Abstract
Until recently, no truly effective treatment options have existed for patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC), a serious disease with poor prognosis. In November 2013, the targeted multikinase inhibitor, sorafenib, was approved for use in these patients based on substantially improved progression-free survival compared with placebo. A number of other targeted agents, including lenvatinib, are being investigated in phase II and phase III trials. With the advent of these new treatment options, practitioners are faced with making important decisions in determining which patients are candidates for systemic treatment and the optimal timing for treatment initiation. Since patients may remain asymptomatic for a protracted period of time, tumor size and growth rate are the primary considerations for making these choices. Proactive management of side effects is also critical in optimizing the effectiveness of treatment. Here we review targeted systemic agents that are either in use or are under investigation for RAI-refractory DTC and provide recommendations on the rationale for initiating systemic treatment and on managing adverse events. Four illustrative case studies are provided.
Keywords: sorafenib, lenvatinib, thyroid cancer
Introduction
Thyroid cancer is the most common endocrine malignancy and its incidence has continuously increased worldwide over the past three decades [Pellegriti et al. 2013]. In the last 10 years, thyroid cancer cases have been rising on average 6.4% each year in the United States, with death rates rising on average 0.9% each year [National Cancer Institute Surveillance, Epidemiology, and End Results program (NCI SEER) stat fact sheet: http://seer.cancer.gov/statfacts/html/thyro.html]. Differentiated thyroid cancer (DTC) accounts for over 90% of all thyroid cancers (NCI SEER cancer statistics review: http://seer.cancer.gov/csr/1975_2010/results_merged/sect_26_thyroid.pdf).
The current treatment for DTC involves thyroidectomy with suppression of thyroid-stimulating hormone (TSH) using levothyroxine [Cooper et al. 2009; NCCN, 2013]. Radioactive iodine (RAI) is often administered postoperatively to ablate remaining thyroid tissue, eliminate any suspected micrometastases, or eliminate recurrent disease [Cooper et al. 2009; Colevas and Shah, 2012; NCCN, 2013]. In some cases, external beam radiation therapy (EBRT) may be employed in the adjuvant setting or for bone or brain metastases [Cooper et al. 2009; Colevas et al. 2012; NCCN, 2013]. For most patients with DTC, these treatments are highly effective and the 5-year relative survival rates are 83–98% in patients under 80 years old, depending on the patient’s age and histologic subgroup [Hundahl et al. 1998].
Because thyroid gland absorption of iodine concentrates exposure to the tumor site, RAI therapy is one of the cornerstones in the management of DTC following surgery; 5–15% of patients, however, become refractory to RAI [Sciuto et al. 2009; Pacini and Castagna, 2012; Xing et al. 2013; Amin et al. 2014] and the prognosis for these patients is poor. The 5-year disease-specific survival rate in DTC that is RAI nonavid is 66% [Nixon et al. 2012] and the 10-year survival rate is only 10% [Durante et al. 2006; Robbins et al. 2006]. A combination of studies appears to support the notion that survival for patients with RAI-refractory DTC and distant metastases is around 2.5–3.5 years [Durante et al. 2006; Robbins et al. 2006].
Until recently, few treatment options have been available for patients with RAI-refractory DTC. Standard chemotherapy has yielded disappointing response rates and significant toxicity [Shimaoka et al. 1985; Matuszczyk et al. 2008; NCCN, 2013]. With the introduction of targeted systemic therapies, the outlook for these patients is dramatically changing. Here we review targeted therapies for RAI-refractory DTC and provide perspectives on optimizing their use.
Targeted systemic therapies for RAI-refractory thyroid cancer
Systemic therapies represent an important treatment option for differentiated thyroid tumors that are nonresectable, not responsive to RAI, and not amenable to EBRT. Based on a study of 30 patients published in 1974 [Gottlieb and Hill, 1974; Colevas and Shah, 2012], doxorubicin became the only US Food and Drug Administration approved agent for patients with metastatic DTC and was thus considered the standard of care. Subsequent studies using doxorubicin have demonstrated disappointing response data and considerable toxicity [Shimaoka et al. 1985; Droz et al. 1990; Matuszczyk et al. 2008].
Over the past couple of decades, improved understanding of oncogenic pathways has enabled the development of targeted therapies with very promising efficacy in many tumor types. Targeted therapies for RAI-refractory DTC, in particular, act primarily through two mechanisms of action: inhibition of angiogenesis, and inhibition of cell proliferation and survival.
Inhibition of angiogenesis is an effective tool since thyroid tumors are highly vascularized and depend on vascularization for provision of nutrients and oxygen for tumor growth. Vascular endothelial growth factor (VEGF) is a major driver of tumor vascularization and has been associated with larger tumor size and poorer prognosis in DTC [Bunone et al. 1999; Klein et al. 2001]. Platelet-derived growth factor (PDGF) is another growth factor whose activity complements that of VEGF in vessel formation (reviewed by Homsi and colleagues) [Homsi and Daud, 2007]. Many of the targeted therapies are multikinase inhibitors that inhibit VEGF or PDGF (often along with other targets) and act, at least in part, through deprivation of the tumor vascular supply.
The mitogen-activated protein kinase (MAPK)/ERK and phosphoinositide 3 kinase/AKT pathways are also key regulators of cell proliferation and survival. Mutations in and abnormal activation of genes encoding constituents of these pathways are prevalent in DTC [Santoro et al. 1992; Kimura et al. 2003; Wang et al. 2007]. A variety of targeted therapies inhibit the active mechanisms driving these paths, or they inhibit their upstream activation (reviewed by Saez and Sacks and Braunstein) [Saez, 2013; Sacks and Braunstein, 2013].
The most extensively evaluated targeted systemic agents for DTC are sorafenib and lenvatinib (Table 1). Sorafenib is a multikinase inhibitor that inhibits RAF, VEGF receptors (VEGFR)1–3, PDGF receptor (PDGFR), and RET (reviewed by Wilhelm and colleagues) [Wilhelm et al. 2008]. Clinical evidence suggests that sorafenib blocks thyroid cancer growth through both antiproliferative and antiangiogenic mechanisms [Kloos et al. 2009]. Lenvatinib inhibits VEGFR1–3, fibroblast growth factor receptors 1–4, RET, c-KIT, and PDGFRβ [Matsui et al. 2008; Okamoto et al. 2013].
Table 1.
Phase II and phase III trials of lenvatinib and sorafenib for RAI-refractory DTC.
| Drug | Phase | Study population | N | Main outcomes | Reference |
|---|---|---|---|---|---|
| Lenvatinib | III | Patients with RAI-refractory DTC with radiographic evidence of disease progression within the prior 12 months | 392 | Estimated completion date February 2015 | [ClinicalTrials.gov identifier: NCT01761266] |
| II | Patients with advanced, RAI-refractory DTC and disease progression during the prior 12 months | 58 | PR: 50% (n = 29) | [Sherman et al. 2011] | |
| SD: NR | |||||
| mPFS: 12.6 months | |||||
| Sorafenib | III | Patients with locally advanced/metastatic RAI-refractory DTC with evidence of disease progression within prior 14 months (RECIST) | 417 | mPFS: | [Brose et al. 2013b; Bayer Healthcare Pharmaceuticals] |
| Sorafenib, 10.8 months (95% CI 9.1–12.9) | |||||
| Placebo, 5.8 months (95% CI 5.3–7.8) p < 0.001 | |||||
| DCR (CR + PR + SD ≥6 months): | |||||
| Sorafenib, 54% (n = 106) | |||||
| Placebo, 34% (n = 68) p < 0.0001 | |||||
| II | Patients with metastatic or unresectable thyroid cancer for which curative measures were no longer effective. Evidence of disease progression in the prior 12 months | 30 | PR: 23% (n = 7) | [Gupta-Abramson et al. 2008] | |
| SD: 53% (n = 16)* | |||||
| mPFS: 84 weeks$ | |||||
| II | Metastatic thyroid cancer patients who had experienced I-131 therapy failure or were not candidates to receive I-131 | 52‡ | PR: 12% (n = 6) | [Kloos et al. 2009] | |
| SD: 65% (n = 34) | |||||
| mPFS: PTC (no prior chemo): 16 months | |||||
| PTC (prior chemo): 10 months | |||||
| FTC/HTC: 4.5 months | |||||
| II | Patients with progressive metastases or unresectable local recurrence of DTC for which RAI therapy was no longer effective | 31 | PR: 31% (n = 8) | [Schneider et al. 2012] | |
| SD: 12% (n = 3) | |||||
| mPFS: 18 months | |||||
| II | Patients with progressive locally advanced/metastatic MTC, or DTC with nonradioiodine-avid disease | 19§ | PR: 18% at 1 year | [Ahmed et al. 2011] | |
| 1-year PFS: 68% | |||||
| II | Patients with progressing advanced, iodine-refractory differentiated TC or PDTC, and MTC and ATC | 55 | PR: 38% (n = 18) | [Keefe et al. 2011] | |
| (47 DTC/PDTC, 5 ATC, 3 MTC) | SD: 47% (n = 22) | ||||
| PFS in DTC/PDTC patients: 96 weeks (95% CI 75.1–135.4) | |||||
| OS in DTC/PDTC patients: 140.9 weeks (95% CI 93.9-) | |||||
| II | Patients with progressive metastatic or locally advanced RAI refractory DTC | 31 | PR: 25% (n = 8)SD: 34% (n = 11)PFS: 58 weeks (95% CI 47–68) | [Hoftijzer et al. 2009] |
Includes one patient with MTC who had SD.
Median PFS for patients with DTC.
Excludes four patients with ATC.
Data reported for 19 patients with DTC.
ATC, anaplastic thyroid cancer; CI, confidence interval; CR, complete response; DCR, disease control rate; DTC, differentiated thyroid cancer; FTC, follicular thyroid cancer; HTC, Hürthle cell thyroid cancer; mPFS, median progression-free survival; MTC, medullary thyroid cancer; NR, not reported; OS, overall survival; PDTC, poorly differentiated thyroid cancer; PFS, progression-free survival; PR, partial response; PTC, papillary thyroid cancer; RAI, radioactive iodine; RECIST, Response Evaluation Criteria In Solid Tumors; SD, stable disease; TC, thyroid cancer.
Sorafenib is the first targeted systemic agent to demonstrate benefit in RAI-refractory thyroid cancer and was recently approved for the treatment of locally recurrent or metastatic, progressive, differentiated thyroid carcinoma refractory to RAI treatment [US Food and Drug Administration, 2013]. It is an established agent that is also used in the treatment of advanced renal cell carcinoma (approved in 2005) and unresectable hepatocellular carcinoma (HCC; approved in 2007). The safety and efficacy of sorafenib in metastatic, RAI-refractory DTC are supported by multiple phase II trials including over 150 patients (Table 1) [Gupta-Abramson et al. 2008; Hoftijzer et al. 2009; Kloos et al. 2009; Ahmed et al. 2011; Keefe et al. 2011; Sherman et al. 2011; Schneider et al. 2012; Brose et al. 2013b; Bayer Healthcare Pharmaceuticals, 2013] [ClinicalTrials.gov identifier: NCT01761266] and a recent phase II trial, DECISION (Study of Sorafenib in Locally Advanced or Metastatic Patients with RAI Refractory Thyroid Cancer), completed in 2013 [Brose et al. 2013b].
In DECISION (N = 417), the median progression-free survival (PFS) was 10.8 months [95% confidence interval (CI) 9.1–12.9] in the patients treated with sorafenib, and 5.8 months in those receiving placebo (95% CI 5.3–7.8) (p < 0.001), a 5-month improvement [hazard ratio (HR) 0.59; 95% CI 0.46–0.76] [Bayer Healthcare Pharmaceuticals, 2013]. The disease control rate, defined as complete response (CR) rate + partial response (PR) rate + stable disease (SD) rate for at least 6 months, was 54% in the sorafenib group [versus 34% in placebo (p < 0.0001)] [Brose et al. 2013b], with PRs observed in 12.2% of patients [versus 0.5% in the placebo group (p < 0.0001) [Brose et al. 2013b]. Thirty percent of patients treated with sorafenib and 75% of those on placebo received open-label sorafenib at progression [Bayer Healthcare Pharmaceuticals, 2013]. Due to extensive crossover, a significant difference in overall survival (OS) is not anticipated; median OS was not reached at the time of data cutoff for PFS [Bayer Healthcare Pharmaceuticals, 2013]. The safety profile was similar to that observed in other disease indications and most adverse events were grade 1/2. However, grade 3/4 rash/desquamation occurred in 5% of patients, and grade 3/4 diarrhea, fatigue, or weight loss occurred in 6% of patients [Bayer Healthcare Pharmaceuticals, 2013]. The incidence of grade 3 hand–foot skin reaction (HFSR) (20%), grade 3/4 hypertension (10%), and grade ≥3 hypocalcaemia (10%) appeared higher than observed in other indications [Bayer Healthcare Pharmaceuticals, 2013].
Lenvatinib has also demonstrated promising activity for patients with RAI-refractory DTC. In a phase II trial, 50% of patients had an objective PR, with a median PFS of 12.6 months reported (Table 1) [Sherman et al. 2011]. Grade 3 adverse events occurring in over 5% of patients included diarrhea, fatigue, and proteinuria [Sherman et al. 2011]. Nine percent of patients experienced grade 4 adverse events [Sherman et al. 2011]. In a recent multicenter phase III trial, median PFS (95% CI) was 18.7 (16.4-NR) months in lenvatinib-treated patients vs 3.6 (2.1-5.3) months in placebo-treated patients (HR 0.2 [0.14-0.27]) [Schlumberger et al. 2014].
A number of other agents with overlapping or distinct target profiles have been tested in phase II trials (Table 2) [Cohen et al. 2008a, 2008b; Pennell et al. 2008; Sherman et al. 2008; Bible et al. 2010; Carr et al. 2010; Hayes et al. 2012; Leboulleux et al. 2012] (reviewed by Sacks and Braunstein, and Brose and colleagues) [Sacks and Braunstein, 2013; Brose et al. 2012], and another randomized trial comparing vandetanib with placebo in RAI-refractory DTC is now accruing patients [ClinicalTrials.gov identifier: NCT01876784].
Table 2.
Additional phase II trials of targeted systemic agents in RAI-refractory DTC.
| Drug | Study population | N* | Main outcomes | Reference |
|---|---|---|---|---|
| Axitinib | Patients with advanced thyroid cancer of any histology for whom RAI was not effective or not appropriate | 45 | mPFS: 18.1 months | [Cohen et al. 2008b] |
| PR: 30% (n = 18) | ||||
| SD: 38% (n = 23) | ||||
| Gefitinib | Patients with locally advanced or metastatic RAI-refractory DTC or medullary or anaplastic TC | 25 | mPFS: 3.7 months | [Pennell et al. 2008] |
| mOS: 17.5 months | ||||
| PR: 0 | ||||
| SD$: 12% (n = 3) | ||||
| Motesanib | Patients with locally advanced or metastatic DTC with evidence of progression in prior 6 months that is not amenable to EBRT, resection or is RAI-refractory | 93 | mPFS: 40 weeks | [Sherman et al. 2008] |
| PR: 14% (n = 13) | ||||
| SD: 67% (n = 62) | ||||
| Pazopanib | Patients with RAI-refractory DTC with progression in prior 6 months | 37 | mPFS: 11.7 months | [Bible et al. 2010] |
| PR: 49% (n = 18) | ||||
| Selumetinib | Patients with RAI-refractory PDTC and documented progression within the prior 12 months | 32 | mPFS: 32 weeks‡ | [Hayes et al. 2012] |
| PR: 3% (n = 1) | ||||
| SD: 66% (n = 21) | ||||
| Sunitinib | Patients with DTC or MTC refractory to curative treatment with evidence of progression in prior 6 months | 31 | PR: 13% (n = 4) | [Cohen et al. 2008a] |
| SD: 68% (n = 21) | ||||
| Patients with metastatic RAI-refractory WDTC or MTC and evidence of FDG-PET uptake | 33 (26 DTC, 7 MTC) | CR: 3% (n = 1) | [Carr et al. 2010] | |
| PR: 28% (n = 10) | ||||
| SD: 46% (n = 16) | ||||
| Vandetanib | Patients with locally advanced or metastatic DTC or poorly differentiated DTC without an anaplastic component | VAN: 72PLC: 73 | mPFS VAN: 11.1 monthsmPFS PLC: 5.9 monthsPR VAN: 8% (n = 6)PR PLC: 5% (n = 4) | [Leboulleux et al. 2012] |
| Vemurafenib | Patients with metastatic or unresectable RAI-refractory papillary thyroid cancer positive for the BRAF V600 mutation | 51 | VEGFR inhibitor naïve patientsmPFS: 15.6 moPR: 35% (n=9)SD: 23 (n=6)VEGF inhibitor pretreated patientsmPFS: 6.3 moPR: 29% (n=6)SD: 10 (n=2) | [Brose et al. 2013a] |
Patients eligible for response assessment.
SD after 12 months of treatment.
Includes all patients.
CR, complete response; DTC, differentiated thyroid cancer; EBRT, external beam radiation therapy; FDG-PET, fluorodeoxyglucose positron emission tomography; mOS, median overall survival; mPFS, median progression-free survival; MTC, medullary thyroid cancer; PLC, placebo; PDTC, poorly differentiated thyroid cancer; PR, partial response; RAI, radioactive iodine; SD, stable disease; TC, thyroid cancer; VAN, vandetanib; WDTC, well differentiated thyroid cancer.
In addition to trials with single-agent targeted systemic therapies, combination strategies are being explored. Notably, combination treatment with sorafenib and mammalian target of rapamycin (mTOR) inhibitors has shown promise. One phase II (N = 41) single-arm study [ClinicalTrials.gov identifier: NCT01141309] combined sorafenib and everolimus in patients with progressive, RAI-refractory, recurrent/metastatic, nonanaplastic, thyroid cancer. PRs were observed in 19 (53%) and SD in 15 (42%) of 36 eligible patients. Among the 19 patients with DTC in this study, PRs were observed in 11 (58%) and SD in 7 (37%) [Sherman et al. 2013]. A second phase II single-arm study [ClinicalTrials.gov identifier: NCT01263951] is examining combination treatment with everolimus and sorafenib in patients with metastatic DTC whose condition has progressed on sorafenib alone. Another mTOR inhibitor, temsirolimus, has been evaluated in a phase II (N = 37) single-arm study [ClinicalTrials.gov identifier: NCT01025453] in patients with progressive recurrent/metastatic nonmedullary thyroid cancer. In this study, PRs were seen in 8 (22%) (including three with anaplastic/poorly differentiated thyroid cancer) and SD in 21 (57%) of 37 patients [Sherman et al. 2012]. Finally, the University of Chicago Phase II Consortium is exploring the combination of lenalidomide with cediranib in patients with metastatic RAI-refractory DTC [ClinicalTrials.gov identifier: NCT1208051].
Ho and colleagues [Ho et al. 2013] investigated a completely different mechanism of action based on observations that activated MAPK/ERK signaling inhibits expression of thyroid hormone biosynthetic genes, including the sodium-iodide symporter and thyroid peroxidase, which are important in iodine uptake and organification. The MAPK/ERK inhibitor, selumetinib, was administered to patients who met one or more of the following criteria: a non-RAI-avid lesion, an RAI-avid metastatic lesion that remained stable or progressed despite RAI treatment 6 months or more prior to entry, or fluorodeoxyglucose (FDG)-avid lesions [ClinicalTrials.gov identifier: NCT00970359]. In this single-arm study, 12 of 20 (60%) evaluable patients had new or increased RAI uptake and 8 (40%) reached the dosimetry threshold for radioiodine therapy, suggesting a possible role for selumetinib in restimulating RAI uptake.
Outstanding questions in the use of systemic agents in RAI-refractory DTC
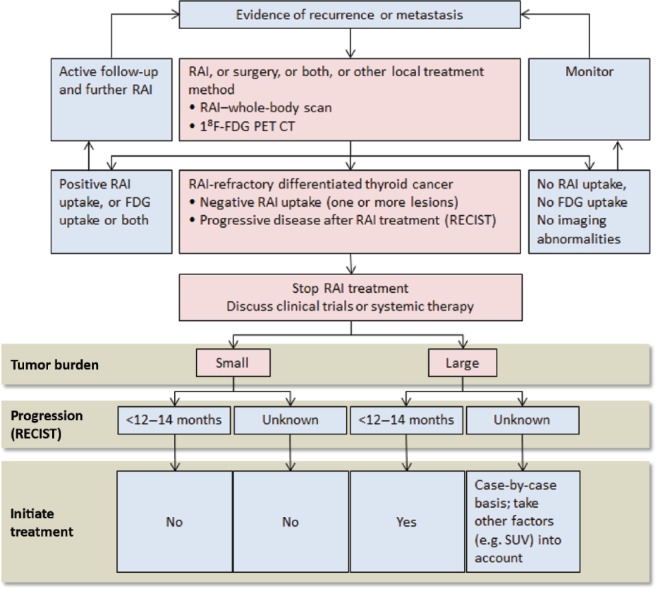
Despite the profound recent advances in the development and use of targeted systemic therapy for patients with RAI-refractory DTC, a number of important questions persist in the effort to establish optimal treatment. Given that RAI-refractory DTC may present in clinically heterogeneous ways based on histology and stage [Sacks and Braunstein, 2013], some patients may have inherently RAI nonavid tumors or may have multiple lesions that differentially take up RAI, including those that initially concentrated RAI, but lose this capability. However, certain RAI-avid lesions progress despite significant uptake of RAI (i.e. they are resistant), and some RAI-avid lesions do not progress, but they are not curable despite multiple courses of RAI treatment. Due to this vast heterogeneity, it is difficult to define RAI-refractory disease. However, it is important to recognize when a patient is no longer deriving benefit from RAI because repeated futile treatments are expensive, create undo morbidity, and may unnecessarily delay initiation of potentially beneficial systemic agents. Furthermore, prolonged exposure to radioactive agents may be associated with increased risk of secondary cancers and leukemia [Ahmed et al. 2011]. In the DECISION trial, RAI-refractory disease was defined as the presence of at least one of the following factors: at least one lesion that does not take up I-131, clinical evidence that I-131 is no longer providing benefit, or a cumulative dose of over 600 millicuries (mCi) of RAI [Brose et al. 2011, 2013b].
Once a patient’s tumor is deemed refractory to RAI treatment, a decision must be made as to whether and when to treat with a systemic agent. Since most patients with RAI-refractory tumors are initially asymptomatic, many do not require immediate treatment. For example, in the DECISION trial, 17% of placebo-treated patients had a PFS longer than 1 year (M. Brose, personal communication). Additionally, in one series of patients, whose tumors were FDG nonavid upon positron emission tomography (PET) imaging, the median survival was 41 months without systemic therapy [Schreinemakers et al. 2012]. Hence, the decision to treat should be based on a combination of factors, including tumor size and growth rate, symptoms, and tumor location. In September 2012, an expert panel met to propose a working definition for RAI-refractory disease and devise a treatment algorithm [Schlumberger et al. 2014]. The considerations for initiation of systemic treatment in my practice are concordant with those outlined by this group. Patients with large, bulky tumors (>3 cm) or those with multiple tumors greater than 1–2 cm in size that are rapidly progressing (progression within <12 months) should be considered for treatment with a systemic agent. In contrast, patients with smaller tumors (<1 cm) or small tumor burdens with only a few scattered lesions that are slow to progress (i.e. have not progressed over 12–14 months) rarely require immediate systemic treatment. Such patients should be carefully observed via imaging every 3–12 months. For patients with smaller tumors that are progressing rapidly (doubling time <6–12 months) or those who have large tumors that are progressing slowly (doubling >12 months), the decision is less clear and may depend largely on such factors as symptoms, tumor location, performance status, and patient preference. If the disease is isolated to one particular location (i.e. a solitary liver or bone lesion), treatment with an isolated modality, such as EBRT or radio frequency ablation, may be attempted prior to initiation of systemic therapy. Whether lesions take up FDG may also be considered since its uptake is associated with poorer tumor differentiation [Feine et al. 1996] and resistance to RAI [Feine et al. 1996; Wang et al. 2001], and is thus indicative of poor prognosis [Robbins et al. 2006]. Finally, it is important to note that an expert panel recently recommended that progressive disease should be defined by growth of lesions using Response Evaluation Criteria In Solid Tumors (RECIST) rather than relying solely on an increase in tumor markers (e.g. thyroglobulin) [Brose et al. 2012].
Case studies
The following case studies provide illustrative examples of patients falling into the above-mentioned categories.
Patients with large tumors or multiple tumors with rapid doubling time
M.C. is a 64-year-old man with a history of T3, N1, M0 papillary thyroid carcinoma, who initially underwent total thyroidectomy with bilateral selective neck dissections (levels II–IV). His pathology revealed extrathyroidal extension with positive anterior and posterior margins as well as 4 out of 64 positive nodes. He underwent tracheostomy for presumed bilateral vocal cord paralysis and anticipated failure to wean from the ventilator. He was adequately suppressed with levothyroxine and an I-131 scan was performed and was negative for uptake, suggesting RAI-refractory disease. He then underwent adjuvant intensity-modulated radiation therapy. Eighteen months later, he presented with complaints of neck pain. Computed tomography (CT)/ PET scans were performed and revealed intense FDG uptake in the anterior neck as well as multiple pulmonary nodules. A repeat I-131 scan was negative for uptake. A CT-guided core lung biopsy was performed 1 month later and his pathology was positive for metastatic papillary thyroid cancer. He then developed a persistent cough and follow-up imaging 6 weeks later demonstrated doubling of his lung nodules. The patient was enrolled on the DECISION trial [ClinicalTrials.gov identifier: NCT00984282]. His disease continued to progress following 2 months of therapy, and he was found to be on the placebo arm, upon unblinding. M.C. opted for treatment with sorafenib. Following 8 weeks of therapy, his target lesions were reduced by over 10%, and his symptoms of cough markedly improved.
Patients with small or few tumors and a slow doubling time
C.B. is a 57-year-old woman with a history of T2, N0, MX papillary thyroid cancer (tall cell variant). She underwent total thyroidectomy and her postsurgical I-131 scan was negative, indicating RAI-refractory disease. A CT scan of the lung showed three small nodules that were too small to biopsy. Eight years after surgery, she developed lower neck discomfort. An ultrasound of the right neck was performed, which revealed a mass in her right peritracheal space, including three pulmonary nodules. A fine needle aspiration was completed and her pathology was positive for metastatic papillary thyroid carcinoma. A follow-up CT of the neck revealed a 2.6 × 2.0 × 0.4 cm3 lesion in the anterior neck with bilateral level II lymph nodes. She then underwent a resection of this mass as well as central neck dissection. Her pathology revealed a positive margin along the right recurrent laryngeal nerve as well as the anterior and lateral tracheal surface; 0 out of 11 lymph nodes were involved. A repeat PET scan revealed residual disease in her neck as well as three pulmonary nodules. She received adjuvant radiation to the neck. Ongoing surveillance imaging at 6-month intervals demonstrates no change in the size of her three pulmonary nodules and no new nodules. Due to the small and nonaggressive nature of this patient’s tumor, low tumor burden, and her lack of symptoms, systemic therapy is not currently contemplated.
Patients with small or few tumors and a rapid doubling time
C.M. is a 53-year-old woman with a history of T3, N1a, M1 follicular thyroid cancer involving her right iliac crest and two pulmonary lung lesions. She underwent total thyroidectomy and four RAI treatments (total cumulative dosage of 930 mCi). Her disease was stable for 4 years, after which CT imaging revealed two enlarged level II lymph nodes. PET imaging demonstrated FDG-avid lesions in her iliac crest and her neck. A biopsy of her neck nodes revealed metastatic follicular thyroid cancer, which may be considered RAI refractory owing to prior treatment with a cumulative dose of over 600 mCi I-131. She underwent EBRT to her right iliac crest. Follow-up imaging 2 months later demonstrated doubling of her chest disease (from 5 to 11 mm) and small progression of the lymph nodes in her neck. Following a discussion with this patient, she elected to start systemic treatment, given her histology of follicular thyroid cancer involving her bone. She was enrolled in the DECISION trial [ClinicalTrials.gov identifier: NCT00984282] and within 8 weeks showed marked improvement in her right hip pain. Imaging revealed near resolution of her lung metastases and stabilization of her boney lesions.
Patients with large tumors and slow doubling time
C.F. is a 64-year-old man with a history of T2, N2, M1 papillary thyroid cancer with extensive metastatic disease to the neck, mediastinum, axillary lymph nodes, lung, and spine. Initially, he underwent total thyroidectomy, followed by postoperative EBRT (31 Gy) to his neck. An I-131 scan demonstrated extensive uptake in his lungs, spine and lymph nodes. He then received RAI therapy using 200 mCi of I-131. CT imaging demonstrated SD for 2 years. He then developed disease progression in his left neck, left thyroid bed, and thoracic inlet causing displacement and narrowing of the trachea. After levothyroxine withdrawal, an I-131 thyroid scan showed an iodine-avid component of his metastatic disease, and 353 mCi of I-131 was administered. The patient’s thyroglobulin level decreased from 3434 pretherapy to 1587 post-treatment. Six months later, his thyroglobulin rose to 2546. Repeat imaging revealed a slight increase in the size of his left thyroid bed mass with stable lung and bone lesions. A repeat I-131 scan was negative for uptake, and an FDG-PET scan was positive, consistent with RAI-refractory disease. He continues to remain asymptomatic, and repeat imaging of his neck and chest every 3 months demonstrates SD defined by RECIST criteria. It should be noted that the value of repeat PET scan in this setting is a matter of debate since it may not be cost-effective in patients whose tumors are known to be RAI refractory. A discussion of treatment either on clinical trial or with sorafenib has been discussed, given the extent of his tumor burden. He elected to remain without therapy with very close monitoring because of concerns related to side effects. The patient noted that he would reconsider systemic treatments once he develops symptoms.
Supportive care and management of side effects in patients receiving targeted systemic therapy
Targeted systemic agents may have significant side effects. To avoid premature or unnecessary withdrawal from treatment, thus depriving patients of the potential for life-prolonging benefits, side effects should be proactively and aggressively managed. In the phase II trial of lenvatinib, 35% of patients required a dose reduction for management of toxicity, and 23% were withdrawn from therapy due to adverse events. In DECISION, 14% of sorafenib-treated patients discontinued due to drug-related adverse events compared with 1% in the placebo group [Bayer Healthcare Pharmaceuticals, 2013].
Because multikinase inhibitors have been used for more than 9 years in other disease indications, practitioners have gained considerable experience in the management of associated adverse events [Brose et al. 2014; Walko and Grande, 2014]. One of the most common side effects is HFSR [Chu et al. 2008; Lacouture et al. 2008; Bellmunt et al. 2011], which usually occurs within the first 45 days of therapy [Grandinetti and Goldspiel, 2007; Lacouture et al. 2008]. Recommendations to prevent and reduce HFSR signs and symptoms include avoiding exposure to hot water and excessive friction on the skin or pressure on the feet. Calluses should be controlled by avoiding pressure points and using well-padded footwear and insole cushions [Lacouture et al. 2008; Bellmunt et al. 2011; Brose et al. 2014; Schlumberger et al. 2014]. Foot soaks with tepid water and Epsom salts may also be useful. Regular use of topical agents is recommended [Lacouture et al. 2008; Bellmunt et al. 2011]. A large randomized, controlled phase II study (N = 868) of patients with HCC treated with sorafenib demonstrated that prophylaxis with urea-based cream over a 12-week period significantly reduced the incidence of all grades of HFSR compared with best supportive care in patients treated with sorafenib (56% versus 74%; p < 0.0001) [Lacouture et al. 2008; Ren et al. 2012]. In another study of patients with HCC receiving sorafenib, use of a corticosteroid ointment significantly improved HFSR score, and the need for treatment modifications was reduced [Lu et al. 2013]. Wearing cotton gloves and socks at night may prevent further injury and help retain moisture [Lacouture et al. 2008; Bellmunt et al. 2011]. For pain control, clinicians may consider topical analgesics such as 2% lidocaine [Lacouture et al. 2008], or oral codeine, pregabalin, or an anti-inflammatory such as ibuprofen [Walko and Grande, 2014]. Temporary dose reductions, and even interruptions, may be considered for moderate or severe cases [Lacouture et al. 2008; Bellmunt et al. 2011]. In the DECISION trial, initial dose reductions from 400 mg twice daily to 600 mg once daily were used to manage toxicities.
Other common side effects include diarrhea, fatigue, anorexia/loss of appetite with weight loss, and hypertension. A recent meta-analysis of 15 phase II and three phase III randomized controlled trials comprising patients with various tumor types demonstrated that the incidence of all-grade fatigue was 27% (95% CI 25%–29%) in patients treated with sorafenib. No significant differences were observed among tumor types. The best empirically supported intervention for cancer-related fatigue is exercise, particularly aerobic exercise such as walking and cycling. In a meta-analysis of 56 studies (4068 participants), exercise was observed to be statistically more effective than any control intervention (standardized mean difference −0.27, 95% CI −0.37 to −0.17) [Cramp and Byron-Daniel, 2012]. Stimulants such as methylphenidate or dexmethylphenidate may be used, although there is limited evidence that these are beneficial (reviewed by Mitchell) [Mitchell, 2010]. Sorafenib-related fatigue is likely to resolve after approximately 6 months of treatment without additional intervention or dose adjustment [Brose et al. 2014]. Additional approaches to managing sorafenib-related fatigue are reviewed by Brose and colleagues [Brose et al. 2014] and Walko and Grande [Walko and Grande, 2014] and general guidelines for managing cancer-related fatigue are provided by the National Comprehensive Cancer Network [NCCN, 2014]. In many cases, diarrhea can be managed with over-the-counter agents such as loperamide [Brose et al. 2014]. Maintaining hydration is critical as dehydration may contribute to fatigue [Walko and Grande, 2014]. For weight loss and loss of appetite, data suggest that appetite stimulants such as megestrol acetate or dronabinol have benefit in patients with cancer [Loprinzi et al. 1993; Jatoi et al. 2002; Pascual Lopez et al. 2004; Chow et al. 2011]. Frequent monitoring of blood pressure is important, particularly in the first 6 weeks of treatment [Brose et al. 2014; Walko and Grande, 2014]. In the DECISION trial, hypertension usually occurred early in the course of sorafenib treatment and was managed with standard antihypertensive therapy [Bayer Healthcare Pharmaceuticals, 2013]
Tyrosine kinase inhibitors may interfere with exogenous thyroid suppression; in DECISION, elevation of TSH level above 0.5 mU/liter was observed in 41% of patients treated with sorafenib compared with 16% of those on placebo. Monthly TSH monitoring should continue while patients are being treated with sorafenib for thyroid cancer, and thyroid replacement medication should be adjusted as needed [Bayer Healthcare Pharmaceuticals, 2013].
For patients with metastases to bone, bisphosphonate may reduce bone pain, improve performance status and have a beneficial impact on quality of life [Wexler, 2011]. One published work recommends treatment of patients with thyroid cancer without pathological fracture twice yearly with 4 mg doses of zoledronic acid ad infinitum [Wexler, 2011]. It was also recommended that, for those who suffer an acute fracture, 4 mg zoledronic acid be administered every 3 months for the first year and then twice yearly thereafter [Wexler, 2011]. This regimen differs from what is typically used in other solid tumors, because patients with DTC may live many years, and represents a compromise between the potential advantages of intravenous bisphosphonate and potential adverse effects, such as atypical femoral shaft fractures and osteonecrosis of the jaw [Wexler, 2011].
Conclusion
The advent of targeted systemic therapies represents a major advance for patients with RAI-refractory DTC, a disease for which there were previously few treatment options. The recent approval of sorafenib and the recent completion of the phase III trial of lenvatinib in these patients foretell the possibility of more widespread use of these agents in the community. However, researchers and practitioners are still learning how best to use targeted therapies in DTC, particularly in determining who to treat (i.e. recognizing RAI-refractory disease) and when to initiate treatment. In my practice, patients with large tumors or multiple tumors with rapid progression are considered for treatment with a targeted systemic agent. For patients with smaller tumors that are progressing rapidly or those who have large tumors that are progressing slowly, tumor location, the presence of symptoms, and the patient’s performance status are additional factors that must be considered and discussed with the patient prior to initiating therapy. Diligent management of side effects is essential to avoid premature or unnecessary withdrawal from treatment. Most adverse events occur early in the course of treatment and can be easily and effectively managed. As new systemic agents become available, it will be important to maintain an awareness of the differences in response and adverse effect profiles of each agent. As a consequence, patient management may differ, at least to some extent, for each targeted agent.
Figure 1.
Management of RAI-refractory differentiated thyroid cancer. Cumulative activity RAI administered to be based on individualized patient presentation and risk assessment. FDG-PET, fluorodeoxyglucose positron emission tomography; RAI, radioactive iodine; RECIST, Response Evaluation Criteria In Solid Tumors; SUV, standardized uptake value on PET scan. Figure adapted with permission from Schlumberger et al. [2014].
Acknowledgments
The author thanks Pamela Foreman, PhD, of Powered 4 Significance, LLC.
Footnotes
Funding: Support for third-party medical writing was provided by Onyx Pharmaceuticals.
Conflict of interest statement: FW has participated in advisory boards for Bayer/Onyx. Otherwise, he reports no conflicts of interest related to thyroid cancer and its treatment.
References
- Ahmed M., Barbachano Y., Riddell A., Hickey J., Newbold K., Viros A., et al. (2011) Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol 165: 315-322 [DOI] [PubMed] [Google Scholar]
- Amin A., Badwey A., El-Fatah S. (2014) Differentiated thyroid carcinoma: an analysis of 249 patients undergoing therapy and aftercare at a single institution. Clin Nucl Med 39: 142-146 [DOI] [PubMed] [Google Scholar]
- Bayer Healthcare Pharmaceuticals (2013) Nexavar (Sorafenib) Prescribing Information. Whippany, NJ: Bayer Healthcare Pharmaceuticals [Google Scholar]
- Bellmunt J., Eisen T., Fishman M., Quinn D. (2011) Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol 78: 24-32 [DOI] [PubMed] [Google Scholar]
- Bible K., Suman V., Molina J., Smallridge R., Maples W., Menefee M., et al. (2010) Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11: 962-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose M. (2013) Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: the phase III Decision Trial [Abstract 4]. Paper presented at American Society of Clinical Oncology, 31 May–4 June 2013; Chicago, IL [Google Scholar]
- Brose M., Cabanillas M., Cohen E., Wirth L., Sherman S., Riehl T., et al. (2013a) An Open-Label, Multicenter Phase 2 Study of the BRAF Inhibitor Vemurafenib in Patients With Metastatic or Unresectable Papillary Thyroid Cancer (PTC) Positive for the BRAF V600 Mutation and Resistant to Radioactive Iodine (NCT01286753, NO25530). Eur J Cancer 49 (Suppl. 3): S1–S19 [Google Scholar]
- Brose M., Frenette C., Keefe S., Stein S. (2014) Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol 41(Suppl. 2): S1-S16 [DOI] [PubMed] [Google Scholar]
- Brose M., Nutting C., Jarzab B., Elisei R., Siena S., Bastholt L., et al. (2013b) Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: the phase III DECISION trial. J Clin Oncol 31: 4. [DOI] [PubMed] [Google Scholar]
- Brose M., Nutting C., Sherman S., Shong Y., Smit J., Reike G., et al. (2011) Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer 11: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose M., Smit J., Capdevila J., Elisei R., Nutting C., Pitoia F., et al. (2012) Regional approaches to the management of patients with advanced, radioactive iodine-refractory differentiated thyroid carcinoma. Expert Rev Anticancer Ther 12: 1137-1147 [DOI] [PubMed] [Google Scholar]
- Bunone G., Vigneri P., Mariani L., Buto S., Collini P., Pilotti S., et al. (1999) Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 155: 1967-1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr L., Mankoff D., Goulart B., Eaton K., Capell P., Kell E., et al. (2010) Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 16: 5260-5268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow P., Machin D., Chen Y., Zhang X., Win K., Hoang H., et al. (2011) Randomised double-blind trial of megestrol acetate vs placebo in treatment-naive advanced hepatocellular carcinoma. Br J Cancer 105: 945-952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D., Lacouture M., Fillos T., Wu S. (2008) Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol 47: 176-186 [DOI] [PubMed] [Google Scholar]
- Cohen E., Needles B., Cullen K., Wong S., Wade J., Ivy S., et al. (2008a) Phase 2 study of sunitinib in refractory thyroid cancer. J Clin Oncol 26: 6025 [Google Scholar]
- Cohen E., Rosen L., Vokes E., Kies M., Forastiere A., Worden F., et al. (2008b) Axitinib Is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26: 4708-4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colevas A., Shah M. (2012) Evaluation of patients with disseminated or locoregionally advanced thyroid cancer: a primer for medical oncologists. Am Soc Clin Oncol Educ Book 32: 384-388 [DOI] [PubMed] [Google Scholar]
- Cooper D., Doherty G., Haugen B., Kloos R., Al E. (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19: 1167-1214 [DOI] [PubMed] [Google Scholar]
- Cramp F., Byron-Daniel J. (2012) Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev 11: CD006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droz J., Schlumberger M., Rougier P., Ghosn M., Gardet P., Parmentier C. (1990) Chemotherapy in metastatic nonanaplastic thyroid cancer: experience at the Institut Gustave-Roussy. Tumori 76: 480-483 [DOI] [PubMed] [Google Scholar]
- Durante C., Haddy N., Baudin E., Leboulleux S., Hartl D., Travagli J., et al. (2006) Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91: 2892-2899 [DOI] [PubMed] [Google Scholar]
- Feine U., Lietzenmayer R., Hanke J., Held J., Wohrle H., Muller-Schauenburg W. (1996) Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med 37: 1468-1472 [PubMed] [Google Scholar]
- Gottlieb J., Hill C., Jr (1974) Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. N Engl J Med 290: 193-197 [DOI] [PubMed] [Google Scholar]
- Grandinetti C., Goldspiel B. (2007) Sorafenib and sunitinib: novel targeted therapies for renal cell cancer. Pharmacotherapy 27: 1125-1144 [DOI] [PubMed] [Google Scholar]
- Gupta-Abramson V., Troxel A., Nellore A., Puttaswamy K., Redlinger M., Ransone K., et al. (2008) Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26: 4714-4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D., Lucas A., Tanvetyanon T., Krzyzanowska M., Chung C., Murphy B., et al. (2012) Phase II efficacy and pharmacogenomic study of selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res 18: 2056-2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A., Grewal R., Leboeuf R., Sherman E., Pfister D., Deandreis D., et al. (2013) Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 368: 623-632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftijzer H., Heemstra K., Morreau H., Stokkel M., Corssmit E., Gelderblom H., et al. (2009) Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol 161: 923-931 [DOI] [PubMed] [Google Scholar]
- Homsi J., Daud A. (2007) Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control 14: 285-294 [DOI] [PubMed] [Google Scholar]
- Hundahl S., Fleming I., Fremgen A., Menck H. (1998) A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 83: 2638-2648 [DOI] [PubMed] [Google Scholar]
- Jatoi A., Windschitl H., Loprinzi C., Sloan J., Dakhil S., Mailliard J., et al. (2002) Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group Study. J Clin Oncol 20: 567-573 [DOI] [PubMed] [Google Scholar]
- Keefe S., Troxel A., Rhee S., Puttaswamy K., O’Dwyer P., Loevner L., et al. (2011) Phase II trial of sorafenib in patients with advanced thyroid cancer. J Clin Oncol 29: 5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E., Nikiforova M., Zhu Z., Knauf J., Nikiforov Y., Fagin J. (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63: 1454-1457 [PubMed] [Google Scholar]
- Klein M., Vignaud J., Hennequin V., Toussaint B., Bresler L., Plenat F., et al. (2001) Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab 86: 656-658 [DOI] [PubMed] [Google Scholar]
- Kloos R., Ringel M., Knopp M., Hall N., King M., Stevens R., et al. (2009) Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27: 1675-1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture M., Reilly L., Gerami P., Guitart J. (2008) Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 19: 1955-1961 [DOI] [PubMed] [Google Scholar]
- Leboulleux S., Bastholt L., Krause T., De La, Fouchardiere C., Tennvall J., Awada A., et al. (2012) Vandetanib in locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 2 trial. Lancet Oncol 13: 897-905 [DOI] [PubMed] [Google Scholar]
- Loprinzi C., Michalak J., Schaid D., Mailliard J., Athmann L., Goldberg R., et al. (1993) Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J Clin Oncol 11: 762-767 [DOI] [PubMed] [Google Scholar]
- Lu S., Lin S., Chen P., Jeng L., Chen S., Hu C., et al. (2013) Randomized investigation of prophylactic corticosteroid versus non-corticosteroid ointment for reducing cutaneous toxicity in Taiwanese patients receiving sorafenib for hepatocellular carcinoma. Presented at the Asia Pacific Association for the Study of the Liver (APASL) Liver Week; 6–10 June 2013; Singapore [Google Scholar]
- Matsui J., Yamamoto Y., Funahashi Y., Tsuruoka A., Watanabe T., Wakabayashi T., et al. (2008) E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer 122: 664-671 [DOI] [PubMed] [Google Scholar]
- Matuszczyk A., Petersenn S., Bockisch A., Gorges R., Sheu S., Veit P., et al. (2008) Chemotherapy with doxorubicin in progressive medullary and thyroid carcinoma of the follicular epithelium. Horm Metab Res 40: 210-213 [DOI] [PubMed] [Google Scholar]
- Mitchell S. (2010) Cancer-related fatigue: state of the science. PM R 2: 364-383 [DOI] [PubMed] [Google Scholar]
- NCCN (2013) NCCN Clinical Practice Guidelines in Oncology. Thyroid carcinoma (version 2.2013). Fort Washington, PA: National Comprehensive Cancer Network [Google Scholar]
- NCCN (2014) NCCN Clinical Practice Guidelines in Oncology. Cancer-related Fatigue (version 1.2014). Fort Washington, PA: National Comprehensive Cancer Network [Google Scholar]
- Nixon I., Whitcher M., Palmer F., Tuttle R., Shaha A., Shah J., et al. (2012) The impact of distant metastases at presentation on prognosis in patients with differentiated carcinoma of the thyroid gland. Thyroid 22: 884-889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Kodama K., Takase K., Sugi N., Yamamoto Y., Iwata M., et al. (2013) Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (e7080) against ret gene fusion-driven tumor models. Cancer Lett 340: 97-103 [DOI] [PubMed] [Google Scholar]
- Pacini F., Castagna M. (2012) Approach to and treatment of differentiated thyroid carcinoma. Med Clin North Am 96: 369-383 [DOI] [PubMed] [Google Scholar]
- Pascual Lopez A., Roque I Figuls M., Urrutia Cuchi G., Berenstein E., Almenar Pasies B., Balcells Alegre M., et al. (2004) Systematic review of megestrol acetate in the treatment of anorexia-cachexia syndrome. J Pain Symptom Manage 27: 360-369 [DOI] [PubMed] [Google Scholar]
- Pellegriti G., Frasca F., Regalbuto C., Squatrito S., Vigneri R. (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013: 965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell N., Daniels G., Haddad R., Ross D., Evans T., Wirth L., et al. (2008) A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid 18: 317-323 [DOI] [PubMed] [Google Scholar]
- Ren Z., Zhu K., Kang H., Lu M., Qu Z., Lu L., et al. (2012) A randomized controlled phase II study of the prophylactic effect of urea-based cream on the hand-foot skin reaction associated with sorafenib in advanced hepatocellular carcinoma. J Clin Oncol 30: 4008. [DOI] [PubMed] [Google Scholar]
- Robbins R., Wan Q., Grewal R., Reibke R., Gonen M., Strauss H., et al. (2006) Real-time prognosis for metastatic thyroid carcinoma based on 2-[18f]fluoro-2-deoxy-d-glucose-positron emission tomography scanning. J Clin Endocrinol Metab 91: 498-505 [DOI] [PubMed] [Google Scholar]
- Sacks W., Braunstein G. (2013) Evolving approaches in managing radioactive iodine-refractory differentiated thyroid cancer. Endocr Pract: 1-36 [DOI] [PubMed] [Google Scholar]
- Saez J. (2013) Treatment directed to signalling molecules in patients with advanced differentiated thyroid cancer. Anticancer Agents Med Chem 13: 483-495 [DOI] [PubMed] [Google Scholar]
- Santoro M., Carlomagno F., Hay I., Herrmann M., Grieco M., Melillo R., et al. (1992) Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J Clin Invest 89: 1517-1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumberger M., Brose M., Elisei R., Leboulleux S., Luster M., Pitoia F., et al. (2014) Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol 13: 70215-70218 [DOI] [PubMed] [Google Scholar]
- Schlumberger M., Tahara M., Wirth L., Robinson B., Brose M., Elisei R., et al. (2014) A phase 3, multicenter, randomized, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with131-I-refractory differentiated thyroid cancer (SELECT) [Abstract LBA6008]. Paper presented at American Society of Clinical Oncology, Chicago, IL, 30 May–3 June 2013 [Google Scholar]
- Schneider T., Abdulrahman R., Corssmit E., Morreau H., Smit J., Kapiteijn E. (2012) Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol 167: 643-650 [DOI] [PubMed] [Google Scholar]
- Schreinemakers J., Vriens M., Munoz-Perez N., Guerrero M., Suh I., Rinkes I., et al. (2012) Fluorodeoxyglucose-positron emission tomography scan-positive recurrent papillary thyroid cancer and the prognosis and implications for surgical management. World J Surg Oncol 10: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciuto R., Romano L., Rea S., Marandino F., Sperduti I., Maini C. (2009) Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol 20: 1728-1735 [DOI] [PubMed] [Google Scholar]
- Sherman E., Ho A., Fury M., Baxi S., Haque S., Korte S., et al. (2012) A phase II study of temsirolimus/sorafenib in patients with radioactive iodine (RAI)-refractory thyroid carcinoma. J Clin Oncol 30: 5514 [Google Scholar]
- Sherman E., Ho A., Fury M., Baxi S., Haque S., Lipson B., et al. (2013) Phase II study of everolimus and sorafenib for the treatment of metastatic thyroid cancer. J Clin Oncol 31: 6024 [Google Scholar]
- Sherman S., Jarzab B., Cabanillas M., Licitra L., Pacini F., Martins R., et al. (2011) A phase II trial of the multitargeted kinase inhibitor E7080 in advanced radioiodine (RAI)-refractory differentiated thyroid cancer (DTC). J Clin Oncol 29: 5503 [Google Scholar]
- Sherman S., Wirth L., Droz J., Hofmann M., Bastholt L., Martins R., et al. (2008) Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med 359: 31-42 [DOI] [PubMed] [Google Scholar]
- Shimaoka K., Schoenfeld D., Dewys W., Creech R., Deconti R. (1985) A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56: 2155-2160 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2013) FDA news release: FDA approves nexavar to treat type of thyroid cancer. Silver Spring, MD [Google Scholar]
- Walko C., Grande C. (2014) Management of common adverse events in patients treated with sorafenib: nurse and pharmacist perspective. Semin Oncol 41(Suppl. 2): S17-S28 [DOI] [PubMed] [Google Scholar]
- Wang W., Larson S., Tuttle R., Kalaigian H., Kolbert K., Sonenberg M., et al. (2001) Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid 11: 1169-1175 [DOI] [PubMed] [Google Scholar]
- Wang Y., Hou P., Yu H., Wang W., Ji M., Zhao S., et al. (2007) High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/Akt pathway in thyroid tumors. J Clin Endocrinol Metab 92: 2387-2390 [DOI] [PubMed] [Google Scholar]
- Wexler J. (2011) Approach to the thyroid cancer patient with bone metastases. J Clin Endocrinol Metab 96: 2296-2307 [DOI] [PubMed] [Google Scholar]
- Wilhelm S., Adnane L., Newell P., Villanueva A., Llovet J., Lynch M. (2008) Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 7: 3129-3140 [DOI] [PubMed] [Google Scholar]
- Xing M., Haugen B., Schlumberger M. (2013) Progress in molecular-based management of differentiated thyroid cancer. Lancet 381: 1058-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]