Summary
Functional abdominal pain syndrome is characterised by frequent or continuous abdominal pain associated with a degree of loss of daily activity. It has a reported population prevalence of between 0.5% and 1.7%, with a female preponderance. The pathophysiology of functional abdominal pain is incompletely understood although it has been postulated that peripheral sensitisation of visceral afferents, central sensitisation of the spinal dorsal horn and aberrancies within descending modulatory systems may have an important role. The management of patients with functional abdominal pain requires a tailored multidisciplinary approach in a supportive and empathetic environment in order to develop an effective therapeutic relationship. Patient education directed towards an explanation of the pathophysiology of functional abdominal pain is in our opinion a prerequisite step and provides the rationale for the introduction of interventions. Interventions can usefully be categorised into general measures, pharmacotherapy, psychological interventions and ‘step-up’ treatments. Pharmacotherapeutic/step-up options include tricyclic antidepressants, serotonin noradrenergic reuptake inhibitors and the gabapentinoids. Psychological treatments include cognitive behavioural therapy and hypnotherapy. However, the objective evidence base for these interventions is largely derived from other chronic pain syndrome, and further research is warranted in adult patients with functional abdominal pain.
Keywords: functional abdominal pain, pathophysiology, investigation, management
Introduction
Visceral pain per se is a prevalent and leading global cause of healthcare expenditure and has been estimated to cost the UK economy in the order of £100 million per annum.1,2 While the majority of those who present with acute visceral pain, following appropriate clinical evaluation and management, have resolution of their symptoms; a significant proportion do not, developing symptom chronicity and a reduction in health-related quality of life.3 In a minority of patients, a demonstrable ‘organic’ cause is not found and patients are often classified as having a functional bowel disorder, the most prevalent of which is irritable bowel syndrome (IBS).4 Functional bowel disorders, as a diagnostic entity, account for in excess of one-third of new patient referrals to gastroenterology clinics in secondary care of the National Health Service and represent a heterogeneous group of disorders.5 This heterogeneous group of disorders is delineated using the multinational Rome symptom/temporal-based criteria, currently in its third iteration.6 Although seemingly similar to IBS, the functional abdominal pain (FAP) syndrome is characterised by frequent or continuous abdominal pain associated with a degree of loss of daily activity, in the absence in change in bowel habit (Table 1).6 The reported population prevalence of FAP varies from 0.5% to 1.7% with a female predilection.7 Although it could be argued that such an ‘artificial’ distinction between these two disorders is purely academic, from a clinical perspective these distinctions are of importance as there are differences in one’s approach to the evaluation and subsequent management of FAP.
Table 1.
The Rome III diagnostic criteria for the diagnosis of functional abdominal pain.
| All the following criteria must be fulfilled, with symptom onset at least six months prior to diagnosis: |
| • Continuous or almost continuous abdominal pain |
| • No relation to physiological events |
| • Some loss of daily functioning |
| • The pain is real and not feigned |
| • Insufficient symptoms to meet the criteria for another functional gastrointestinal disorder |
Aims and methods
The aims of this review are three-fold. First, to provide an overview of the pathways emanating from the gastrointestinal (GI) tract that transduce noxious stimuli. Second, to provide a review of the salient peripheral and central mechanisms implicated in the genesis and maintenance of FAP. Third, we propose a clinical approach to such patients and finally describe treatments, encompassing pharmacological and psychological interventions in addition to describing their evidence base, where available. We interrogated the PubMed interface of Medline to identify salient publications concerning FAP and other relevant functional bowel disorders and chronic pain syndromes.
Normal visceral pain transduction
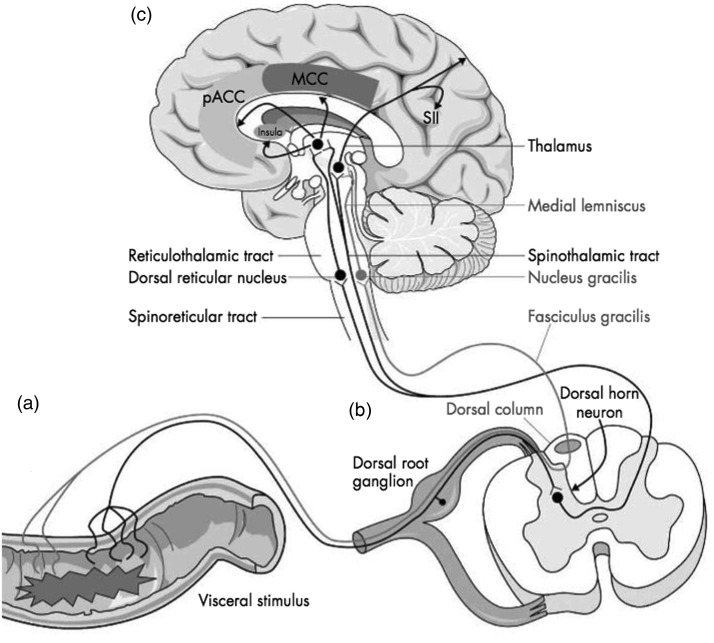
A noxious stimulus, when applied to the GI tract, causes the activation of peripheral nerve receptors that are sensitive to chemical, mechanical or inflammatory stimuli.8 This signal is then transduced via spinal visceral afferents, synapsing at the dorsal horn of the spinal cord, and is communicated to the brain via the spinothalamic, spinoreticular and spinomesencephalic tracts. The spinothalamic tract terminates in the thalamus, with thalamocortical fibres subsequently projecting both to the primary and secondary somatosensory cortices, which form the neural substrate of the sensory discriminative aspect of the pain experience. Unlike somatic sensation, which has homuncular representation in the primary somatosensory cortex, the representation of visceral sensation is less well organised. The spinoreticular and spinomesencephalic pathways terminate in the medial thalamus with subsequent third-order thalamocortical fibres primarily ascending to the anterior cingulate cortex and insula. These areas are salient in the affective-motivational aspects of visceral pain. In addition to these ascending pathways, a variety of descending inhibitory pathways variably influence the perception of normal visceral sensation. For instance, pathways arising from anterior cingulate cortex may transmit inhibitory signals to the periaqueductal grey, located in close proximity to cerebral aqueduct within the midbrain, either directly or via second-order neurons from the amygdala. Ensuing third-order neurons complete a dynamically and functional interface with neurons in the spinal dorsal horn where modulation, or gating, of ascending visceral afferent signals may occur.9 When considered in totality, these pathways can be usefully thought of as representing the visceral pain neuraxis (Figure 1).
Figure 1.
A highly schematic representation of the visceral pain neuraxis, illustrating the major pain pathways from the viscera to the central nervous system.
pACC: perigenual anterior cingulate cortex, MCC: midcingulate cortex, SII: secondary somatosensory cortex. Reprinted with permission from Mathews and Aziz, Postgrad Med J 2005.10
Pathophysiological mechanisms in FAP
The contemporaneous definition of FAP is not based absolutely on a fundamental understanding of the underlying pathophysiology, as a significant proportion of the postulated basic mechanisms have been elucidated from other chronic pain syndromes, most commonly from somatic pain research. Considering the marked variability in an individual’s experience of visceral pain both in health and disease,11,12 it is not an unreasonable proposition to return to first principles to conceptualise the source of such pain arising at any, or several concomitant, levels of the visceral pain neuraxis. Dysfunction, culminating in FAP, within this neuraxis may therefore be a consequence of (a) peripheral augmentation of the visceral pain afferent signal, (b) central sensitisation of the spinal dorsal horn, (c) alterations in descending modulation or finally by (d) central amplification.
Peripheral sensitisation of visceral afferents
Heightened ascending visceral afferent signalling, termed peripheral sensitisation, may occur after repeated injury or inflammation to the GI tract.13 For instance, approximately one-third of people who develop IBS report that their symptoms are initiated following an episode of acute infection, an epiphenomenon widely referred to as postinfectious IBS (PI-IBS). PI-IBS has been the focus of a considerable academic effort directed at elucidating the pathophysiological features therein.14,15 For instance, it has been reproducibly associated with the presence of a low-grade inflammatory infiltrate.16 This inflammatory infiltrate has been theorised to cause increased peripheral receptor sensitivity and field, the latter through recruitment and activation of hitherto silent nociceptors resulting in hyperalgesia. Furthermore, stress, as indexed by traumatic life events, and a neurotic personality trait, were found to be the best predictors of who might develop PI-IBS.16 These converging lines of evidence add weight to the postulation that injury and/or inflammation in a psychological predisposed individual may lead to the peripheral sensitisation of visceral afferents, thus augmenting the ascending volley of nociceptive information to the spinal dorsal horn.
Central sensitisation at the spinal dorsal horn
The sensitisation of peripheral nociceptors results in an increased volley of signals reaching the spinal dorsal horn. This increase in amplitude and frequency of peripheral signalling reaching the spinal dorsal horn can cause central sensitisation. Central sensitisation occurs due to an increase in presynaptic glutamate secretion, itself leading to the removal of the magnesium ion block of the N-methyl-d-aspartate (NMDA) receptor. In association with activation of other key enzymes, the overall consequence is increased responsiveness of the dorsal horn neurons often outlasting the initiating insult. In a model of acid-induced oesophageal pain, Sarkar et al.17 have demonstrated the concept of central sensitisation. In this model, hydrochloric acid infused into distal oesophagus induces hyperalgesia in the acid-exposed distal region, as would be expected, but also in the unexposed proximal oesophagus due to central sensitisation. Within the lower GI tract, repetitive experimental stimulation of the sigmoid colon causes secondary hyperalgesia in the rectum due to central sensitisation.18 Pharmacological studies have demonstrated that antagonism of the NMDA receptor prevents the development of, and can reverse, central sensitisation within the oesophagus.19 In a recent paper by Walker et al.,20 it was demonstrated that in a subgroup of FAP patients, termed therein as high pain dysfunctional patients, showed significantly greater thermal wind-up, i.e. the perception of an increase in pain intensity when a thermal painful stimulus is repeatedly delivered, thus suggesting that at least a subgroup have pathophysiology consistent with the development of central sensitisation.
Descending modulation of nociceptive pathways
As previously alluded to, central descending modulatory systems, largely reside in the anterior cingulate cortex. These modulatory systems, which interface with the spinal dorsal horn, allow the gating of afferent signals from the periphery, thereby allowing amplification, or indeed curtailment, of this signal. Aberrancies within the descending pain modulatory system are increasingly considered to be central to the development of the pro-nociceptive state encountered in many chronic visceral pain syndromes.21 A recent study has evaluated visceral sensory function in a small sample of FAP patients in comparison to IBS and healthy controls.22 These preliminary data demonstrated that rectal perceptual thresholds were significantly lower in patients with IBS but interestingly not in FAP, suggesting that pain reporting in FAP is less likely to be attributable to visceral hypersensitivity, which may be due to differential descending modulation in the two patient groups, thus potentially offering an objective biomarker. Wilder Smith23 has proposed that alterations in modulatory balance may well be a unifying pathophysiological mechanism across functional bowel disorders as it can be driven by both top-down (i.e. central nervous system pathology) and bottom-up (i.e. peripheral immune activation/infection) influences. However, further validation is needed in larger patient cohorts although targeted manipulation of these modulatory systems offer an attractive therapeutic target for the future.
Clinical evaluation in FAP patients
Clinical history
A comprehensive history should be sought from the FAP patient that explores in detail the chronology of pain events particularly in relation to surgery, infection or traumatic life events. In addition, the pattern or distribution of pain maybe widespread and one of several pains complained about, thus raising the possibility of a concomitant somatisation disorder such as fibromyalgia. Typically, the intensity of abdominal pain seldom varies in FAP with maximal pain being experienced for the majority of the time.7 In addition, the patient’s behavioural traits, belief systems and previous patterns of healthcare seeking, such as poor coping skills, concomitant depression, challenging social circumstances and multiple attendances, maybe useful in suggesting a functional disorder within an initial differential diagnosis.
Physical examination and investigational strategy
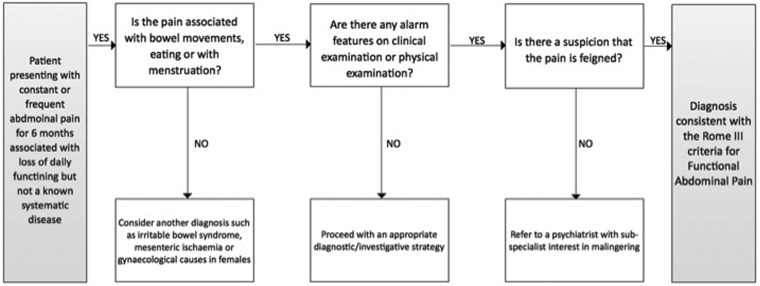
By definition, the clinical examination in a patient with FAP should be normal. However, it is worth scrutinising for presence of abdominal scars relating to previous surgeries or investigations as initiating factors. Likewise, Carnett’s test may be useful; this is where a painful area is palpated before and after the patient tenses his/her abdominal wall. Here, the patient performs a sit up against the resistance of the clinician’s hand on the subject’s forehead. If the subject experiences pain with palpation against tense abdominal musculature, it suggests the cause of the pain is musculoskeletal (i.e. arising from the anterior abdominal wall) rather than emanating from the visceral contents per se. A targeted investigational strategy to include standard haematological, biochemical and immunological parameters is appropriate in the majority. In patients with alarm features, then an alternative diagnosis should be considered and investigated accordingly. In terms of making a positive diagnosis of FAP, the Rome foundation has produced a useful diagnostic algorithm (Figure 2).
Figure 2.
A suggested diagnostic algorithm for the diagnosis of functional abdominal pain, reproduced with kind permission of the Rome Foundation.
Management
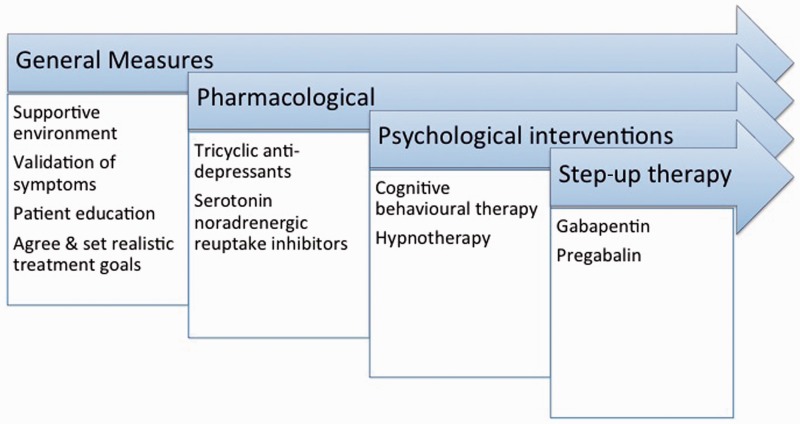
There is no absolute consensus regarding the optimal management of FAP in adults. Therefore, interventions that are currently used are largely based on evidence, and anecdotal experience, derived from other functional bowel disease and chronic pain syndromes. Treatment modalities can be usefully divided into general measures, pharmacological treatments and psychological interventions. A summary overview of management steps for FAP is given in Figure 3.
Figure 3.
A suggested step wise management/treatment algorithm for functional abdominal pain.
General measures
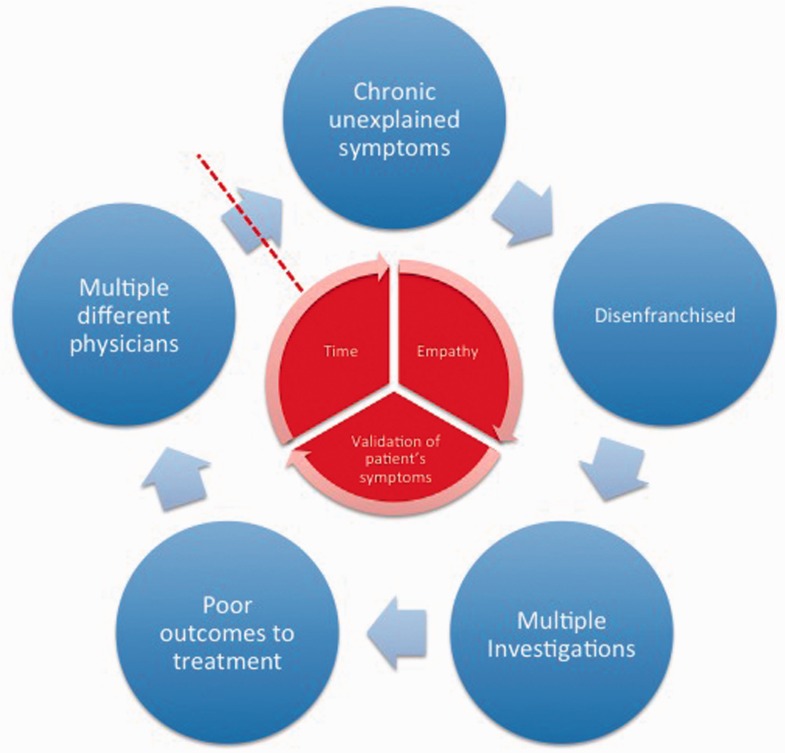
Central to a successful outcome in the management of the patient with FAP is the doctor–patient relationship. In particular, validation of a patient’s symptoms in a supportive multidisciplinary environment is an absolute cornerstone of treatment. For instance, many, if not most, of these patients may have been hitherto labelled with a myriad of diagnoses, such as IBS or even been accused of being a malingerer. Likewise, many patients in our experience have encountered negative attitudes towards their symptoms from clinicians often for many years prior to a definitive diagnosis of FAP being made. Not unsurprisingly, therefore, many patients are distrusting of clinicians and feel disenfranchised and disengaged. This frequently leads to onward referral to another clinician who performs more investigations, which are usually negative, and so the cycle begins again (Figure 4). We believe that the most challenging aspect to managing the patient with FAP ab initio is gaining their trust in order to establish a therapeutic relationship. Likewise, it is our opinion that patient education as to the pathophysiology of FAP is a prerequisite step before therapeutic interventions are commenced, thereby giving patients a rationale for a particular treatment choice, for instance using low-dose antidepressants as analgesics rather than as antidepressants per se. The clinician and the patient should also agree upon, and set, reasonable treatment goals in the context of regular outpatient reviews. The absolute regularity of such reviews maybe limited by local service provision, but they do allow definition of response or non-response, facilitating earlier escalation of intervention as appropriate, and also leaves the patients with a sense of confidence that they are not going to be left to ‘fend for themselves’. While this approach is relatively ‘resource intensive’, it does reduce the likelihood of patients seeking further advice/consultations from other clinicians in the intervening period and is therefore arguably more cost effective in the longer term.
Figure 4.
A patient's negative experience often leads to diagnostic uncertainty, multiple unnecessary investigations, patient dissatisfaction and presentation to another physician. In order to break this cycle, the patient needs to be offered time, empathy in the context of validation of their symptoms.
Pharmacological interventions
Pharmacological interventions in FAP are primarily targeted towards neuromodulation of the putative pathophysiological mechanisms.
Antidepressants
Antidepressants are generally accepted as the first line of pain management in FAP. Although, to date, there is a lack of robust data in FAP, a recent systematic review and meta-analysis in IBS has provided evidence of a beneficial effect for antidepressants over placebo for improvement of abdominal pain (number needed to treat [NNT] – 5), global assessment (NNT – 4) and overall symptoms (NNT – 4).24 Within FAP, there are currently two broad classes of antidepressant used: the tricyclic antidepressants (TCA) and the serotonin noradrenergic reuptake inhibitors (SNRIs).
While the antinociceptive mechanism of action of TCAs, such as amitriptyline, remains incompletely understood, three distinct possibilities have been proposed.25 First, the modulation of descending inhibitory pathways; second, through binding at the NMDA receptor on spinal dorsal horn and finally via direct inhibition of Na+/K+ channels on spinal afferents. In our practice, we utilise low-dose TCAs at night, for instance amitriptyline 10 mg at night, as first-line pharmacotherapy. Higher doses may lead to somnolence and anticholinergic side effects, such as constipation and dry mouth, which may result in poor adherence. Nightly dosing can negate some of these problematic side effects. However, while amitriptyline has been utilised as a first-line treatment for many years, somewhat surprisingly there is no supportive unbiased evidence for a beneficial effect in chronic neuropathic and musculoskeletal pain syndromes, although this assertion must be balanced against the clinical experience of successful outcomes in many patients.26 Thus, there is a need not to over-estimate the potential beneficial treatment effects with amitriptyline.
Serotonin and noradrenaline have been implicated as central mediators of endogenous analgesic mechanisms. Evidence indicates that SNRIs, such as duloxetine and venlafaxine, are among the most promising modern agents for treating many types of chronic pain.27 Pooled data from two randomised controlled trials (RCTs) containing 538 patients with fibromyalgia demonstrated the efficacy of duloxetine in reducing pain, functional impairment and increasing quality of life.28 These results have been replicated in a further six-month RCT indicating a degree of durability of analgesic effect.29 Based on these data, it is our clinical practice to commence an SNRI (e.g. duloxetine 60 mg MR once daily) as second-line treatment in those patients who have failed to respond to TCA therapy ab initio or whose response has become abated over time although to date there is a paucity of empirical data to directly support this.
Psychological therapies
While the literature is devoid of any controlled trials examining the efficacy of psychological therapies in the treatment of adult FAP, within paediatric populations of FAP and other adult functional gastrointestinal disorders, a diverse array of psychological treatments, such as cognitive behavioural therapy, relaxation therapy, hypnotherapy, dynamic psychotherapy and stress management, have been systematically evaluated (NNT – 4) although the reported trials are generally of low quality.30 We would refer the reader to an excellent recent primer concerning the nature of each of these therapies by Palsson and Whitehead.31 In our experience, the specific type of psychological therapy that is chosen is often largely dependent on local service provision and therapist availability that has specialist expertise and interest in this area. Considering these limitations, it is therefore important to stratify and rationalise which patients one refers for such therapies. We would suggest that patients who have recalcitrant symptoms after three to six months of medical therapy or who have co-morbid psychiatric disorders and/or stressful life events, which trigger, or exacerbate, symptoms should be referred.
Interventions for patients with symptoms refractory to standard pharmacological and psychological interventions
A small, but significant, proportion of FAP patients may remain refractory to standard interventions, and ‘step-up’ therapy should be considered in addition to standard interventions. We would strongly argue that ‘step-up’ therapy should not include the use of opiate analgesics, as over time this may result in the development of opioid-induced bowel dysfunction, hyperalgesia and potentially the narcotic bowel syndrome.32,33
Gabapentinoids
Gabapentin and pregabalin are used in the treatment of a number of chronic pain syndromes.34 These compounds bind with high affinity to α2δ subunits of voltage-gated calcium channels in areas of the central nervous system involved in pain signalling. Both gabapentin and pregabalin have been demonstrated to alter pain and sensory thresholds to rectal distension in IBS patients.35,36 They should therefore be considered as adjunctive therapies in patients with refractory symptoms.
Conclusions
FAP is a relatively uncommon disorder characterised by chronic unexplained visceral pain that should be considered by clinicians as a distinct entity from other syndromes such as IBS. FAP is likely to represent a heterogeneous group of patients whose symptoms are likely to be attributable to multiple and variable pathophysiologies. A complete mechanistic understanding of these remains incomplete. Clinical evaluation should encompass a detailed history with only targeted investigations undertaken in a supportive multidisciplinary environment. Treatment options often necessitate a variable combination of pharmacological, psychological and step-up interventions. However, there is a pressing need for further well-designed controlled trials specifically addressing the distinct pathophysiological features of FAP as well the efficacy of interventions.
Declarations
Competing interests
None declared
Funding
ADF is supported by the Whitechapel Society for the Advancement of Gastroenterology, which had no input on the content or writing of this paper.
Ethical approval
Not applicable
Guarantor
ADF
Contributorship
ADF wrote the paper. QA revised the paper for intellectual content.
Acknowledgements
None
Provenance
Not commissioned; peer-reviewed by Klara Garsed
References
- 1.Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms, 2nd ed. Seattle, WA: IASP Press, 1994 [Google Scholar]
- 2.Collett B. Visceral pain: the importance of pain management services. Br J Pain 2013; 7: 6–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer AD, Aziz Q. Recent advances in chronic visceral pain. Curr Opin Support Palliat Care 2008; 2: 116–121 [DOI] [PubMed] [Google Scholar]
- 4.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012; 10: 712–721 e4 [DOI] [PubMed] [Google Scholar]
- 5.Shivali UN, Ford AC. Prevalence of functional gastrointestinal disorders among consecutive new patient referrals to a gastroenterology clinic. Frontline Gastroenterol 2014; 4.: DOI: 10.1136/flgastro-2013-100426–DOI: 10.1136/flgastro-2013-100426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drossman DA. Rome III: the functional gastrointestinal disorders, 3rd ed. McLean, VA: Degnon Associates, 2006, pp. xli, 1048 p–xli, 1048 p [Google Scholar]
- 7.Clouse RE, Mayer EA, Aziz Q, et al. Functional abdominal pain syndrome. Gastroenterology 2006; 130: 1492–1497 [DOI] [PubMed] [Google Scholar]
- 8.Knowles CH, Aziz Q. Basic and clinical aspects of gastrointestinal pain. Pain 2009; 141: 191–209 [DOI] [PubMed] [Google Scholar]
- 9.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol 2007; 13: 3699–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews PJ, Aziz Q. Functional abdominal pain. Postgrad Med J 2005; 81: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer AD, Coen SJ, Kano M, et al. Psychophysiological responses to pain identify reproducible human clusters. Pain 2013; 154: 2266–2276 [DOI] [PubMed] [Google Scholar]
- 12.Farmer AD, Coen SJ, Kano M, et al. Psychophysiological responses to visceral and somatic pain in functional chest pain identify clinically relevant pain clusters. Neurogastroenterol Motil 2014; 26: 139–148 [DOI] [PubMed] [Google Scholar]
- 13.Aziz Q, Furlong PL, Barlow J, et al. Topographic mapping of cortical potentials evoked by distension of the human proximal and distal oesophagus. Electroencephalogr Clin Neurophysiol 1995; 96: 219–228 [DOI] [PubMed] [Google Scholar]
- 14.Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil 2012; 18: 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhuiyan MR, Majumder TK, Raihan AA, Roy PK, Farha N, Kamal M. Histopathological alterations in post-infectious irritable bowel syndrome in Bangladeshi population. Mymensingh Med J 2010; 19: 275–281 [PubMed] [Google Scholar]
- 16.Gwee KA. Postinfectious irritable bowel syndrome. Curr Treat Options Gastroenterol 2001; 4: 287–291 [DOI] [PubMed] [Google Scholar]
- 17.Sarkar S, Aziz Q, Woolf CJ, Hobson AR, Thompson DG. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet 2000; 356: 1154–1159 [DOI] [PubMed] [Google Scholar]
- 18.Munakata J, Naliboff B, Harraf F, et al. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology 1997; 112: 55–63 [DOI] [PubMed] [Google Scholar]
- 19.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology 2004; 126: 683–692 [DOI] [PubMed] [Google Scholar]
- 20.Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 2012; 153: 1798–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004; 53: 1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nozu T, Kudaira M. Altered rectal sensory response induced by balloon distention in patients with functional abdominal pain syndrome. Biopsychosoc Med 2009; 3: 13–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut 2011; 60: 1589–1599 [DOI] [PubMed] [Google Scholar]
- 24.Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2011; 10(8): CD003460–CD003460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dharmshaktu P, Tayal V, Kalra BS. Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol 2012; 52: 6–17 [DOI] [PubMed] [Google Scholar]
- 26.Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2012; 12: CD008242–CD008242 [DOI] [PubMed] [Google Scholar]
- 27.Pergolizzi JV, Jr, Raffa RB, Taylor R, Jr, Rodriguez G, Nalamachu S, Langley P. A review of duloxetine 60 mg once-daily dosing for the management of diabetic peripheral neuropathic pain, fibromyalgia, and chronic musculoskeletal pain due to chronic osteoarthritis pain and low back pain. Pain Pract 2013; 13: 239–252 [DOI] [PubMed] [Google Scholar]
- 28.Arnold LM, Pritchett YL, D'Souza DN, Kajdasz DK, Iyengar S, Wernicke JF. Duloxetine for the treatment of fibromyalgia in women: pooled results from two randomized, placebo-controlled clinical trials. J Women's Health 2007; 16: 1145–1156 [DOI] [PubMed] [Google Scholar]
- 29.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain 2008; 136: 432–444 [DOI] [PubMed] [Google Scholar]
- 30.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut 2009; 58: 367–78 [DOI] [PubMed] [Google Scholar]
- 31.Palsson OS, Whitehead WE. Psychological treatments in functional gastrointestinal disorders: a primer for the gastroenterologist. Clin Gastroenterol Hepatol 2013; 11: 208–216; quiz e22–e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunkemeier DM, Cassara JE, Dalton CB, Drossman DA. The narcotic bowel syndrome: clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007; 5: 1126–1139; quiz 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farmer AD, Ferdinand E, Aziz Q. Opioids and the gastrointestinal tract – a case of narcotic bowel syndrome and literature review. J Neurogastroenterol Motil 2013; 19: 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Mao J. Update on neuropathic pain treatment: ion channel blockers and gabapentinoids. Curr Pain Headache Rep 2013; 17: 359–359 [DOI] [PubMed] [Google Scholar]
- 35.Lee KJ, Kim JH, Cho SW. Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Alimentary Pharmacol Ther 2005; 22: 981–988 [DOI] [PubMed] [Google Scholar]
- 36.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut 2007; 56: 1218–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]