Abstract
Patients with advanced stage or recurrent cervical cancer represent a population with limited chemotherapeutic options. More specifically, patients with recurrent disease have a poor salvage rate, with a 5-year survival rate of less than 10%. This year, the first prospective phase III clinical trial exploring the anti-angiogenic agent, bevacizumab, was published, meeting its primary endpoint, with a significant improvement in overall survival. As such, a review of anti-angiogenic therapy in the treatment of this disease is warranted.
Keywords: angiogenesis, bevacizumab, cervical cancer
Introduction
In 2011 an estimated 529,800 cases of cervical cancer were diagnosed worldwide, with 275,100 deaths [Jemal et al. 2011]. Regionally, in the United States, an estimated 12,360 cases will be diagnosed in 2014, with 4020 deaths [Siegel et al. 2014]. In the European Union, the crude incidence of cervical cancer is 13.2/100,000, and the crude mortality is 5.9/100,000 women/year [Lynge et al. 2009]. This discrepancy between global and regional disease burden is attributable to the disproportionately high burden of cervical cancer in developing, resource-poor countries lacking adequate healthcare infrastructure and screening programs. Nonetheless, despite advances in screening, vaccination and treatment of early stage disease, a proportion of patients will be diagnosed with advanced stage (stage IVB), recurrent or persistent cervical cancer.
Chemotherapeutic options for patients with advanced stage or recurrent disease have been explored and are based on phase II and III clinical trials completed under the auspices of cooperative groups, most notably the Gynecologic Oncology Group (GOG). In this setting, goals of care are centered on disease control and palliation of symptoms, as cure is exceedingly rare [Greer et al. 2010]. Since Dr Thigpen’s initial paper in 1981, a number of chemotherapeutic agents, both single drug and combination regimens, have been studied in the treatment of advanced and metastatic cervical cancer with limited gains in overall survival (OS) [Thigpen et al. 1981, 1995; McGuire et al. 1989, 1996; Sutton et al. 1989; Look et al. 1998; Bookman et al. 2000; Schilder et al. 2000; Curtin et al. 2001; Fracasso et al. 2003; Muggia et al. 2005; Garcia et al. 2007; Omura et al. 1997; Bloss et al. 2002; Moore et al. 2004; Long et al. 2005; Monk et al. 2009b]. Importantly, cisplatin + paclitaxel has been established as the backbone for future trials, although OS approaches only 13 months in highly selected populations [Thigpen et al. 1981].
The poor oncologic outcome in this patient population represents and unmet clinical need, and catalyzed the exploration of novel treatment paradigms. In an era of molecular medicine, the development of biologic therapies, to be used solely or in conjunction with cytotoxic chemotherapy, is implicit. Following publication of the results of GOG 240, a renewed interest in utilization of anti-angiogenic therapies for the treatment of cervical cancer has emerged. This review article discusses the concept of angiogenesis within the tumor microenvironment, describes the biologic rational of anti-angiogenic therapy in cervical cancer and subsequently details clinical trials investigating various anti-angiogenic agents in the treatment of this disease.
Angiogenesis
Targeting the cancer microenvironment, specifically neovascularization, via vascular endothelial growth factor (VEGF) pathway inhibition, evolved as our understanding of tumor biology was refined over the past three decades. Following several, novel, in vitro and in vivo studies, it became evident that angiogenesis was essential for tumor invasion and metastasis, and was required for tumor growth beyond 1–2 mm3 [Folkman, 1971; Eskander et al. 2011]. This process requires the recruitment of vasculature, circulating endothelial cells and pro-angiogenic mediators (Yancopoulos et al. 2000).
All members of the VEGF family of ligands stimulate cellular responses by binding to tyrosine kinase receptors on the cell surface causing dimerization and activation through trans-phosphorylation. The three main subtypes of the VEGF receptors (VEGFRs) are numbered VEGFR-1, VEGFR-2 and VEGFR-3. Typically membrane-bound, the VEGFRs have an extracellular portion consisting of seven immunoglobulin-like domains, a single transmembrane spanning region and an intracellular portion containing the split tyrosine kinase domain. Through alternative splicing, cytoplasmic VEGFRs can also exist.
In 1993, following several years of collaborative laboratory efforts between the University of California San Francisco and Genentech, Dr Ferrara’s laboratory reported that anti-VEGF monoclonal antibodies exerted a potent inhibitory effect on the growth of three tumor cell lines injected subcutaneously into nude mice[Kim et al. 1993]. Interestingly, the antibody had no effects on the cell lines in vitro. Several parallel studies confirmed in vivo growth inhibition, which correlated with decrease tumor microvessel density and inhibition of tumor angiogenesis[Kim et al. 1993; Warren et al. 1995; Borgstrom et al. 1996, 1998, 1999]. Ultimately, bevacizumab, a humanized monoclonal antibody directed against VEGF, was synthesized and used in early proof of concept studies. Bevacizumab neutralizes VEGF-A and blocks its signal transduction through both VEGFR-1 and VEGFR-2, as demonstrated by the inhibition of VEGF-induced cell proliferation, survival, permeability, nitric oxide production, migration and tissue factor production.
The first phase I clinical trial assessing the safety, pharmacokinetics and tolerability of bevacizumab was conducted in 1997 [Margolin et al. 2001]. In the United States, bevacizumab gained Food and Drug Administration (FDA) approval in February 2004, following a randomized double-blind phase III clinical trial assessing the impact of addition of bevacizumab to irinotecan, 5-fluorouracil and leucovorin (IFL) in the upfront treatment of patients with unresectable metastatic colorectal cancer (OS survival advantage) [Hurwitz et al. 2004]. Additional phase III trials were conducted in metastatic nonsmall cell lung cancer (OS advantage) [Sandler et al. 2006], metastatic breast cancer (mBC) [Miller et al. 2007], renal cell carcinoma (progression-free survival advantage), and recurrent glioblastoma multiforme (progression-free survival advantage), all of which met their primary endpoints, thus supporting FDA approval of bevacizumab for these indications [Escudier et al. 2007]. Importantly, in November 2011, FDA commissioner Margaret Hamburg revoked accelerated approval of bevacizumab in HER2-negative mBC, given lack of an OS advantage and a lack of improvement in quality of life.
Angiogenesis and cervical cancer: the biologic rationale
The limited success achieved with traditional cytotoxic chemotherapy in patients with recurrent and metastatic cervical cancer is multifactorial. Patients with cervical cancer represent a unique population, as radiation and chemotherapy exposure for primary therapy are hypothesized to alter the disease biology. Upfront chemosensitizing radiation therapy may select for radio-resistant and/or chemotherapy-resistant cell populations, particularly if there is crossover with respect to mechanisms of drug resistance. Additionally, these patients commonly suffer from obstructive uropathy and acute kidney injury, limiting clearance of cytotoxic compounds from the systemic circulation. Lastly, cancer foci recurring in the previously irradiated field may have compromised blood supply and relative hypoxia, limiting delivery of cytotoxic drugs. These unique characteristics may explain the limited response to retreatment with traditional chemotherapy and highlight the importance of studying novel biologic strategies.
Biologically, tumor neovascularization imparts an aggressive course in cervical cancer, where aberrant and abnormal vascularity identified on colposcopic examination may indicate invasive disease. Mechanistically, this is explained by the effects of E6/E7 on downstream angiogenic pathways. Upregulation of the E6 oncoprotein is hypothesized to directly stimulate VEGF production [Lopez-Ocejo et al. 2000; Toussaint-Smith et al. 2004]. In elegant transgenic mice experiments, Coussens and colleagues were able to reproduce progressive invasive squamous cell carcinoma of the epidermis with HPV 16, E6 and E7 oncogene expression [Coussens et al. 1996]. Additionally, angiogenic proliferation along the basement membrane of premalignant cervical lesions, infected with high risk human papilloma virus (HPV) subtypes, was demonstrated in archival tissue specimens, further validating activation of the angiogenic switch as mediated by HPV infection [Smith-Mccune and Weidner, 1994]. Ultimately, E6 mediated degradation of p53 and E7 inactivation of pRb result in increased VEGF and hypoxia inducible factor 1α (HIF1α), promoting angiogenesis and tumor growth (Figure 1).
Figure 1.
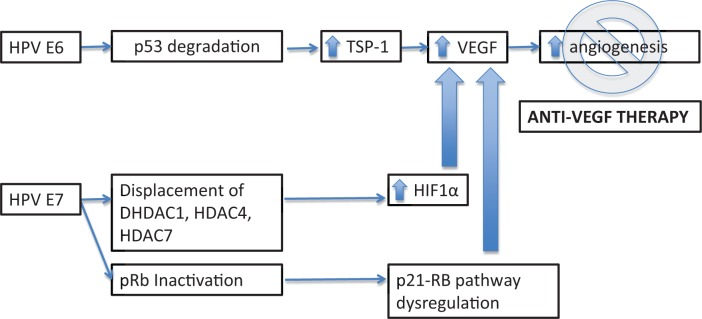
Biologic rational of anti-angiogenic therapy in the treatment of cervical cancer.
HIF1α, hypoxia inducible factor 1α; HPV, human papilloma virus; TSP-1, thrombospondin-1; VEGF, vascular endothelial growth factor.
Clinical evidence that angiogenesis plays an important role in locally advanced cervical cancer has accumulated over the past decade. Specifically, overexpression of VEGF has been associated with adverse oncologic outcomes in numerous solid tumors, including cervical cancer [Kudelka et al. 1997, 1998; Cooper et al. 1998; Ferrara et al. 2004; Ferrara, 2005; Ferrara and Kerbel, 2005]. In an early study, Cooper and colleagues assessed tumor angiogenesis in 111 archived tumor specimens by measuring intratumor microvessel density (IMD) [Cooper et al. 1998]. High IMD was associated with a poor prognosis and remained a significant prognostic factor within a Cox multivariate analysis. More recently, intratumoral VEGF was shown to be upregulated in cervical cancer specimens relative to control cervical tissues, with higher VEGF levels being associated with advanced stage, increase risk of nodal metastasis, and worse progression-free survival (PFS) and OS [Guidi et al. 1995; Cheng et al. 2000; Loncaster et al. 2000; Lee et al. 2005]. Additionally, cluster of differentiation 31 (CD31) expression, found on endothelial cell surfaces, and used as an immunohistochemical marker of angiogenesis, has been shown to be significantly associated with tumor size and the presence of lymph vascular space involvement in patients with clinical stage 1B squamous cell cervical cancer [Silva-Filho et al. 2006]. In a post-trial ad hoc analysis of GOG protocol 109, a total of 173 tumor tissue specimens were assessed for expression of markers of tumor angiogenesis including VEGF, thrombospondin-1 (TSP-1, anti-angiogenesis factor), CD31 and CD105 (tumor-specific endothelial marker) [Randall et al. 2009]. After adjusting for prognostic clinical covariates, high CD31 microvessel density was an independent prognostic factor for PFS [hazard ratio (HR) = 0.36; 95% confidence interval (CI): 0.17–0.75; p = 0.006) and OS (HR = 0.36; 95% CI: 0.17–0.79; p = 0.010).
To date, several parallel strategies targeting angiogenesis have been explored, with VEGF dependent and non-VEGF dependent downstream targets (Monk et al. 2013).
Bevacizumab and cervical cancer
Anti-angiogenic therapy in cervical cancer has shown promising results compared with historical cohorts. In the first case series describing the use of bevacizumab in patients with recurrent cervical cancer, despite heavy pretreatment (the patients had a median of 3 prior regimens), there was a 67% overall response rate [Wright et al. 2006]. Treatment was well tolerated, with only one grade 4 adverse event (AE) noted (table 1).
Table 1.
Bevacizumab studies in cervical cancer.
| Study | Drug | n | Eligibility | Pathology | OS (months) | PFS (months) | RR (%) | Grade 3–4 AEs |
|---|---|---|---|---|---|---|---|---|
| Wright et al. [2006] | 5-FU or capecitabine + bevacizumab (5-15 mg/kg IV Q3 weeks) | 6 | Retrospective series | Squamous, adenosquamous, poorly differentiated | 5.1 | NR | 33 | Neutropenia (17%), anemia (17%), thrombocytopenia (17%), fatigue (33%), diarrhea (17%), nausea (33%), bowel obstruction (17%) |
| Monk et al. [2009a] | bevacizumab 15 mg/kg Q3 weeks | 46 | Second line (74%); third line (26%); GOG PS 0-2 | Squamous, adenosquamous | 7.3 | 3.4 | 35 | HTN (15%), thromboembolism (11%), anemia (4%), vaginal bleeding (2%), neutropenia (2%), pain (13%), GI (8.7%), cardiovascular (4.3%), pulmonary (2%), fistula (2%) |
| Schefter et al. [2014] | cisplatin 40 mg/m2+ radiation therapy + brachytherapy + bevacizumab 10 mg/kg Q2 weeks for 3 cycles | 49 | Untreated patients with stage 1B–3B cervical cancer | Squamous (80%) | 3-year OS: 81.3% | 3-year DFS 68.7% | NR | No treatment related SAEs; hematalogic AE 80% |
| Zighelboim et al. [2013] | cisplatin 50 mg/m2 day 1 + Topotecan 0.75 mg/m2 days 1, 2, 3 + bevacizumab 15 mg/kg day 1 Q3 weeks | 27 | First recurrence; GOG PS 0-1 | Squamous (67%), adenocarcinoma (33%) | 13.2 | 7.1 | 35 | Leukopenia (74%), neutropenia (56%), thrombocytopenia (81%), anemia (63%), GI (19%), pain (33%), metabolic (48%), infection (19%) |
AEs = adverse events; DFS = disease-free survival; GI = gastrointestinal; GOG = Gynecologic Oncology Group; HTN = hypertension; n = number of subjects; NR = not reported; OS = overall survival; PFS = progression-free survival; PS = performance status; Q = every; RR = response rate; SAE, serious adverse effect.
These preliminary results catalyzed the synthesis and opening of GOG protocol 227C, a phase II trial designed to evaluate the efficacy and tolerability of bevacizumab in the treatment of recurrent cervical cancer [Monk et al. 2009a] (Table 1). Amongst the 46 eligible and evaluable patients, 38 (82.6%) received prior pelvic radiation as well as either one (n = 34; 74%) or two (n = 12; 26%) cytotoxic regimens for recurrent disease. A total of 11 patients (23.9%; 2-sided 90% CI: 14–37%) survived progression free for at least 6 months, and five patients (10.9%; 2-sided 90% CI, 4–22%) had partial responses, with a median response duration of 6.2 months (range: 2.83–8.28 months). The median PFS and OS were 3.40 months (95% CI: 2.53–4.53 months) and 7.29 months (95% CI, 6.11–10.41 months), respectively. These results compared favorably with historical phase II GOG trials in this setting [Tewari and Monk, 2005].
Given the clinical activity noted in the pretreated population, Radiation Therapy Oncology Group (RTOG) protocol 0417 was developed, exploring the safety and efficacy of the addition of bevacizumab to standard chemoradiation (CRT) [Schefter et al. 2012] (Table 1). Between 2006 and 2009 a total of 60 patients were enrolled. The median follow up was 12.4 months (range: 4.6–31.4 months). Most patients had (International Federation of Gynecology and Obstetrics (FIGO) stage 2B (63%) disease and with a Zubrod performance status (PS) of 0 (67%). A total of 80% of cases were squamous. There were no treatment-related serious AEs. More recently, oncologic outcomes were published, with 81% 3-year OS and a 23% locoregional failure rate [Schefter et al. 2014].
Another phase II clinical trial exploring the combination of cisplatin 50 mg/m2 day 1 + topotecan 0.75 mg/m2 days 1, 2, 3 + bevacizumab 15 mg/kg day 1 on a 21 day cycle was recently published [Zighelboim et al. 2013]. A total of 27 patients with persistent or recurrent cervical cancer, with no prior chemotherapy for recurrence, were enrolled. The 6-month PFS was 59% (80% CI: 46–70%), with median PFS and OS of 7.1 months and 13.2 months respectively. Unfortunately, grade 3–4 hematologic toxicity was common (thrombocytopenia 82%, leukopenia 74%, anemia 63%, neutropenia 56%) on this treatment regimen. The majority of patients (78%) required unanticipated hospital admissions for supportive care and/or management of toxicities.
Prospective phase III clinical trial incorporating bevacizumab in the treatment of cervical cancer: GOG 240
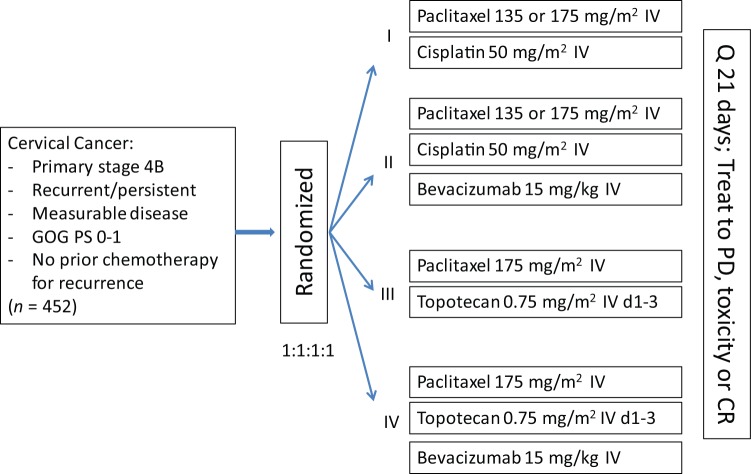
Following the results of GOG 227C (described earlier), advancement of bevacizumab into the phase III arena was deemed a scientific priority. Ultimately, GOG 240, a four-arm prospective randomized trial exploring platinum and nonplatinum doublets with and without the anti-angiogenic agent bevacizumab was designed, meeting its accrual goal in less than 3 years (Figure 2) [Tewari et al. 2014].
Figure 2.
Gynecologic Oncology Group protocol 240 schema.
CR, complete response; IV, intravenous; PD, progressive disease; PS = performance status; Q = every.
From April 2009 to January 2012, the trial accrued 452 patients. Over 220 patients were treated with each of the chemotherapy backbones and patients were well matched for histology (p = 0.308), ethnicity (p = 0.800), as well as disease status: recurrent versus persistent versus advanced (p = 0.298). Notably, the majority of patients on each chemotherapy backbone had a GOG PS of 0 (PS 0–1 required for enrollment). As anticipated, 75% of the entire study group had previously received platinum and this was evenly distributed between the 2 backbones (p = 0.666). The topotecan + paclitaxel arm was shown to not be superior or inferior to the cisplatin + paclitaxel arm (HR 1.20; 95% CI: 0.82–1.76). Median OS in the topotecan containing doublet was 12.5 months versus 15 months in the cisplatin + paclitaxel arm. These results were interpreted as indicating that the nonplatinum chemotherapy doublet was not superior to cisplatin-paclitaxel for efficacy.
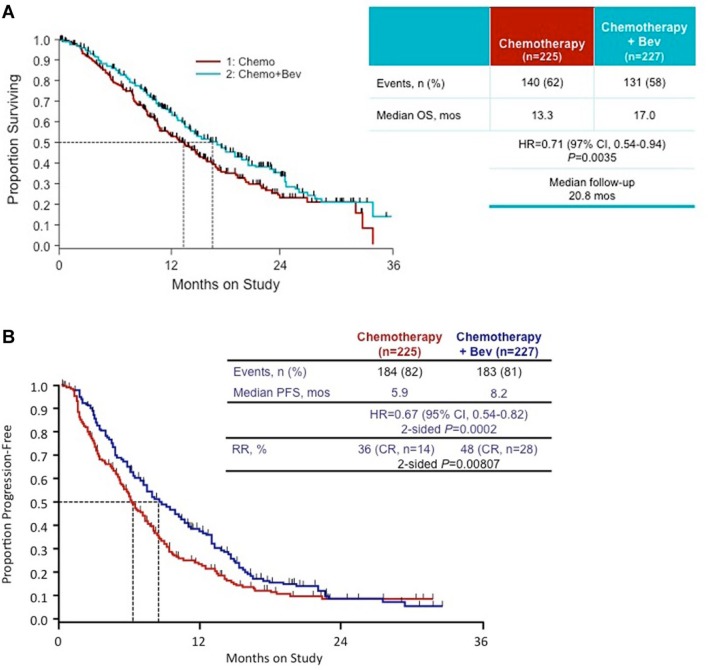
Importantly, the investigators showed a significant improvement in OS in the bevacizumab containing arms relative to nonbevacizumab controls (17 months versus 13.3 months respectively; HR = 0.71; 95% CI: 0.54–0.95; p = 0.0035) (Figure 3). Analogous improvements in PFS were identified (8.2 months bevacizumab containing arm and 5.9 months in control arm) (HR = 0.67; 95% CI: 0.54–0.82; p = 0.0002). Exploratory sub-analysis further indicated the beneficial effects of bevacizumab in patients with prior platinum exposure, recurrent or persistent disease and squamous histology. Importantly, the benefits of bevacizumab persisted in patients with recurrent disease in a previously irradiated field, which was hypothesized to be relatively hypoxic. These findings represent the first time a targeted anti-angiogenic agent has shown an improvement in OS in patients with gynecologic cancer.
Figure 3.
Overall survival (3A) and progression-free survival (3B) in chemotherapy alone versus chemotherapy + bevacizumab arms on Gynecologic Oncology Group 240 [reproduced with permission from Tewari et al. 2014].
bev, bevacizumab; CR, complete response; CI, confidence interval; HR, hazard ratio; mos, months; OS, overall survival; PFS, progression-free survival; RR, response rate.
As with all novel agents, therapeutic benefits must be weighed against potential toxicity and impact on quality of life. Within the bevacizumab-containing arms, there was an increase in grade ≥3 GI and genito-urinary (GU )fistula (n = 5), as well as grade ≥2 hypertension, grade ≥4 neutropenia and grade ≥3 thrombocytopenia. This did not translate into a significant deterioration in health-related quality of life (QoL). The full QOL data have yet to be published and the effects of bevacizumab on symptom palliation are yet to be determined. Additionally, the impact of bevacizumab on GI and GU fistula formation is being investigated in an ancillary data study in an effort to identify cervical cancer patients at risk for this life altering complication.
The significant improvement in OS identified in GOG protocol 240 is linked to the limited postprogression survival in this patient population due to lack of effective salvage strategies. As explained by Broglio and colleagues, a significant PFS advantage (HR = 0.73, p = 0.03) translates into a significant OS advantage (HR = 0.61, p = 0.008) when median survival postprogression on study is estimated to be 6 months [Broglio and Berry, 2009]. Conversely, in the setting where alternate treatment options exist, the postprogression survival is extended and the survival advantage is diluted. With an analogous calculated PFS, in a simulated study, the HR for OS would rise to 1.29 (p = 0.262) if postprogression survival was estimated to be 18 months.
As detailed above, the burden of cervical cancer is greatest in impoverished, resource-poor countries lacking effective screening modalities. The cost implications and feasibility of incorporating anti-angiogenic agents into the treatment algorithm of advanced cervical cancer on a global level are understood. Traditionally, cost-effectiveness studies for novel agents do not perform in favor of the drug primarily due to drug costs and expenditures associated with marketing, which partially offset the extensive costs encountered during the drug development and clinical trial phases. Unless the agent can effectively reduce treatment-related toxicities and prolong survival at a reasonable cost, the new drug will likely be cost-ineffective. However, with time the costs of new therapies are expected to decrease, resulting in improvement in the cost-effectiveness ratio. During the early days of highly active antiretroviral therapy for acquired immunodeficiency syndrome (AIDS), drug costs appeared prohibitive, but currently protease inhibitors and zidovudine (AZT) are widely distributed in resource poor, impoverished countries in Africa [Montaner et al. 2006].
Multitargeted VEGFR tyrosine kinase inhibitors (TKIs)
Based on the promising results identified with use of bevacizumab, exploration of alternate, novel anti-angiogenic agents, targeting parallel pathways commenced. Preclinical models illustrated that HPV-associated cervical cancer-derived cell lines co-expressed the receptor tyrosine kinase (RTK) cKit, implicating cKit in cervical carcinogenesis [Caceres-Cortes et al. 2001].
Additionally, fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) have been identified as targets in the angiogenic cascade. PDGF binding to the PDGF receptor β (PDGFR-β) is essential for pericyte recruitment and blood vessel maturation[Jain and Booth, 2003]. The FGF family of ligands activates angiogenesis via interaction with FGF receptors 1 and 2 [Rusnati and Presta, 2007]. Interestingly, it is thought that signaling via these alternate pathways (PDGF, FGF) may mediate resistance to VEGF inhibition, supporting a multi-targeted approach[Benjamin et al. 1998, Erber et al. 2004, Casanovas et al. 2005, Batchelor et al. 2007, Lu et al. 2008]. In response to receptor-ligand binding, downstream signaling pathways, PI3K-Akt-mTOR and Ras-MEK-Erk are activated and have been identified as potential targets for drug development [Ferrara et al. 2003; Ferrara and Kerbel, 2005].
To date, the largest study exploring nonbevacizumab anti-angiogenic agents in the treatment of cervical cancer was reported in August 2010. Monk and colleagues studied pazopanib and lapatinib as single agents and in combination in patients with stage 4B persistent/recurrent cervical carcinoma not amenable to curative therapy and at least one prior regimen in the metastatic setting [Monk et al. 2010] (Table 2). The primary endpoint was PFS, and secondary endpoints were overall OS, response rate (RR) and safety.
Table 2.
Nonbevacizumab anti-angiogenic trials in cervical cancer.
| Study | Drug | n | Eligibility | Pathology | OS (months) | PFS (months) | RR (%) | Grade 3–4 AEs |
|---|---|---|---|---|---|---|---|---|
| Monk et al. [2010] | pazopanib 800 mg PO QD | 74 | Stage 4B cervical cancer with >1 prior regimen; ECOG PS 0-1 | Squamous, adenocarcinoma, adenosquamous, other | 12.4 | 4.5 | 9 | Diarrhea, nausea, anorexia, emesis, rash, fatigue, anemia, back pain, HTN (41–54% rate of grade 3-4 AEs in the single drug arms) |
| lapatinib 1500 mg PO QD | 78 | 11 | 4.3 | 5 | ||||
| pazopanib 1000 or 1500 mg PO QD + lapatinib 400 or 800 mg PO QD | 78 | NR | NR | NR | ||||
| Mackay et al. [2010] | sunitinib 25-50 mg/day for 4 weeks, 2 weeks off (6 week cycle) | 19 | Unresectable, locally advanced, metastatic or recurrent; ≤ 1 prior line of chemotherapy; ECOG PS 0-1 | Squamous, adenocarcinoma, adenosquamous | NR | 3.5 | 0 | Fatigue (16%), diarrhea (16%), HTN (11%), hand-foot syndrome (11%), anemia (24%), fistula (26%) |
AEs = adverse events; ECOG, Eastern Cooperative Oncology Group; GI = gastrointestinal; HTN = hypertension; n = number of subjects; NR = not reported; OS = overall survival; PFS = progression free survival; PO, by month; PS = performance status; RR = response rate; QD = daily.
Use of this combination regimen was based on preliminary data suggesting at least additive effects with combination of pazopanib and lapatinib in the treatment of solid cancers [Reardon et al. 2013]. Pazopanib is a potent, selective, oral multi targeted RTK inhibitor that targets VEGFR, PDGF receptor (PDGFR) and c-Kit [Monk et al. 2010]. Prior studies confirmed its tolerability in the treatment of solid malignancies, and it is currently FDA approved for use in metastatic soft tissue sarcomas and metastatic renal cell carcinoma [Sloan and Scheinfeld, 2008, Hurwitz et al. 2009]. Conversely, lapatinib is an oral, small-molecule, dual TKI of epidermal growth factor receptor (EGFR) and Her2/neu and is approved by the FDA and the European Medicines Agency (EMA) for use in combination with capecitabine for the treatment of patients with Her2/neu-overexpressing mBC who have received prior therapy including an anthracycline, a taxane, and trastuzumab [Ryan et al. 2008].
Of 230 patients enrolled, 152 were randomly assigned to the monotherapy arms: pazopanib (n = 74) or lapatinib (n = 78). Importantly, the futility boundary was crossed at the planned interim analysis for combination therapy compared with lapatinib therapy and the combination arm was terminated. Most patients (62%) had recurrent cancer. Patients were randomly assigned to pazopanib daily, lapatinib daily, or lapatinib plus pazopanib combination therapy until progression or withdrawal because of AEs. Pazopanib improved PFS (HR = 0.66; 90% CI: 0.48–0.91; p = 0.013) and OS (HR = 0.67; 90% CI: 0.46–0.99; p = 0.045). Median OS was 50.7 weeks and 39.1 weeks, and RRs were 9% and 5% for pazopanib and lapatinib, respectively. The only grade 3 AE >10% was diarrhea (11% pazopanib and 13% lapatinib). Grade 4 AEs were 9% (lapatinib) and 12% (pazopanib). The results of this phase II study confirmed the activity of anti-angiogenic agents in advanced and recurrent cervical cancer, and demonstrated the benefit of pazopanib based on the prolonged PFS and favorable toxicity profile.
Sunitinib, an analogous, oral multi-TKI, exerts its anti-angiogenic effects via inhibition of VEGFR-1, -2 and -3, PDGF α and β, and related RTKs [Chow and Eckhardt, 2007]. Currently, sunitinib is FDA approved for use in patients with metastatic renal cell carcinoma and gastrointestinal stromal tumors. Given the central role RTKs play in tumor cell proliferation and angiogenesis, a phase II clinical trial was developed investigating the efficacy and safety of sunitinib in patients with unresectable, locally advanced or metastatic cervical carcinoma [Mackay et al. 2010] (Table 2). A total of 19 subjects were enrolled on this multicenter phase II study. Unfortunately, there were no documented objective responses on therapy, but morbidity was significant (fistula rate of 26%). Median time to progression was reported as 3.5 months. Given lack of signal, it was determined that sunitinib has insufficient activity as a single agent in cervical cancer to warrant further investigation.
Anti-vascular strategies in the treatment of cervical cancer
Interest into the study of vascular disrupting agents (VDAs) emerged in an effort to circumvent acquired resistance to traditional anti-angiogenic therapies. VDAs result in a rapid and selective shutdown of tumor vasculature via destruction of endothelial cells [Chaplin et al. 1996]. In an analogous, but unique manner to the TKIs described above, these agents interrupt tumor blood flow, depriving the tumor of necessary nutrients and oxygen [Siemann et al. 2004, Tozer et al. 2005]. In addition, VDAs have the added ability to target established blood vessels and are not limited to inhibition of new vessel formation, targeting genetically stable cell populations and potentially decreasing the chances of acquired drug resistance [Lippert, 2007]. One of the best-studied agents within this class is combretastatin A-4 phosphate (CA4P), a synthetic, phosphorylated prodrug of the natural product combretastatin A-4 (CA4) [Bilenker et al. 2005]. This drug functions by binding β-tubulin subunits, preventing microtubule formation resulting in cytoskeletal changes within endothelial cells [Salmon and Siemann, 2006]. The anti-vascular effects of CA4P have been demonstrated in both in vitro and in vivo models, and appear to be the result of endothelial damage, leading to increased vascular resistance, reduced tumor blood flow and central tumor necrosis [Bilenker et al. 2005; Salmon and Siemann, 2006]. Furthermore, in mouse models, CA4P has been shown to act synergistically with 5-fluorouracil, cisplatin and carboplatin [El-Zayat et al. 1993; Bilenker et al. 2005].
The most extensively studied VDA in the treatment of cervical cancer is the investigational anticancer drug 5,6-dimethylxanthenone-4-acetic acid (DMXAA) [Zhou et al. 2002]. This drug was developed by the Auckland Cancer Society Research Centre (ACSRC) and has recently completed phase I studies in New Zealand and the UK under the direction of the Cancer Research Campaign’s Phase I/II Clinical Trials Committee. The effects of DMXAA include induction of cytokines, tumor necrosis factor (TNF-α), serotonin and nitric oxide (NO). In a phase I trial exploring DMXAA in the treatment of several solid tumors, DMXAA (22 mg/kg by intravenous infusion over 20 min) resulted in a partial response in 1 patient with metastatic cervical squamous carcinoma. Given the clinical and preclinical data, 6 separate VDAs have been synthesized and are in various stages of phase I and II clinical trials exploring their efficacy in patients with solid tumors [Lippert, 2007]. As with other novel agents, a unique AE profile exists amongst VDA and includes cardiovascular disease and tumor pain [Cai et al. 2011].
Conclusion
Publication of GOG protocol 240, which detailed an OS advantage with the addition of bevacizumab to cytotoxic chemotherapy, is practice changing and represents the first and only study within the gynecologic cancer arena exhibiting an OS advantage with addition of a targeted anti-angiogenic agent. The significance of this 3.7 month improvement in OS is most clear when placed in context of prior clinical trials in this setting. Unlike patients with advanced stage ovarian or mBC, where salvage therapy commonly translates into partial or complete response, patients with metastatic or recurrent cervical cancer have failed to show any meaningful response to multimodal therapy in prior studies. With these results, and an evolution in the molecular therapy of this disease, it is anticipated that patients with previous poor prognosis cervical cancer may achieve durable and meaningful remission improving quality of life.
As our understanding of the tumor microenvironment evolves and the contribution of the angiogenic switch is further delineated, novel therapeutic paradigms are likely to emerge. More recently, immune modulation has gained interest in the treatment of cervical cancer and combined therapy targeting tumor vasculature, while enhancing the body’s innate response to this immunogenic malignancy may translate into improved survival outcomes. It is a scientific priority to continue to develop phase II/III clinical trials in an effort to provide this vulnerable population with effective therapeutic options.
Footnotes
Funding: The research was supported by a National Cancer Institute T32 Training Grant awarded to the Division of Gynecologic Oncology at the University of California, Irvine.
Conflict of interest statement: K.S.T discloses that he has served as a consultant for Genentech/Roche and his institution has been awarded a research grant from Genentech for contracted research.
Contributor Information
Ramez N. Eskander, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of California Irvine Medical Center, Orange CA, USA
Krishnansu S. Tewari, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, University of California, Irvine, 101 The City Dr. South, Building 56 Room 264, 101 The City Dr., Orange, CA 92868, USA
References
- Batchelor T., Sorensen A., Di Tomaso E., Zhang W., Duda D., Cohen K., et al. (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11: 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin L., Hemo I., Keshet E. (1998) A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125: 1591–1598 [DOI] [PubMed] [Google Scholar]
- Bilenker J., Flaherty K., Rosen M., Davis L., Gallagher M., Stevenson J., et al. (2005) Phase I trial of combretastatin a-4 phosphate with carboplatin. Clin Cancer Res 11: 1527–1533 [DOI] [PubMed] [Google Scholar]
- Bloss J., Blessing J., Behrens B., Mannel R., Rader J., Sood A., et al. (2002) Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol 20: 1832–1837 [DOI] [PubMed] [Google Scholar]
- Bookman M., Blessing J., Hanjani P., Herzog T., Andersen W. (2000) Topotecan in squamous cell carcinoma of the cervix: a phase II study of the gynecologic oncology group. Gynecol Oncol 77: 446–449 [DOI] [PubMed] [Google Scholar]
- Borgstrom P., Bourdon M., Hillan K., Sriramarao P., Ferrara N. (1998) Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumors in vivo. Prostate 35: 1–10 [DOI] [PubMed] [Google Scholar]
- Borgstrom P., Gold D., Hillan K., Ferrara N. (1999) Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res 19: 4203–4214 [PubMed] [Google Scholar]
- Borgstrom P., Hillan K., Sriramarao P., Ferrara N. (1996) Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res 56: 4032–4039 [PubMed] [Google Scholar]
- Broglio K., Berry D. (2009) Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 101: 1642–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres-Cortes J., Alvarado-Moreno J., Waga K., Rangel-Corona R., Monroy-Garcia A., Rocha-Zavaleta L., et al. (2001) Implication of tyrosine kinase receptor and steel factor in cell density-dependent growth in cervical cancers and leukemias. Cancer Res 61: 6281–6289 [PubMed] [Google Scholar]
- Cai Y., Zou Y., Ye Y., Sun H., Su Q., Wang Z., et al. (2011) Anti-tumor activity and mechanisms of a novel vascular disrupting agent, (z)-3,4′,5-trimethoxylstilbene-3′-o-phosphate disodium (M410). Invest New Drugs 29: 300–311 [DOI] [PubMed] [Google Scholar]
- Casanovas O., Hicklin D., Bergers G., Hanahan D. (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8: 299–309 [DOI] [PubMed] [Google Scholar]
- Chaplin D., Pettit G., Parkins C., Hill S. (1996) Antivascular approaches to solid tumour therapy: evaluation of tubulin binding agents. Br J Cancer 27(Suppl.): S86–88 [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Chen C., Lee C., Wei L., Hsieh F., Hsieh C. (2000) Vascular endothelial growth factor and prognosis of cervical carcinoma. Obstet Gynecol 96: 721–726 [DOI] [PubMed] [Google Scholar]
- Chow L., Eckhardt S. (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25: 884–896 [DOI] [PubMed] [Google Scholar]
- Cooper R., Wilks D., Logue J., Davidson S., Hunter R., Roberts S., et al. (1998) High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clin Cancer Res 4: 2795–2800 [PubMed] [Google Scholar]
- Coussens L., Hanahan D., Arbeit J. (1996) Genetic predisposition and parameters of malignant progression in K14-HPV16 transgenic mice. Am J Pathol 149: 1899–1917 [PMC free article] [PubMed] [Google Scholar]
- Curtin J., Blessing J., Webster K., Rose P., Mayer A., Fowler W., Jr., et al. (2001) Paclitaxel, an active agent in nonsquamous carcinomas of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol 19: 1275–1278 [DOI] [PubMed] [Google Scholar]
- El-Zayat A., Degen D., Drabek S., Clark G., Pettit G., Von Hoff D. (1993) In vitro evaluation of the antineoplastic activity of combretastatin A-4, a natural product from combretum caffrum (arid shrub). Anticancer Drugs 4: 19–25 [DOI] [PubMed] [Google Scholar]
- Erber R., Thurnher A., Katsen A., Groth G., Kerger H., Hammes H., et al. (2004) Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 18: 338–340 [DOI] [PubMed] [Google Scholar]
- Escudier B., Pluzanska A., Koralewski P., Ravaud A., Bracarda S., Szczylik C., et al. (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370: 2103–2111 [DOI] [PubMed] [Google Scholar]
- Eskander R., Randall L. (2011) Bevacizumab in the treatment of ovarian cancer. Biologics 5: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. (2005) VEGF as a therapeutic target in cancer. Oncology 69(Suppl. 3): 11–16 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Gerber H., Lecouter J. (2003) The biology of VEGF and its receptors. Nat Med 9: 669–676 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Hillan K., Gerber H., Novotny W. (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3: 391–400 [DOI] [PubMed] [Google Scholar]
- Ferrara N., Kerbel R. (2005) Angiogenesis as a therapeutic target. Nature 438: 967–974 [DOI] [PubMed] [Google Scholar]
- Folkman J. (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186 [DOI] [PubMed] [Google Scholar]
- Fracasso P., Blessing J., Wolf J., Rocereto T., Berek J., Waggoner S. (2003) Phase II evaluation of oxaliplatin in previously treated squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 90: 177–180 [DOI] [PubMed] [Google Scholar]
- Garcia A., Blessing J., Vaccarello L., Roman L. (2007) Phase II clinical trial of docetaxel in refractory squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Am J Clin Oncol 30: 428–431 [DOI] [PubMed] [Google Scholar]
- Greer B., Koh W., Abu-Rustum N., Apte S., Campos S., Chan J., et al. (2010) Cervical cancer. J Natl Compr Canc Netw 8: 1388–1416 [DOI] [PubMed] [Google Scholar]
- Guidi A., Abu-Jawdeh G., Berse B., Jackman R., Tognazzi K., Dvorak H., et al. (1995) Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in cervical neoplasia. J Natl Cancer Inst 87: 1237–1245 [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Dowlati A., Saini S., Savage S., Suttle A., Gibson D., et al. (2009) Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res 15: 4220–4227 [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342 [DOI] [PubMed] [Google Scholar]
- Jain R., Booth M. (2003) What brings pericytes to tumor vessels? J Clin Invest 112: 1134–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Bray F., Center M., Ferlay J., Ward E., Forman D. (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90 [DOI] [PubMed] [Google Scholar]
- Kim K., Li B., Winer J., Armanini M., Gillett N., Phillips H., et al. (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362: 841–844 [DOI] [PubMed] [Google Scholar]
- Kudelka A., Levy T., Verschraegen C., Edwards C., Piamsomboon S., Termrungruanglert W., et al. (1997) A Phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin Cancer Res 3: 1501–1505 [PubMed] [Google Scholar]
- Kudelka A., Verschraegen C., Loyer E. (1998) Complete remission of metastatic cervical cancer with the angiogenesis inhibitor TNP-470. N Engl J Med 338: 991–992 [DOI] [PubMed] [Google Scholar]
- Lee C., Shrieve D., Zempolich K., Lee R., Hammond E., Handrahan D., et al. (2005) Correlation between human epidermal growth factor receptor family (EGFR, HER2, HER3, HER4), phosphorylated Akt (P-Akt), and clinical outcomes after radiation therapy in carcinoma of the cervix. Gynecol Oncol 99: 415–421 [DOI] [PubMed] [Google Scholar]
- Lippert J., 3rd. (2007) Vascular disrupting agents. Bioorg Med Chem 15: 605–615 [DOI] [PubMed] [Google Scholar]
- Loncaster J., Cooper R., Logue J., Davidson S., Hunter R., West C. (2000) Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer 83: 620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H., 3rd, Bundy B., Grendys E., Jr., Benda J., Mcmeekin D., Sorosky J., et al. (2005) Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol 23: 4626–4633 [DOI] [PubMed] [Google Scholar]
- Look K., Blessing J., Levenback C., Kohler M., Chafe W., Roman L. (1998) A Phase II trial of CPT-11 in recurrent squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 70: 334–338 [DOI] [PubMed] [Google Scholar]
- Lopez-Ocejo O., Viloria-Petit A., Bequet-Romero M., Mukhopadhyay D., Rak J., Kerbel R. (2000) Oncogenes and tumor angiogenesis: the HPV-16 e6 oncoprotein activates the vascular endothelial growth factor (VEGF) gene promoter in a p53 independent manner. Oncogene 19: 4611–4620 [DOI] [PubMed] [Google Scholar]
- Lu C., Thaker P., Lin Y., Spannuth W., Landen C., Merritt W., et al. (2008) Impact of vessel maturation on antiangiogenic therapy in ovarian cancer. Am J Obstet Gynecol 198: 477 e471–479; discussion 477 e479–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynge E., Antilla A., Arbyn M., Segnan N., Ronco G. (2009) What’s next? Perspectives and future needs of cervical screening in Europe in the era of molecular testing and vaccination. Eur J Cancer 45: 2714–2721 [DOI] [PubMed] [Google Scholar]
- Mackay H., Tinker A., Winquist E., Thomas G., Swenerton K., Oza A., et al. (2010) A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG trial IND.184. Gynecol Oncol 116: 163–167 [DOI] [PubMed] [Google Scholar]
- Margolin K., Gordon M., Holmgren E., Gaudreault J., Novotny W., Fyfe G., et al. (2001) Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol 19: 851–856 [DOI] [PubMed] [Google Scholar]
- McGuire W., 3rd, Arseneau J., Blessing J., Disaia P., Hatch K., Given F., Jr., et al. (1989) A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol 7: 1462–1468 [DOI] [PubMed] [Google Scholar]
- McGuire W., Blessing J., Moore D., Lentz S., Photopulos G. (1996) Paclitaxel has moderate activity in squamous cervix cancer. a Gynecologic Oncology Group study. J Clin Oncol 14: 792–795 [DOI] [PubMed] [Google Scholar]
- Miller K., Wang M., Gralow J., Dickler M., Cobleigh M., Perez E., et al. (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357: 2666–2676 [DOI] [PubMed] [Google Scholar]
- Monk B., Mas Lopez L., Zarba J., Oaknin A., Tarpin C., Termrungruanglert W., et al. (2010) Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J Clin Oncol 28: 3562–3569 [DOI] [PubMed] [Google Scholar]
- Monk B., Poveda A., Vergote I., Raspagliesi F., Fujiwara K., Bae D., et al. (2013) A phase III, randomized, double-blind trial of weekly paclitaxel plus the angiopoietin 1 and 2 inhibitor, trebananib, or placebo in women with recurrent ovarian cancer: Trinova-1. Lancet Oncol. Jun 17 2014 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Monk B., Sill M., Burger R., Gray H., Buekers T., Roman L. (2009a) Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol 27: 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk B., Sill M., McMeekin D., Cohn D., Ramondetta L., Boardman C., et al. (2009b) Phase III trial of four cisplatin-containing doublet combinations in stage ivb, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol 27: 4649–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J., Hogg R., Wood E., Kerr T., Tyndall M., Levy A., et al. (2006) The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 368: 531–536 [DOI] [PubMed] [Google Scholar]
- Moore D., Blessing J., Mcquellon R., Thaler H., Cella D., Benda J., et al. (2004) Phase III study of cisplatin with or without paclitaxel in stage IVb, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol 22: 3113–3119 [DOI] [PubMed] [Google Scholar]
- Muggia F., Blessing J., Waggoner S., Berek J., Monk B., Sorosky J., et al. (2005) Evaluation of vinorelbine in persistent or recurrent nonsquamous carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol 96: 108–111 [DOI] [PubMed] [Google Scholar]
- Omura G., Blessing J., Vaccarello L., Berman M., Clarke-Pearson D., Mutch D., et al. (1997) Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol 15: 165–171 [DOI] [PubMed] [Google Scholar]
- Randall L., Monk B., Darcy K., Tian C., Burger R., Liao S., et al. (2009) Markers of angiogenesis in high-risk, early-stage cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol 112: 583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon D., Groves M., Wen P., Nabors L., Mikkelsen T., Rosenfeld S., et al. (2013) A Phase I/Ii trial of pazopanib in combination with lapatinib in adult patients with relapsed malignant glioma. Clin Cancer Res 19: 900–908 [DOI] [PubMed] [Google Scholar]
- Rusnati M., Presta M. (2007) Fibroblast growth factors/fibroblast growth factor receptors as targets for the development of anti-angiogenesis strategies. Curr Pharm Des 13: 2025–2044 [DOI] [PubMed] [Google Scholar]
- Ryan Q., Ibrahim A., Cohen M., Johnson J., Ko C., Sridhara R., et al. (2008) FDA drug approval summary: lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses Her-2. Oncologist 13: 1114–1119 [DOI] [PubMed] [Google Scholar]
- Salmon H., Siemann D. (2006) Effect of the second-generation vascular disrupting agent OXI4503 on tumor vascularity. Clin Cancer Res 12: 4090–4094 [DOI] [PubMed] [Google Scholar]
- Sandler A., Gray R., Perry M., Brahmer J., Schiller J., Dowlati A., et al. (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355: 2542–2550 [DOI] [PubMed] [Google Scholar]
- Schefter T., Winter K., Kwon J., Stuhr K., Balaraj K., Yaremko B., et al. (2012) A phase II study of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: preliminary results of RTOG 0417. Int J Radiat Oncol Biol Phys 83: 1179–1184 [DOI] [PubMed] [Google Scholar]
- Schefter T., Winter K., Kwon J., Stuhr K., Balaraj K., Yaremko B., et al. (2014) RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int J Radiat Oncol Biol Phys 88: 101–105 [DOI] [PubMed] [Google Scholar]
- Schilder R., Blessing J., Morgan M., Mangan C., Rader J. (2000) Evaluation of gemcitabine in patients with squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol 76: 204–207 [DOI] [PubMed] [Google Scholar]
- Siegel R., Ma J., Zou Z., Jemal A. (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9–29 [DOI] [PubMed] [Google Scholar]
- Siemann D., Chaplin D., Horsman M. (2004) Vascular-targeting therapies for treatment of malignant disease. Cancer 100: 2491–2499 [DOI] [PubMed] [Google Scholar]
- Silva-Filho A., Traiman P., Triginelli S., Reis F., Pedrosa M., Miranda D., et al. (2006) Association between CD31 expression and histopathologic features in stage Ib squamous cell carcinoma of the cervix. Int J Gynecol Cancer 16: 757–762 [DOI] [PubMed] [Google Scholar]
- Sloan B., Scheinfeld N. (2008) Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr Opin Investig Drugs 9: 1324–1335 [PubMed] [Google Scholar]
- Smith-Mccune K., Weidner N. (1994) Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res 54: 800–804 [PubMed] [Google Scholar]
- Sutton G., Blessing J., Adcock L., Webster K., Deeulis T. (1989) Phase II study of ifosfamide and mesna in patients with previously-treated carcinoma of the cervix. A Gynecologic Oncology Group study. Invest New Drugs 7: 341–343 [DOI] [PubMed] [Google Scholar]
- Tewari K., Monk B. (2005) Gynecologic Oncology Group trials of chemotherapy for metastatic and recurrent cervical cancer. Curr Oncol Rep 7: 419–434 [DOI] [PubMed] [Google Scholar]
- Tewari K., Sill M., Long H., 3rd, Penson R., Huang H., Ramondetta L., et al. (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen T., Blessing J., Gallup D., Maiman M., Soper J. (1995) Phase II trial of mitomycin-C in squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecol Oncol 57: 376–379 [DOI] [PubMed] [Google Scholar]
- Thigpen T., Shingleton H., Homesley H., Lagasse L., Blessing J. (1981) Cis-Platinum in treatment of advanced or recurrent squamous cell carcinoma of the cervix: a phase II study of the Gynecologic Oncology Group. Cancer 48: 899–903 [DOI] [PubMed] [Google Scholar]
- Toussaint-Smith E., Donner D., Roman A. (2004) Expression of human papillomavirus type 16 e6 and e7 oncoproteins in primary foreskin keratinocytes is sufficient to alter the expression of angiogenic factors. Oncogene 23: 2988–2995 [DOI] [PubMed] [Google Scholar]
- Tozer G., Kanthou C., Baguley B. (2005) Disrupting tumour blood vessels. Nat Rev Cancer 5: 423–435 [DOI] [PubMed] [Google Scholar]
- Warren R., Yuan H., Matli M., Gillett N., Ferrara N. (1995) Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 95: 1789–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J., Viviano D., Powell M., Gibb R., Mutch D., Grigsby P., et al. (2006) Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecol Oncol 103: 489–493 [DOI] [PubMed] [Google Scholar]
- Yancopoulos G., Davis S., Gale N., Rudge J., Wiegand S., Holash J. (2000) Vascular-specific growth factors and blood vessel formation. Nature 407: 242–248 [DOI] [PubMed] [Google Scholar]
- Zhou S., Kestell P., Baguley B., Paxton J. (2002) 5,6-Dimethylxanthenone-4-acetic acid (DMXAA): a new biological response modifier for cancer therapy. Invest New Drugs 20: 281–295 [DOI] [PubMed] [Google Scholar]
- Zighelboim I., Wright J., Gao F., Case A., Massad L., Mutch D., et al. (2013) Multicenter phase II trial of topotecan, cisplatin and bevacizumab for recurrent or persistent cervical cancer. Gynecol Oncol 130: 64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]