niche that teriflunomide will occupy is unclear. Furthermore, teriflunomide’s relative efficacy compared with existing first-line agents is not definitive, but based on its performance against placebo in clinical trial settings; it seems to be a modestly effective DMA that is similar in efficacy to existing first-line parenteral agents. As a result, given its convenience of administration and safety and tolerability, teriflunomide will likely occupy a small but certain position as a first-line treatment agent for CIS or relapsing MS.
Ongoing large-scale phase III clinical trials and open-label extensions will answer lingering questions regarding teriflunomide and postmarketing surveillance will be essential to more definitively characterize the long-term safety and efficacy of teriflunomide in the treatment of MS.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Funding: This work was supported by the Multiple Sclerosis Society of Canada’s Decker Family Transitional Career Development Award (to J.O.).
Contributor Information
Jiwon Oh, The MS Clinic at St. Michael’s Hospital, Division of Neurology, Department of Medicine, University of Toronto, Shuter 3-003, 30 Bond St, Toronto, ON M5B 1W8, Canada.
Paul W. O’Connor, St Michael’s Hospital, Division of Neurology, Department of Medicine, University of Toronto, Toronto, Canada
References
- Bar-Or A., Freedman M.S., Kremenchutzky M., Menguy-Vacheron F., Bauer D., Jodl S., et al. (2013) Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology 81(6): 552–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A., Larouche R., Legrand B., Miller B., Benamor M., Truffinet P., et al. (2013b) Immune response to neoantigen and recall antigens in healthy subjects receiving teriflunomide. In: Proceedings of the 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, October, Copenhagen [Google Scholar]
- Brent R. (2001) Teratogen update: reproductive risks of leflunomide (Arava); a pyrimidine synthesis inhibitor: counseling women taking leflunomide before or during pregnancy and men taking leflunomide who are contemplating fathering a child. Teratology 63: 106–112 [DOI] [PubMed] [Google Scholar]
- Bruneau J.M., Yea C.M., Spinella-Jaegle S., Fudali C., Woodward K., Robson P.A., et al. Purification of human dihydro-orotate dehydrogenase and its inhibition by A77 1726, the active metabolite of leflunomide. The Biochemical journal. 1998; 336 (Pt 2):299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina M., Johnson D.L., Robinson L.K., Braddock S.R., Xu R., Jimenez J.L., et al. (2012) Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis and rheumatism 64(7): 2085–94 [DOI] [PubMed] [Google Scholar]
- Chambers C.D., Johnson D.L., Robinson L.K., Braddock S.R., Xu R., Lopez-Jimenez J., et al. (2010) Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis and rheumatism 62(5): 1494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H.M., Cohn R.G., Cheung P., Webster D.J., Xu Y.Z., Caulfield J.P., et al. (1995) The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. The Journal of pharmacology and experimental therapeutics 275(2): 1043–9 [PubMed] [Google Scholar]
- Confavreux C., Li D.K., Freedman M.S., Truffinet P., Benzerdjeb H., Wang D., et al. Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Multiple sclerosis (Houndmills, Basingstoke, England) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C., O’Connor P., Comi G., Freedman M.S., Miller A.E., Olsson T.P., et al. (2014) Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet neurology 13(3): 247–56 [DOI] [PubMed] [Google Scholar]
- Davenport L., Czich A., Turpault S. (2013) Teriflunomide: no effects on sperm DNA. In: Proceedings of the 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, October, Copenhagen [Google Scholar]
- Elder R., Xu X., Williams J., Gong H., Finnegan A., Chong A. (1997) The immunosuppressive metabolite of leflunomide, A77 1726, affects murine T cells through two biochemical mechanisms. J Immunol 159: 22–27 [PubMed] [Google Scholar]
- Fairbanks L., Bofill M., Ruckemann K., Simmonds H. (1995) Importance of ribonucleotide availability to proliferating T-lymphocytes from healthy humans. Disproportionate expansion of pyrimidine pools and contrasting effects of de novo synthesis inhibitors. J Biol Chem 270: 29682–29689 [PubMed] [Google Scholar]
- Fox R.I., Herrmann M.L., Frangou C.G., Wahl G.M., Morris R.E., Strand V., et al. (1999) Mechanism of action for leflunomide in rheumatoid arthritis. Clinical immunology (Orlando, Fla) 93(3): 198–208 [DOI] [PubMed] [Google Scholar]
- Freedman M., Wolinsky J., Comi G., Kappos L., Olsson T., Miller A., et al. (2013) Long-term safety and efficacy of teriflunomide in patients with relapsing forms of multiple sclerosis in the TEMSO extension trial. In: Proceedings of the 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, October, Copenhagen [Google Scholar]
- Freedman M., Wolinsky J., Frangin G., Confavreux C., Comi G., Byrnes W., et al. (2010) Oral teriflunomide or placebo added to glatiramer acetate for 6 months in patients with relapsing multiple sclerosis: safety and efficacy results. In: Proceedings of the 62nd Annual Meeting of the American Academy of Neurology, Toronto, abstract S21.001. [Google Scholar]
- Freedman M.S., Wolinsky J.S., Wamil B., Confavreux C., Comi G., Kappos L., et al. (2012) Teriflunomide added to interferon-beta in relapsing multiple sclerosis: A randomized phase II trial. Neurology 78(23): 1877–1885 [DOI] [PubMed] [Google Scholar]
- Fukushima R., Kanamori S., Hirashiba M., Hishikawa A., Muranaka R.I., Kaneto M., et al. (2007) Teratogenicity study of the dihydroorotate-dehydrogenase inhibitor and protein tyrosine kinase inhibitor Leflunomide in mice. Reproductive toxicology (Elmsford, NY) 24(3-4): 310–6 [DOI] [PubMed] [Google Scholar]
- Gold R., Wolinsky J. (2011) Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand 124: 75–84 [DOI] [PubMed] [Google Scholar]
- Hamilton L., Vojnovic I., Warner T. (1999) A771726, the active metabolite of leflunomide, directly inhibits the activity of cyclo-oxygenase-2 in vitro and in vivo in a substrate-sensitive manner. Br J Pharmacol 127: 1589–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Schleyerbach R., Kirschbaum B. (2000) Leflunomide: an immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology 47: 273–289 [DOI] [PubMed] [Google Scholar]
- Kieseier B., Stuve O., Benamor M., Delhay J., Truffinet P. (2013) Updated pregnancy outcomes from the teriflunomide clinical development programme: retrospective analysis of the teriflunomide clinical trial database. In: Proceedings of the 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, October, Copenhagen [Google Scholar]
- Korn T., Magnus T., Toyka K., Jung S. (2004) Modulation of effector cell functions in experimental autoimmune encephalomyelitis by leflunomide–mechanisms independent of pyrimidine depletion. J Leuk Biol 76: 950–960 [DOI] [PubMed] [Google Scholar]
- Korn T., Toyka K., Hartung H., Jung S. (2001) Suppression of experimental autoimmune neuritis by leflunomide. Brain 124: 1791–1802 [DOI] [PubMed] [Google Scholar]
- Leist T., Freedman M., Kappos L., Olsson T., Miller A., Wolinsky J., et al. (2013) Pooled safety data from three placebo-controlled teriflunomide studies. In: Proceedings of the 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, October, Copenhagen [Google Scholar]
- Limsakun T., Menguy-Vacheron F. (2010) Pharmacokinetics of oral teriflunomide, a novel oral disease-modifying agent under investigation for the treatment of multiple sclerosis. In: Proceedings of the 62nd Annual Meeting of the American Academy of Neurology, Toronto, abstract P.05.032 [Google Scholar]
- Marriott J., O’Connor P. (2010) Emerging therapies in relapsing-remitting multiple sclerosis. Rev Recent Clin Trials 5: 179–188 [DOI] [PubMed] [Google Scholar]
- Mattar T., Kochhar K., Bartlett R., Bremer E., Finnegan A. (1993) Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett 334: 161–164 [DOI] [PubMed] [Google Scholar]
- Miller A., Wolinksy J., Kappos L., Comi G., Freedman M., Olsson T., et al. (2013) TOPIC main outcomes: efficacy and safety of once-daily oral teriflunomide in patients with clinically isolated syndrome. In: Proceedings of the 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, October, Copenhagen [Google Scholar]
- O’Connor P.W., Li D., Freedman M.S., Bar-Or A., Rice G.P., Confavreux C., et al. (2006) A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66(6): 894–900 [DOI] [PubMed] [Google Scholar]
- O’Connor P.W., Lublin F.D., Wolinsky J.S., Confavreux C., Comi G., Freedman M.S., et al. (2013) Teriflunomide reduces relapse-related neurological sequelae, hospitalizations and steroid use. Journal of neurology 260(10): 2472–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor P., Wolinsky J.S., Confavreux C., Comi G., Kappos L., Olsson T.P., et al. (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. The New England journal of medicine 365(14): 1293–1303 [DOI] [PubMed] [Google Scholar]
- Osiri M., Shea B., Robinson V., Suarez-Almazor M., Strand V., Tugwell P., et al. (2003) Leflunomide for treating rheumatoid arthritis. Cochrane database of systematic reviews (Online) (1): CD002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmlow M., Shuster E.A., Dominik J., Deen H.G., Jr., Dickson D.W., Aksamit A.J., Jr., et al. (2008) Leflunomide-associated progressive multifocal leukoencephalopathy. Archives of neurology 65(11): 1538–9 [DOI] [PubMed] [Google Scholar]
- Ruckemann K., Fairbanks L.D., Carrey E.A., Hawrylowicz C.M., Richards D.F., Kirschbaum B., et al. (1998) Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. The Journal of biological chemistry 273(34): 21682–91 [DOI] [PubMed] [Google Scholar]
- Sanofi (2012) Teriflunomide data on file at Sanofi, Sanofi S.A., Paris [Google Scholar]
- Siemasko K., Chong A., Jack H., Gong H., Williams J., Finnegan A. (1998) Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. J Immunol 160: 1581–1588 [PubMed] [Google Scholar]
- Tallantyre E., Evangelou N., Constantinescu C. (2008) Spotlight on teriflunomide. Int Mult Scler J 15: 6–68 [PubMed] [Google Scholar]
- Vermersch P., Czlonkowska A., Grimaldi L.M., Confavreux C., Comi G., Kappos L., et al. (2014) Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Multiple sclerosis (Houndmills, Basingstoke, England) 20(6): 705–16 [DOI] [PubMed] [Google Scholar]
- Warnatz K., Peter H.H., Schumacher M., Wiese L., Prasse A., Petschner F., et al. (2003) Infectious CNS disease as a differential diagnosis in systemic rheumatic diseases: three case reports and a review of the literature. Annals of the rheumatic diseases 62(1): 50–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. (1980) A heteroskedasticity consistent covariace matrix estimator and a direct test for heteroscedasticity. Econometrica 48: 817–830 [Google Scholar]