Abstract
Abatacept is recommended by several expert consensus groups including the 2013 update of the EULAR recommendations for the pharmacologic management of rheumatoid arthritis (RA), as a potential choice for biologic therapy for patients with RA. Initially developed, studied, and approved as an intravenous (IV) formulation, abatacept is now also available as a subcutaneous (SC) injection. Having both options available makes abatacept a particularly versatile agent for the management of RA, greatly expanding the population of patients who could benefit from this treatment. This review provides a summary of the most important clinical trials that have investigated this molecule in both of its formulations, with a focus on the more recent trials evaluating the SC formulation, specifically the AMPLE study, the first major trial evaluating two biologic agents (abatacept and the tumor necrosis factor (TNF)-inhibitor adalimumab) in a head-to-head manner. In that study, SC abatacept was found to have an efficacy profile similar to that of SC adalimumab, both in combination with methotrexate.
Keywords: abatacept, biologic, costimulation modulator, rheumatoid arthritis, subcutaneous
Introduction
Rheumatoid arthritis (RA) affects approximately 1% of adults worldwide [Gibofsky, 2012], with prevalence rates varying somewhat from country to country and region to region. Evidence suggests that incidence rates for this autoimmune inflammatory disease are rising in the developed world [Myasoedova et al. 2010; Bombardier et al. 2011].
The management of RA has undergone a remarkable transformation over the past 15 years as clinicians and their patients have gained access to a growing number of biologic disease-modifying agents that have proven to be efficacious and safe options for reducing disease activity and lowering the inflammatory burden of RA. Concurrent with this evolution in therapeutic options, clinical practice guidelines around the world have recognized the greater expectations for therapeutic success with the expanded therapeutic armamentarium and have advocated the early, intensive treatment, using a treat-to-target approach and achievement of remission (or low disease activity [LDA]) as a realistic goal of therapy [Smolen et al. 2010, 2013; Singh et al. 2012; Bykerk et al. 2012].
The biologic agents currently available for RA treatment vary somewhat from country to country. In Canada, for example, there are currently nine biologic agents approved for the treatment of RA: abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, and tocilizumab. The order in which the agents became available also varies, which has influenced the order in which the agents have been used (e.g. as first-, second-, or subsequent-line biologic). However, sufficient experience and evidence has accumulated with most of the currently available agents that the authors of clinical practice recommendations now list abatacept, tumor necrosis factor (TNF) inhibitors, rituximab, and tocilizumab as potential choices for first-line biologic [Smolen et al. 2013; Bykerk et al. 2012].
One of the considerations in the selection of a particular biologic is its route of administration. Some of the biologics are administered only by intravenous (IV) infusion, while others are administered by subcutaneous (SC) injection. One of the potential first-line biologics, abatacept, was initially one of those agents available only as an IV formulation. More recently, however, an SC formulation has also become available. In addition to the initial clinical trial program with IV abatacept, the SC formulation has also undergone extensive clinical study.
The goal of this review paper is to provide summaries of the relevant studies that have completed with the SC formulation of abatacept, to familiarize clinicians with this pharmacotherapeutic option for their patients with RA. First, however, a brief overview of the initial research with IV abatacept is presented.
Abatacept: initial evidence with IV formulation
The efficacy of IV abatacept has been established in different and distinct RA patient populations; the efficacy findings are primarily from four randomized controlled trials (RCTs): the Abatacept Trial in Treatment of Anti-TNF Inadequate Responders (ATTAIN) trial [Genovese et al. 2005] among RA patients after inadequate response to anti-TNF therapy; the Abatacept in Inadequate Responders to Methotrexate (AIM) [Kremer et al. 2006] and Abatacept or Infliximab Versus Placebo, a Trial for Tolerability, Efficacy and Safety in Treating RA (ATTEST) [Schiff et al. 2008] trials in RA patients after inadequate response to MTX; and the Abatacept Study to Gauge Remission and Joint Damage Progression in Methotrexate-naïve Patients with Early Erosive Rheumatoid Arthritis (AGREE) trial in methotrexate (MTX)-naïve patients, early RA patients [Westhovens et al. 2009].
ATTAIN
This study included 391 RA patients with an inadequate response to etanercept, infliximab, or both (at least 3 months treatment duration) [Genovese et al. 2005]. The subjects were randomized to receive abatacept or placebo on days 1, 15, and 29 and every 28 days thereafter for 6 months, each added to at least one traditional disease-modifying antirheumatic drug (DMARD).
The two key efficacy assessments for this study were both significantly in favor of abatacept. The rates of ACR20 (20% improvement in American College of Rheumatology [ACR] response criteria) response at 6 months were 50.4% and 19.5% for abatacept and placebo, respectively (p < 0.001), while the proportions of patients with clinically meaningful improvement in physical function (noted as improvement of ≥0.3 points from baseline on the Health Assessment Questionnaire Disability Index [HAQ-DI]) were 47.3% and 23.3% for abatacept and placebo, respectively (p < 0.001).
AIM
The AIM study included 652 patients with persistent, active RA despite treatment with MTX [Kremer et al. 2006]. They were randomized to receive IV abatacept (~10 mg/kg) or placebo in addition to background MTX. Over the study’s 1-year duration, abatacept plus MTX was found to have significantly improved all three of the trial’s coprimary endpoints (ACR20 at 6 months, clinically meaningful improvements in physical function, and change from baseline in joint erosion score at 1 year) compared with placebo plus MTX. The overall incidence of adverse events (AEs) was similar in both arms, but the rates of prespecified serious infections (2.5% versus 0.9%) and infusion reactions were higher with abatacept plus MTX compared with placebo plus MTX.
ATTEST
ATTEST was a 6-month study that also included a population of 431 RA patients with inadequate response to MTX [Schiff et al. 2008]. The patients were randomized to receive IV abatacept (~10 mg/kg), IV infliximab (3 mg/kg), or placebo, each in addition to background MTX. Mean baseline 28-point disease activity score (DAS28) was 6.8.
The mean change from baseline in DAS28 score at 6 months (primary efficacy endpoint) was −2.53 for abatacept, –2.25 for infliximab, and −1.48 for placebo. Both the active treatments were significantly superior to placebo (p < 0.001), but the study was not powered to detect differences in efficacy between the two active treatment arms. During the entire double-blind period of 12 months (day 1 to day 365), serious AEs (SAEs), serious infections, and discontinuations due to AEs were lower with abatacept than infliximab.
AGREE
This study involved 509 patients with early RA (2 years or less disease duration) and poor prognostic factors (≥12 tender and 10 swollen joints, C-reactive protein [CRP] ≥0.45 mg/dl or greater, rheumatoid factor [RF] and/or anti-cyclic citrullinated peptide [anti-CCP] antibody seropositivity and radiographic evidence of bone erosion of the hands/wrists/feet) [Westhovens et al. 2009]. The mean DAS28 at baseline was 6.3.
Patients were randomized to receive abatacept (~10 mg/kg) plus MTX, or placebo plus MTX. For the primary endpoint (DAS28-defined remission) abatacept plus MTX was found to be significantly superior to placebo plus MTX, with 41.4% and 23.3% of patients, respectively, achieving DAS28 remission after 1 year (p < 0.001). The investigators also observed significantly less radiographic progression with abatacept plus MTX relative to placebo plus MTX. Safety and tolerability were comparable for both arms.
Safety and tolerability
The safety and tolerability of the abatacept IV formulation across the clinical trial program has been reviewed [Khraishi et al. 2010]. This analysis included a review of the long-term extensions of the controlled studies. The authors reported that, across the studies, discontinuation rates due to AEs ranged from 1.8% to 8.7%, serious infections were reported in 3.0% of patients (compared with 1.9% in placebo groups) and malignancies in 3.7% (compared with 2.9% in placebo groups). The safety findings in the long-term extensions were similar to those of the original controlled studies.
SC abatacept: clinical trial program
Just as the studies described above were instrumental in the approval of the IV formulation of abatacept for the treatment of RA, a substantial body of evidence has also been compiled to support the approval of the SC abatacept formulation. The following section provides an overview of the key findings of the research conducted to date with the SC formulation. Pharmacokinetic variables and dose-finding data will be briefly summarized [Corbo et al. 2009]. The major clinical trial research summarized below includes head-to-head comparisons of SC abatacept against IV abatacept (Abatacept Comparison of Subcutaneous versus Intravenous in Inadequate Responders to Methotrexate [ACQUIRE] study [Genovese et al. 2011]) and against SC adalimumab (Abatacept Versus Adalimumab Comparison in Biologic-Naive RA Subjects With Background Methotrexate [AMPLE] study [Weinblatt et al. 2013]). The data from the ACQUIRE and AMPLE trials has also been analyzed to determine whether or not there is utility in including a loading dose of abatacept in the dosing regimen for the SC formulation [Schiff et al. 2012]. In addition, researchers have evaluated the feasibility of switching from the abatacept IV formulation to the SC formulation (Abatacept in Subjects who Switch from Intravenous to Subcutaneous Therapy [ATTUNE] study [Keystone et al. 2012]) and of withdrawing therapy and restarting treatment in the event of relapse (Evaluation of Abatacept Administered SubcutaneousLy in Adults With Active Rheumatoid Arthritis: Impact of Withdrawal and Reintroduction on Immunogenicity, Efficacy and Safety [ALLOW] study [Kaine et al. 2012]. The impact of MTX on the immunogenicity of SC abatacept has been independently evaluated [Nash et al. 2013]. Finally, pooled safety data for the SC formulation have also been presented [Alten et al. 2011].
Pharmacokinetics and dose-finding
A double-blind, randomized, placebo-controlled phase IIa study was conducted examining various dosing regimens for SC abatacept in patients with active RA [Corbo et al. 2009]. In parallel, pharmacokinetic parameters with SC abatacept were evaluated among normal healthy volunteers (NHVs).
Among 68 RA patients, the SC abatacept was administered in flat (125 mg weekly) and weight-tiered (75–200 mg weekly) dosing regimens. For their initial treatment, all patients received an IV loading dose 1 hour prior to SC dosing. The treatment duration was 12 weeks.
In the open-label, randomized, parallel-group, single-dose study in NHVs, receptor occupancy of CD86 was assessed by flow cytometry of whole blood samples after a single 750 mg IV infusion of abatacept.
The investigators found that flat SC dosing of 125 mg/week was associated with trough plasma concentrations similar to the approved IV dosing regimen across all weight ranges investigated. Data from the NHVs suggested that receptor saturation was optimal at these trough plasma concentrations. As such, the flat 125 mg weekly dose was selected as the therapeutic dose.
ACQUIRE
The objective of the ACQUIRE study [Genovese et al. 2011] was to compare the efficacy and safety of SC abatacept with that of IV abatacept in patients with RA and inadequate response to MTX. A total of 1457 patients were randomized to receive either abatacept 125 mg SC on days 1 and 8 and weekly thereafter, or abatacept ∼10 mg/kg IV on days 1, 15, and 29 and every 4 weeks thereafter. The SC group also had an IV loading dose of ~10 mg/kg administered on day 1. The study was designed to demonstrate noninferiority of the SC formulation compared with the IV formulation, with noninferiority defined as a maximum difference in the ACR20 response of −2.1% at Month 6. A number of other efficacy endpoints were assessed in this study, as were immunogenicity and safety.
The baseline characteristics and demographics were similar in the two treatment arms. With respect to the primary analysis, the proportions of patients achieving an ACR20 response at 6 months were 76.0% in the SC group and 75.8% in the IV group (Figure 1).
Figure 1.

AQUIRE: noninferiority of subcutaneous (SC) abatacept relative to intravenous (IV) abatacept.
ACR20, 20% improvement in American College of Rheumatology [ACR] response criteria; CI, confidence interval.
This estimated difference of 0.3% (95% confidence interval [CI] –4.2 to +4.8) confirmed the noninferiority of SC abatacept to IV abatacept.
Furthermore, the curves documenting the kinetics for ACR20 response, as well as those for ACR50 and ACR70 responses and the proportions of patients with HAQ-DI responses and LDA were largely super-imposable for both the IV and SC abatacept treatment arms.
Patients in both arms of the ACQUIRE study were also stratified by body weight (specified in the study protocol). The three prespecified subgroups were body weight less than 60 kg, 60–100 kg and greater than 100 kg. For each body-weight subgroup, there were no significant differences observed in ACR response rates (ACR20, ACR50, or ACR70) between the SC and IV treatment arms.
The proportions of AEs and SAEs were comparable in both treatment arms. The safety summary is shown in Table 1.
Table 1.
AQUIRE: SC abatacept versus IV abatacept, safety summary.
| Safety variable |
n (%) |
|
|---|---|---|
| SC abatacept + MTX (n = 736) | IV abatacept + MTX (n = 736) | |
| AEs | 493 (67.0) | 470 (65.2) |
| SAEs | 31 (4.2) | 35 (4.9) |
| Discontinuations due to AEs | 15 (2.0) | 25 (3.5) |
| Discontinuations due to SAEs | 8 (1.1) | 14 (1.9) |
| Infections | 234 (31.8) | 221 (30.7) |
| Serious infections | 5 (0.7) | 10 (1.4) |
| Discontinuations due to serious infections | 0 | 4 (0.6) |
| Malignancies | 3 (0.4) | 5 (0.7) |
| Autoimmune events | 7 (1.0) | 6 (0.8) |
| SC injection-site reactions | 19 (2.6) | 18 (2.5) |
| Deaths | 2 (0.3) | 5 (0.7) |
AE, adverse event; IV, intravenous; MTX, methotrexate; SAE, serious adverse event; SC, subcutaneous.
Discontinuations due to SAEs occurred in 1.9% of patients in the IV group and 1.1% of the SC group. This difference was attributed to the between-group difference in discontinuations due to serious infections (four patients in the IV group [0.6%] and none in the SC group). Abatacept-induced antibodies were detected in 1.1% of those in the SC group and 2.3% of those in the IV group [Genovese et al. 2011].
The ACQUIRE population has subsequently been evaluated in a long-term extension study [Genovese et al. 2012]. At the conclusion of the initial 6-month RCT, patients could opt to enter the open-label, long-term extension study, in which all patients received the SC formulation at a dose of 125 mg weekly. Data from this extension were presented at the 2012 meeting of the ACR. A total of 1372 patients had entered the long-term extension phase and 1134 patients remained on therapy [Genovese et al. 2012]. The median duration of exposure to abatacept in this analysis was 33 months (range 8–44 months). The investigators reported that the rates of serious adverse events, infections, serious infections and malignancy in the extension did not increase compared to those observed in the SC group in the initial 6-month study. Efficacy rates were also maintained during the extension period.
AMPLE
The AMPLE study [Weinblatt et al. 2013] was the first major RCT comparing two SC biologic medications for the treatment of RA. The study included 646 patients with active RA who had an inadequate response to MTX and were previously naïve to biologic therapy. The subjects were randomized to receive either SC abatacept (125 mg weekly) or SC adalimumab (40 mg every other week), each in combination with background MTX, for a period of 2 years.
The study was designed to show noninferiority of SC abatacept to SC adalimumab, with the primary efficacy endpoint of ACR20 response at 1 year. The maximum allowable difference in ACR20 response to establish noninferiority was −4.7%. A number of other efficacy endpoints were assessed, as were safety, tolerability, and immunogenicity.
In the primary outcome analysis, the proportions of patients with an ACR20 response at 1 year were 64.8% in the SC abatacept group and 63.4% in the SC adalimumab group (Figure 2). The estimated difference between treatment groups was 1.8% (95% CI −5.6% to +9.2%), which established the noninferiority of abatacept relative to adalimumab.
Figure 2.
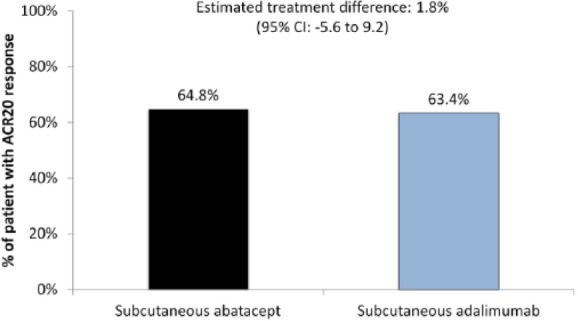
AMPLE: noninferiority of subcutaneous (SC) abatacept relative to SC adalimumab.
ACR20, 20% improvement in American College of Rheumatology [ACR] response criteria; CI, confidence interval.
Analysis of the kinetics of ACR20, ACR50, and ACR70 responses showed that the curves were similar in both groups. Similarly, the kinetics of change in DAS28 scores and the proportion of patients experiencing a response on the HAQ-DI were similar. The mean changes DAS28 scores at one year (from a baseline of 5.5 in each group) were −2.3 in the SC abatacept group and ‒2.27 in the SC adalimumab group. The proportions achieving remission and LDA as defined by DAS28 (scores of ≤2.6 and ≤3.2, respectively) were 43.3% and 59.3% for the SC abatacept group and 41.9% and 61.4% for the SC adalimumab group, respectively. In terms of tolerability and safety, discontinuation rates due to AEs were higher in the adalimumab groups, as was the incidence of injection-site reactions. The tolerability and safety summary is shown in Table 2.
Table 2.
AMPLE: SC abatacept versus SC adalimumab - 1-year safety summary.
| Safety variable |
n (%) |
|||
|---|---|---|---|---|
| One-year analysis | Two-year analysis | |||
| SC abatacept + MTX (n = 318) | SC adalimumab + MTX (n = 328) | SC abatacept + MTX (n = 318) | SC adalimumab + MTX (n = 328) | |
| AEs | 280 (88.1) | 283 (86.3) | 295 (92.8) | 300 (91.5) |
| SAEs | 32 (10.1) | 30 (9.1) | 44 (13.8) | 54 (16.5) |
| Discontinuations due to AEs | 11 (3.5) | 20 (6.1) | 12 (3.8) | 31 (9.5) |
| Discontinuations due to SAEs | 4 (1.3) | 10 (3.0) | 5 (1.6) | 16 (4.9) |
| Serious infections and infestations | 7 (2.2) | 9 (2.7) | 12 (3.8) | 19 (5.8) |
| Discontinuations due to serious infections | 0 | 5 (1.5) | 0 | 9 (2.7) |
| Malignancies | 5 (1.6) | 4 (1.2) | 7 (2.2) | 7 (2.1) |
| Autoimmune events | 10 (3.1) | 4 (1.2) | 12 (3.8) | 6 (1.8) |
| Local injection-site reactions | 12 (3.8) | 30 (9.1) | 13 (4.1) | 34 (10.4) |
| Deaths | 1 (0.3) | 0 | 1 (0.3) | 1 (0.3) |
AE, adverse event; MTX, methotrexate; SAE, serious adverse event; SC, subcutaneous.
The data for the complete 2-year randomized period of AMPLE have also been published [Schiff et al. 2014]. Of the patients originally randomized, 79.2% of the abatacept arm completed year 2, as did 74.7% of the adalimumab arm. All of the efficacy outcomes remained comparable between the two arms after 2 years. ACR20, ACR50, and ACR70 rates were 59.7%, 44.7%, and 31.1% in the abatacept arm and 60.1%, 46.6%, and 29.3% in the adalimumab arm. Radiographic progression over the 2-year period was assessed by change in Total Sharp Score (TSS). For the SC abatacept group, the mean change was 0.9 points from baseline; in the SC adalimumab group, the mean change from baseline was 1.1 points.
The same safety patterns observed in the 1-year analysis continued in the 2-year analysis. There were similar rates of AEs and SAEs in both groups, but a higher incidence of injection-site reactions, serious infections, and discontinuations due to AEs in the adalimumab arm (Table 2).
Loading dose or no loading dose?
The data from the ACQUIRE and AMPLE studies were used to determine whether or not an IV loading dose should be included in the dosing protocol for SC abatacept [Schiff et al. 2012]. As outlined above, all patients randomized to SC abatacept in each of these two studies received SC abatacept at a dose of 125 mg per week, in addition to background MTX. In ACQUIRE, patients received an IV loading dose of ~10 mg/kg, while no loading dose was administered in AMPLE.
Efficacy, assessed in a post-hoc analysis by ACR20 response rates and HAQ-DI scores, was found to be similar at days 15, 29, 57, 85, 113, 141, and 169. The investigators concluded that their findings suggest that SC abatacept can be effectively administered without an IV loading dose.
ATTUNE
This study addressed a practical question with respect to abatacept therapy [Keystone et al. 2012]: what is the impact of switching to SC abatacept among patients who are controlled on the IV formulation? This was a single-arm study in which 123 patients who had completed at least 4 years of therapy with IV abatacept (drawn from long-term extensions of the AIM and ATTAIN studies) were switched to SC abatacept 125 mg weekly, without an IV loading dose. The first SC dose was administered within 30 days of the last IV dose. The investigators assessed safety as the primary endpoint, along with immunogenicity and efficacy during the first 3 months following the switch.
At the time of the switch, the mean DAS28 score was 3.4 and the mean HAQ-DI was 0.94. At 3 months after the switch, 120 of the 123 subjects (97.6%) continued to receive weekly SC abatacept. None of the three patients who discontinued did so for lack of efficacy.
During the first 3 months post-switch, 49 patients (39.8%) reported AEs. There was one reported SAE and one patient discontinued due to an AE. There were two reported injection-site reactions (1.6% of patients). In terms of immunogenicity, eight patients were found to be seropositive during the 3 months after the switch. Six of these patients were seropositive prior to the switch to SC therapy.
After the switch, there was no significant change in clinical efficacy; DAS28 and HAQ-DI scores remained stable in this population that was clinically stable at baseline. Limited impact on immunogenicity was observed when switching routes of administration.
ALLOW
One of the current trends in research with biologic medications is to determine the impact of medication discontinuation and re-introduction of the agent. The ALLOW study [Kaine et al. 2012] assessed the impact of discontinuation and re-introduction of SC abatacept among 120 patients who had initially responded to 3 months of SC abatacept.
In this study, 167 patients were initially treated with SC abatacept 125 mg weekly plus MTX. After 12 weeks on this open-label treatment, those subjects who had achieved a response (reduction of at least 0.6 points on the DAS28) were randomized to continue with SC abatacept treatment or have their abatacept replaced with placebo for the subsequent 12 weeks (double-blind). After that 12-week period, those who had abatacept discontinued were again randomized to placebo or a retreatment with SC abatacept (with an IV loading dose).
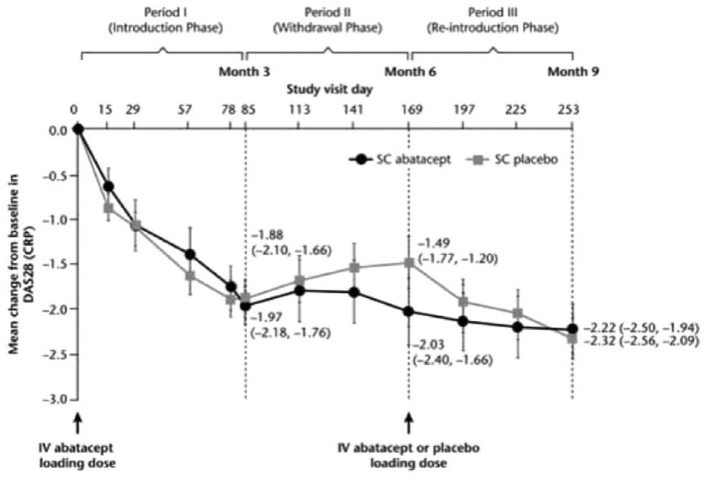
A total of 120 patients (72%) achieved the DAS28 response of at least 0.6 points during the initial 12 weeks. During the subsequent two phases of the study, the investigators noted a worsening of efficacy among those who had their SC abatacept discontinued, while those who received continuous SC abatacept continued to improve. After re-introduction of the SC abatacept, efficacy was restored in these patients (Figure 3).
Figure 3.
ALLOW: withdrawal and re-introduction of SC abatacept, impact on efficacy.
CRP, C-reactive protein; DAS28, 28-point disease activity score; IV, intravenous; SC, subcutaneous.
Among those patients who had their SC abatacept discontinued, the rates of immunogenicity were 7/73 among the discontinued group and 0/38 among the continuously treated patients (p = 0.119). Safety was comparable regardless of whether or not SC abatacept was withdrawn or continued.
Abatacept in subjects with rheumatoid arthritis administered plus or minus background MTX subcutaneously (ACCOMPANY)
This study was designed to evaluate the impact of concomitant MTX on immunogenicity with SC abatacept immunogenicity. Safety and efficacy were also assessed as secondary endpoints. This was an open-label study in which 100 patients were stratified to receive SC abatacept (125 mg weekly), with or without concomitant MTX.
During the first 4 months, the rate of immunogenicity was 3.9% among those receiving both SC abatacept and MTX, and 4.1% among those receiving SC abatacept monotherapy. All of these cases proved to be transient, as there were no patients who were antibody positive at month 4 [Nash et al. 2013].
Integrated safety analysis
At the 2011 annual meeting of the European League Against Rheumatism (EULAR), researchers presented an integrated safety analysis of SC abatacept, using clinical trial data available up to that time [Alten et al. 2011]. This analysis included data from five clinical trials (short-term periods and long-term extensions), totaling 1879 patients with 3086 patient-years of exposure to SC abatacept, with treatment durations up to four-and-a-half years. The subjects included in the studies were both DMARD- and anti-TNF-inadequate responders.
Table 3 shows the safety summary of this integrated analysis, in comparison with the experience in the IV abatacept clinical trial program (n = 4149, with 12,132 patient-years of exposure to IV abatacept) [Becker et al. 2010].
Table 3.
Integrated safety analysis from the SC abatacept and IV abatacept clinical trial programs.
| SC abatacept n = 1879 3086 patient-years |
IV abatacept n = 4149 12,132 patient-years |
|||
|---|---|---|---|---|
| N | Events/100 patient-years (95% confidence interval) |
N | Events/100 patient-years (95% confidence interval) |
|
| Deaths | 17 | 0.55 (0.34–0.89) | 73 | 0.60 (0.47–0.76) |
| Overall SAEs | 274 | 9.53 (8.46–10.72) | 1373 | 14.61 (13.85–15.41) |
| Infections | 1013 | 53.91 (50.69–57.33) | 2998 | 75.68 (73.00–78.44) |
| Serious infections* | 59 | 1.94 (1.50–2.50) | 332 | 2.87 (2.57–3.19) |
| Malignancies, excluding nonmelanoma skin cancer | 21 | 0.68 (0.45–1.05) | 88 | 0.73 (0.58–0.89) |
| Autoimmune events | 39 | 1.28 (0.93–1.75) | 232 | 1.99 (1.74–2.26) |
Subset of infections.
AE, adverse event; IV, intravenous; SAE, serious adverse event; SC, subcutaneous.
Conclusion
Abatacept, one of the biologic disease-modifying agents currently recommended as a first-line biologic for the treatment of RA [Smolen et al. 2013; Bykerk et al. 2012], is the first of these agents to become available to clinicians and their patients in both an SC and an IV formulation.
As documented in this overview, both the IV and the SC formulations have undergone a rigorous evaluation in clinical trial programs. The studies evaluating the SC route have demonstrated that this formulation has pharmacokinetics, efficacy, and safety similar to the IV formulation. In addition, the AMPLE study has shown that SC abatacept has an efficacy profile similar to that of SC adalimumab, with lower rates of discontinuation and injection-site reactions.
Research with TNF-blocking agents has shown that for some patients, the SC route of administration is preferred, while for others, the IV route may be preferable [Scarpato et al. 2010]. Given that abatacept’s mechanism of action (selective costimulation modulator) is unique among available biologic agents for the treatment of RA, having this molecule available in both IV and SC formulations is a welcome expansion of the therapeutic armamentarium for RA.
Acknowledgments
Assistance with the writing and editing of the manuscript was provided by medical writer Scott Moffatt on behalf of STA HealthCare Communications, Montreal, Québec, and funded by Bristol-Myers Squibb.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Khraishi received a research grant from BMS Canada.
References
- Alten R., Kaine J., Keystone E., Nash P., Delaet I., Qi K., et al. (2011) Safety of subcutaneous abatacept in patients with rheumatoid arthritis (RA): Integrated analysis of five clinical trials up to 4.5 years [EULAR abstract #SAT0292]. Ann Rheum Dis 70(Suppl. 3): 617 [Google Scholar]
- Becker J., Westhovens R., Hochberg M., Qi K., Kelly S., Smitten A., et al. (2010) The long-term safety of abatacept in the treatment of rheumatoid arthritis: Integrated safety analyses from the abatacept clinical trial program [EULAR abstract #FRI0189]. Ann Rheum Dis 69(Suppl. 3): 377 [Google Scholar]
- Bombardier C., Hawker G., Mosher D. (2011) The impact of arthritis in Canada: today and the next 30 years. Toronto, ON: Arthritis Alliance of Canada [Google Scholar]
- Bykerk V., Akhavan P., Hazlewood G., Schieir O., Dooley A., Haraoui B., et al. (2012) Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol 39: 1559–1582 [DOI] [PubMed] [Google Scholar]
- Corbo M., Valencia X., Raymond R., Summerill R., Agrawal S., Townsend R., et al. (2009) Subcutaneous administration of abatacept in patients with rheumatoid arthritis: Pharmacokinetics, safety and immunogenicity [EULAR abstract SAT0101]. Ann Rheum Dis 68(Suppl. 3): 574 [Google Scholar]
- Genovese M., Becker J., Schiff M., Luggen M., Sherrer Y., Kremer J., et al. (2005) Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med 353: 1114–1123 [DOI] [PubMed] [Google Scholar]
- Genovese M., Covarrubias A., Leon G., Mysler E., Keiserman M., Valente R., et al. (2011) Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum 63: 2854–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M., Pacheco-Tena C., Covarrubias A., Leon G., Mysler E., Keiserman M., et al. (2012) Subcutaneous Abatacept: long-term data from the Acquire trial [ACR abstract #462]. Arthritis Rheum 10(Suppl.): S201 [Google Scholar]
- Gibofsky A. (2012) Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care 18: S295–S302 [PubMed] [Google Scholar]
- Kaine J., Gladstein G., Strusberg I., Robles M., Louw I., Gujrathi S., et al. (2012) Evaluation of abatacept administered subcutaneously in adults with active rheumatoid arthritis: impact of withdrawal and reintroduction on immunogenicity, efficacy and safety (phase IIIb ALLOW study). Ann Rheum Dis 71: 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone E., Kremer J., Russell A., Box J., Abud-Mendoza C., Elizondo M., et al. (2012) Abatacept in subjects who switch from intravenous to subcutaneous therapy: results from the phase IIIb ATTUNE study. Ann Rheum Dis 71: 857–861 [DOI] [PubMed] [Google Scholar]
- Khraishi M., Russell A., Olszynski W. (2010) Safety profile of abatacept in rheumatoid arthritis: a review. Clin Ther 32: 1855–1870 [DOI] [PubMed] [Google Scholar]
- Kremer J., Genant H., Moreland L., Russell A., Emery P., Abud-Mendoza C., et al. (2006) Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann Intern Med 144: 865–876 [DOI] [PubMed] [Google Scholar]
- Myasoedova E., Crowson C., Kremers H., Therneau T., Gabriel S. (2010) Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum 62: 1576–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash P., Nayiager S., Genovese M., Kivitz A., Oelke K., Ludivico C., et al. (2013) Immunogenicity, safety, and efficacy of abatacept administered subcutaneously with or without background methotrexate in patients with rheumatoid arthritis: results from a phase III, international, multicenter, parallel-arm, open-label study. Arthritis Care Res (Hoboken) 65: 718–728 [DOI] [PubMed] [Google Scholar]
- Scarpato S., Antivalle M., Favalli E., Nacci F., Frigelli S., Bartoli F., et al. (2010) Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology (Oxford) 49: 289–294 [DOI] [PubMed] [Google Scholar]
- Schiff M., Keiserman M., Codding C., Songcharoen S., Berman A., Nayiager S., et al. (2008) Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 67: 1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M., Alten R., Weinblatt M., Nash P., Fleischmann R., Durez P., et al. (2012) Weekly subcutaneous abatacept confers comparable onset of treatment response and magnitude of efficacy improvement over 6 months when administered with or without an intravenous abatacept loading dose [ACR abstract #2547]. Arthritis Rheum 10(Suppl.): S1076 [Google Scholar]
- Schiff M., Weinblatt M., Valente R., van der Heijde D., Citera G., Elegbe A., et al. (2014) Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis 73: 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J., Furst D., Bharat A., Curtis J., Kavanaugh A., Kremer J., et al. (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 64: 625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J., Aletaha D., Bijlsma J., Breedveld F., Boumpas D., Burmester G., et al. (2010) Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 69: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J., Landewé R., Breedveld F., Buch M., Burmester G., Dougados M., et al. (2013) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. DOI: 10.1136/annrheumdis-2013-204573 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhovens R., Robles M., Ximenes A., Nayiager S., Wollenhaupt J., Durez P., et al. (2009) Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann Rheum Dis 68: 1870–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt M., Schiff M., Valente R., van der Heijde D., Citera G., Zhao C., et al. (2013) Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum 65: 28–38 [DOI] [PMC free article] [PubMed] [Google Scholar]