Abstract
Aims and objectives:
We conducted an analysis to explore whether the cardiovascular outcomes associated with nonsteroidal anti-inflammatory drugs (NSAIDs), when used in licensed doses by patients with osteoarthritis or rheumatoid arthritis, was class or compound dependent.
Methods:
Using the Ovid technology search engine, we conducted a search of the literature for relevant studies published between 1995 and 2011. We also retrieved further studies following manual searches. The primary endpoint was major vascular events and the secondary endpoints were stroke, hypertension and congestive heart failure. A total of 19 studies were analysed. Studies conducted in the osteoarthritis and rheumatoid arthritis patients’ population that reported on cardiovascular events were included in the analysis. The analysis was conducted using the software Review Manager 5.1 and Cochrane methodology.
Results:
Using the primary endpoint of major vascular events (MVE) and a prespecified cutoff point of 1.30, diclofenac (versus 1 comparator) and rofecoxib (versus 2 comparators) had increased risk for MVE [odds ratio (OR) >1.30]. Using the same criteria, diclofenac (versus 1 comparator) had an increased risk of myocardial infarction (MI). Although celecoxib had a slightly increased risk for MI (OR 1.33, versus 1 comparator), the confidence interval included 1 and was not significant. For the secondary endpoints, etoricoxib and rofecoxib were significantly worse off for HT (versus 1 comparator each) and naproxen was significantly worse off for stroke (versus 1 comparator). Although ibuprofen was worse off for HT (versus 1 comparator) the increased risk was not significant.
Conclusion:
From the analysis conducted, it appears that the risk for cardiovascular events in arthritis patients on licensed doses of NSAIDs varies considerably and is likely to depend on the individual compound.
Keywords: cardiovascular risk, nonsteroidal anti-inflammatory drugs, NSAIDs
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often used for the treatment of both noninflammatory as well as inflammatory conditions [Burke et al. 2006]. These drugs comprise the traditional NSAIDs (tNSAIDs) and the NSAIDs or coxibs that are selective for cyclooxygenase (COX)-2). The ‘coxibs’ were developed in the late 1990s to reduce the risk of serious gastrointestinal (GI) adverse effects, features of which were associated with the inhibition of COX-1 [Fitzgerald and Patrono, 2001]. The main therapeutic effects (anti-inflammatory and analgesic) of NSAIDs (coxibs and traditional) are mostly due to the inhibition of COX-2 dependent prostanoids.
The results from placebo-controlled randomized clinical trials (RCTs) showed that coxibs were associated with increased relative risk (RR) of cardiovascular (CV) events by between 1 and 2.7 fold [Bresalier et al. 2005; White et al. 2007; Ott et al. 2003; Solomon et al 2008; Nussmeir et al. 2005]. However, results from meta-analysis and observational studies obtained from trials with coxibs have shown that the CV events are not restricted to NSAIDs that are selective for COX-2, but also applies to some traditional NSAIDs, such as diclofenac [Hernández-Diaz et al. 2006; Kearney et al. 2006].
According to a memorandum submitted to the US Food and Drug Administration (FDA) in April 2005 on the analysis and recommendations for NSAIDs and CV risk, the Centre for Drug Evaluation and Research (CDER) review committee felt that it was not possible to conclude at that point that the COX-2 selective drugs are associated with higher CV outcomes compared to nonselective NSAIDs in chronic use [Jenkins and Seligman, 2005]. The FDA believed then that it was reasonable to conclude that there is a ‘class effect’ for increased CV outcomes for all NSAIDs pending the availability of data from long-term controlled clinical trials that defined the true relationships more clearly.
Most of the available analyses to date on this topic have included trials where supratherapeutic doses of tNSAIDs or COX-2s were used or included patient population other than arthritis patients.
In this analysis, we examined the CV outcomes attributable to currently available and commonly used compounds (NSAIDs/COX-2s) in Europe used at licensed doses in patients with rheumatoid arthritis (RA) and osteoarthritis (OA) (as opposed to patients who were taking the medication for preventive purposes, such as patients with polyps or for the prevention of Alzheimer’s disease).
Although it is a common assumption that drugs within the same class will have similar pharmacological properties and clinical effects, this may not always be the case [McAllister et al. 1999]. It would appear reasonable to accept that drugs within the same class exert similar effects, unless there is clear evidence of important differences. However, as rightly suggested by McAllister and colleagues, this assumption can lead to significant errors arising from extrapolation with profound clinical consequences.
In the first instance when agents in a class of drugs, such as angiotensin-converting-enzyme (ACE) inhibitors, all produce similar pharmacological effects (lowering of blood pressure) and similar clinical outcome (reduction in myocardial infarction and major CV events), a second class of drugs (for example, the calcium channel blockers) that produce the same pharmacological actions might be assumed to produce the same clinical benefits. However, a meta-analysis of observational studies by Pahor and colleagues comparing the effects of ACE-inhibitor based therapy versus calcium antagonist based therapies as first-line drugs for hypertension suggested a significantly higher risk of acute myocardial infarction (MI), congestive heart failure (CHF) and major CV events in those assigned to the calcium antagonists arm of treatment [Pahor et al. 2000]. In essence, in the absence of RCTs confirming that final assumption, the extrapolation of pharmacological effects to clinical benefit or risk may be misleading.
Secondly, drugs within the same class may have physiologic effects, which are different from the mechanism of action that defined them as being from the same class.
There are conflicting data in the literature regarding the association between the use of NSAIDs and the development of adverse cardiac events [Sooriakumaran, 2006]. It has been proposed by some authors [Fitzgerald, 2004; Cipollone et al. 2008] that COX-2 selective NSAIDs may adversely influence the prostacyclin (antithrombotic) to thromboxane (prothrombotic) ratio in the walls of the blood vessels. They argued this is possible because coxibs may inhibit the production of prostacyclin but leave the generation of thromboxane unaffected, thereby leading to platelet aggregation and atherosclerosis [Fitzgerald, 2004; Cipollone et al. 2008]. Furthermore, inhibition of prostacyclin in the kidney might lead to sodium and water retention, leading to hypertension [Krum et al. 2004]. Hypertension and water retention can in turn increase the risk of CV events, including CHF and MI. Other authors [Kirkby et al.2012] have argued that the issue of imbalance between vascular prostacyalin and thromboxane as a reason for selective COX-2 CV toxicity has not been convincingly demonstrated, it is COX-1 and not COX-2 that drives prostacyclin production in the CV system. The mechanism proposed for naproxen’s possible lack of CV adverse effect is its inhibition of thromboxane by 95% with consequent inhibition of platelet aggregation by about 88% [Van Hecken et al. 2000].
Major vascular event was defined a priori as any incident of deep vein thrombosis (DVT), pulmonary embolism (PE), angina, transient ischemic attack(TIA), rhythm disorders, valvular heart disease, acute coronary syndrome (excluding MI if it is specifically mentioned) or other thrombo-embolic disorders.
CV risk associated with NSAIDs: regulatory background
In 2004, the pharmaceutical company Merck (now MSD) voluntarily withdrew rofecoxib (Vioxx®) from the market. It decided to withdraw the drug based on the clinical trial, APPROVe (Adenomatous Polyp Prevention on Vioxx), which was a three-year randomized, placebo controlled trial [Bresalier et al. 2005]. The trial was an efficacy trial evaluating Vioxx in the prevention of the recurrence colorectal polyp in patients with a history of colorectal adenomas. However, the trial was stopped when interim safety results showed an increased relative risk for CV events after about 1½ years of treatment in the patients taking Vioxx compared with those taking placebo.
The removal of Vioxx from the market increased public and FDA scrutiny of COX-2 inhibitors. The FDA’s Arthritis and Drug Safety and Risk Management Advisory Committees met in February 2005 to discuss the safety of COX-2 drugs and other NSAIDs. Among the issues discussed was whether another COX-2 inhibitor, valdecoxib (Bextra®), should remain on the market following the withdrawal of Vioxx. The FDA Advisory Committee was in favour of valdecoxib remaining on the market, with 17 voting yes, 13 voting no, and 2 abstentions [Jenkins and Seligman, 2005]. However, in April 2005, the FDA requested the manufacturer of valdecoxib (Pfizer Ltd) to voluntarily suspend the sales of valdecoxib. The FDA’s position was that there was an increased risk of CV events with all prescription NSAIDs for arthritis, and that the additional increased risk of rare, serious skin reactions with valdecoxib warranted the suspension of sales of the drug [Jenkins and Seligman, 2005].
In Europe, the European Medicines Agency (EMA) and the Committee for Medicinal Products for Human Use (CHMP) have indicated that an increased risk of MI and stroke may be a class effect of all COX-2 inhibitors. As such, the EMA advised that these medicines should not be used in patients with stroke or ischaemic heart disease, that prescribers should exercise caution when prescribing COX-2 inhibitors for patients with risk factors such as peripheral arterial disease and diabetes, and that doctors should use the lowest effective dose of COX-2 for the shortest possible duration. Currently, there are no published large RCTs that are specifically designed and powered to compare COX-2 inhibitors with tNSAIDs with CV events as endpoints, so it is difficult to ascertain with confidence whether the tNSAIDs have similar risk of leading to a CV event compared with COX-2 inhibitors. The available literature on the topic has shown conflicting results so far.
The recent report from CHMP issued in October 2012 (EMA 2012a) reiterates the previous conclusion that an increased CV risk for NSAIDs as a class cannot be excluded. What will be most useful for clinicians is to know the absolute and relative risk for the specific NSAID they prescribe for their patients. This important clinical information is not available yet.
Methods
Search strategy
Using the Ovid technology search engine which included the following publications: OVID MEDLINE(R) and OVID MEDLINE(R) In Process, Ovid Medline(R) without revisions, BIOSIS Review, OVID MEDLINE(R) In Process and other CAB abstracts, EMBASE Daily alerts and EMBASE, we conducted a search using the words and connectors below. We also performed a couple of manual searches looking at abstracts, posters and presentations from scientific meetings and international congresses.
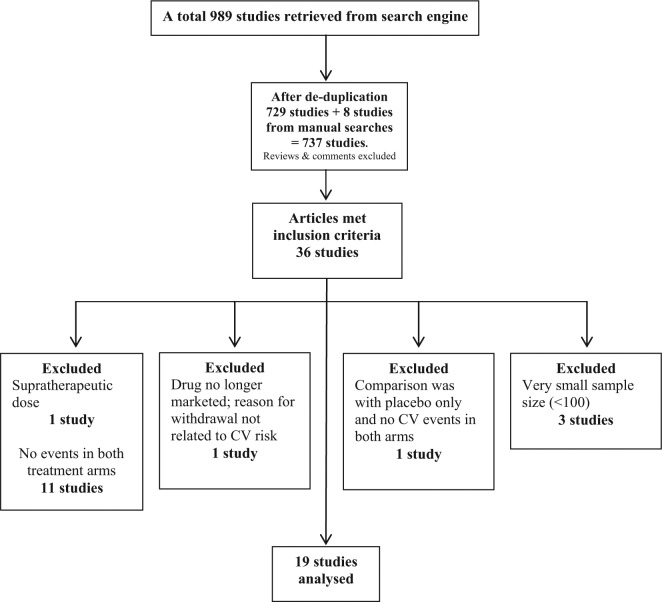
We sought to compare the CV risk associated with the most commonly used NSAIDs in Europe (EMA 2012b). The search words and connectors were ibuprofen or celecoxib or diclofenac or etoricoxib or naproxen and arthritis and efficacy and safety and trial. A total of 989 articles were retrieved (Figure 1). After removing duplicates, the search returned 823 results. Following the specification of year range from 1995 to 2011, there were 729 results. There were 8 further studies retrieved following manual searches making a total of 735 studies. The abstracts of these studies were reviewed with the inclusion criteria list.
Figure 1.
Overview of studies included in the nonsteroidal anti-inflammatory drugs (NSAIDs) analysis.
CV, cardiovascular.
Selection criteria
Population
The patient population of interest is RA and OA.
Inclusion criteria:
RCTs
Study duration of at least 4 weeks
Study involves a COX-2 inhibitor or tNSAID used at licensed doses
Publication reported CV outcome, e.g. events of MI, stroke CV death or other major vascular events like thrombo-embolism, CHF or hypertension
Exclusion criteria:
Trials conducted in patients with other conditions apart from arthritis (such as polyps and Alzheimer’s disease)
Doses of tNSAIDs or COX-2 inhibitors higher than licensed doses
Studies not involving tNSAIDs or COX-2 inhibitors
Trials less than 4 weeks’ duration
Trials conducted only in paediatric population
No extractable data (comments, review articles, letters, etc.)
Very small sample size (<100)
Article selection and data entry
The articles selected for analysis were those that met the inclusion criteria and did not meet any of the exclusion criteria. Trials of less than 4 weeks’ duration and those with sample size <100 were excluded because 4 weeks was considered to be too short to monitor these patients for the development of CV events of interest and a sample size of <100 is unlikely to pick up these uncommon adverse events. Initially, we planned to conduct this analysis using studies where there were head to head data for NSAIDs as well as studies where the NSAIDs were compared with placebo. However, following the review of the literature and suitable studies, it was clear that almost all the studies where NSAIDs were compared with placebo did not have or report any CV events in both treatment arms, and so these studies would not provide any data for analysis. As such, we limited the analysis to studies where there where head to head comparison of the NSAIDs.
Data extraction and entry for CV events which were collected include MI, high blood pressure (hypertension), strokes, CHF pulmonary embolism, DVT, rhythm disorders, acute coronary syndromes and angina. Other data that was collected include the study type, sample size, active treatment and control, dose, duration of treatment and patient population. We presented confidence levels, which can be interpreted as confidence that a drug is associated with an increase risk that is higher than a specified threshold. This is the confidence that the increase in CV risk associated with the evaluated NSAID exceeds 30%, i.e. the value for risk increase used as noninferiority margin in the Multinational Etoricoxib and Diclofenac Arthritis Long-term Programme [Cannon et al. 2006; Trelle et al. 2011].
Where there were more than two different studies we conducted a meta-analyses of the relevant studies using the Cochrane methodology. However, where there was just one relevant study for analysis, the data was presented separately in table format.
The recommended doses for the NSAIDs selected for review are highlighted in Table 1.
Table 1.
Recommended dose range for NSAIDs for OA and RA in UK.*
| NSAIDs | Recommended adult dose range in UK (OA) | Recommended adult dose range in UK (RA) |
|---|---|---|
| Etoricoxib | 30–60 mg once a day | 90 mg once a day |
| Naproxen | 0.5–1g per day in 2 divided doses | 0.5–1g per day in 2 divided doses |
| Celecoxib | 200 mg once a day or in 2 divided doses, maximum 400 mg daily | 200–400 mg daily in 2 divided doses, maximum 400 mg daily |
| Diclofenac | 75–150 mg per day in 2–3 divided doses | 75–150 mg per day in 2–3 divided doses |
| Ibuprofen | 1200–1800 mg per day, maximum 2400 mg per day | 1200–1800 mg per day, maximum 2400 mg per day |
| Rofecoxib$ | 25 mg once daily, maximum 50 mg once daily | 25 mg once daily, maximum 50 mg once daily |
Data from MIMS website, www.mims.co.uk (accessed 4 April 2004).
No longer available.
NSAIDs, nonsteroidal anti-inflammatory drugs; OA, osteoarthritis; RA, rheumatoid arthritis.
Studies that were analysed included:
Ibuprofen versus tNSAIDs/COX-2 inhibitors
Celecoxib versus tNSAIDs/COX-2 inhibitors
Naproxen versus tNSAIDs/COX-2 inhibitor
Eetoricoxib versus tNSAIDs/COX-2 inhibitors
Diclofenac versus tNSAIDs/COX-2 inhibitors
Primary endpoint
Major vascular events: this was defined a priori as any incident of pulmonary embolism, DVT, other thromboembolic disorders and acute coronary syndromes (except MI which was analysed separately as a co-primary endpoint).
Secondary endpoints
Stroke, hypertension and CHF
Statistical analysis
The analysis was conducted using Review Manager 5.1. For the dichotomous outcome in this analysis, the preferred method for each outcome was the Peto odds ratio (OR) as opposed to using the fixed-effect Mantel–Haenszel OR for dichotomous outcomes. The Peto OR is particularly useful here when the events under consideration are not very common and the studies have similar numbers in the experimental and control arms. It has the added advantage of not needing a correction for zero cell count [Green, 2008]. The 95% confidence interval, chi squared and I2 statistic for heterogeneity were also calculated by the software.
Results
The review analysed 19 studies (Appendix 1). The results are shown as follows: etoricoxib versus diclofenac (Tables 2–6 and Figures 2–6), celecoxib versus diclofenac (Tables 7–10 and Figures 7–10), etoricoxib versus naproxen (Table 11 and Figure 11), rofecoxib versus naproxen (Tables 12–13 and Figures 12–13), rofecoxib versus diclofenac (Tables 14–15), rofecoxib versus naproxen (Tables 16–17), etoricoxib versus ibuprofen (Table 18) and rofecoxib versus ibuprofen (Table 19).
Table 2.
Etoricoxib versus diclofenac for major vascular events.
| Study or subgroup | Etoricoxib |
Diclofenac |
Weight | Peto OR, fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| EDGE 2005 [Baraf et al. 2007] | 14 | 3593 | 17 | 3518 | 3.90% | 0.81 [0.40, 1.63] |
| EDGE II [Kreuger et al. 2008] | 21 | 2032 | 15 | 2054 | 4.50% | 1.41 [0.73, 2.73] |
| MEDAL 2009 [Coombe et al. 2009] | 394 | 11,787 | 363 | 11,717 | 91.60% | 1.08 [0.94, 1.25] |
| Zacher et al. [2003] | 0 | 256 | 1 | 260 | 0.10% | 0.14 [0.00, 6.93] |
| Total (95% CI) | 17,668 | 17,549 | 100.00% | 1.08 [0.94, 1.24] | ||
| Total events | 429 | 396 | ||||
Heterogeneity: chi squared = 2.37, degrees of freedom = 3 (p = 0.50); I2 = 0%. Test for overall effect: Z = 1.08 (p = 0.28).
CI, confidence interval; OR, odds ratio
Table 3.
Etoricoxib versus diclofenac for myocardial infarction.
| Study or subgroup | Etoricoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| EDGE 2005 [Baraf et al. 2007] | 19 | 3593 | 13 | 3518 | 47.2% | 1.43 [0.71, 2.86] |
| EDGE II [Kreuger et al. 2008] | 14 | 2032 | 22 | 2054 | 52.8% | 0.65 [0.34, 1.24] |
| Total (95% CI) | 5625 | 5572 | 100.00% | 0.94 [0.58, 1.51] | ||
| Total events | 33 | 35 | ||||
Heterogeneity: chi squared = 2.65, degrees of freedom = 1 (p = 0.10); I2 = 62%. Test for overall effect: Z = 0.26 (p = 0.79).
CI, confidence interval; OR, odds ratio.
Table 4.
Etoricoxib versus diclofenac for stroke.
| Study or Subgroup | Etoricoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| EDGE 2005 [Baraf et al. 2007] | 6 | 3593 | 10 | 3518 | 44.5% | 0.59 [0.22, 1.58] |
| EDGE II [Kreuger et al. 2008] | 8 | 2032 | 12 | 2054 | 55.5% | 0.68 [0.28, 1.63] |
| Total (95% CI) | 5625 | 5572 | 100.00% | 0.64 [0.33, 1.23] | ||
| Total events | 14 | 22 | ||||
Heterogeneity: chi squared = 0.04, degrees of freedom = 1 (p = 0.85); I2 = 0%. Test for overall effect: Z = 1.35 (p = 0.18).
CI, confidence interval; OR, odds ratio.
Table 5.
Etoricoxib versus diclofenac for hypertension.
| Study or subgroup | Etoricoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| EDGE 2005 [Baraf et al. 2007] | 210 | 3593 | 95 | 3518 | 41.8% | 2.15 [1.71, 2.71] |
| EDGE II [Krueger et al. 2008] | 240 | 2032 | 197 | 2054 | 55.9% | 1.26 [1.03, 1.54] |
| ZACHER 2003 | 8 | 256 | 9 | 260 | 2.4% | 0.90 [0.34, 2.36] |
| Total (95% CI) | 5881 | 5832 | 100.0% | 1.56 [1.35, 1.81] | ||
| Total events | 458 | 301 | ||||
Heterogeneity: chi squared = 13.17, degrees of freedom = 2 (p = 0.001); I2 = 85%. Test for overall effect: Z = 5.91 (p = 0.00001).
CI, confidence interval; OR, odds ratio.
Table 6.
Etoricoxib versus diclofenac for congestive heart failure.
| Study or subgroup | Etoricoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| EDGE 2005 [Baraf et al. 2007] | 14 | 3593 | 17 | 3518 | 45.7% | 0.81 [0.40, 1.63] |
| EDGE II [Krueger et al. 2008] | 21 | 2032 | 15 | 2054 | 52.8% | 1.41 [0.73, 2.73] |
| ZACHER 2003 | 0 | 256 | 1 | 260 | 1.5% | 0.14 [0.00, 6.93] |
| Total (95% CI) | 5881 | 5832 | 100.00% | 1.06 [1.66, 1.70] | ||
| Total events | 35 | 33 | ||||
Heterogeneity: chi squared = 2.37, degrees of freedom = 2 (p = 0.31); I2 = 15%. Test for overall effect: Z = 0.23 (p = 0.82).
CI, confidence interval; OR, odds ratio.
Figure 2.
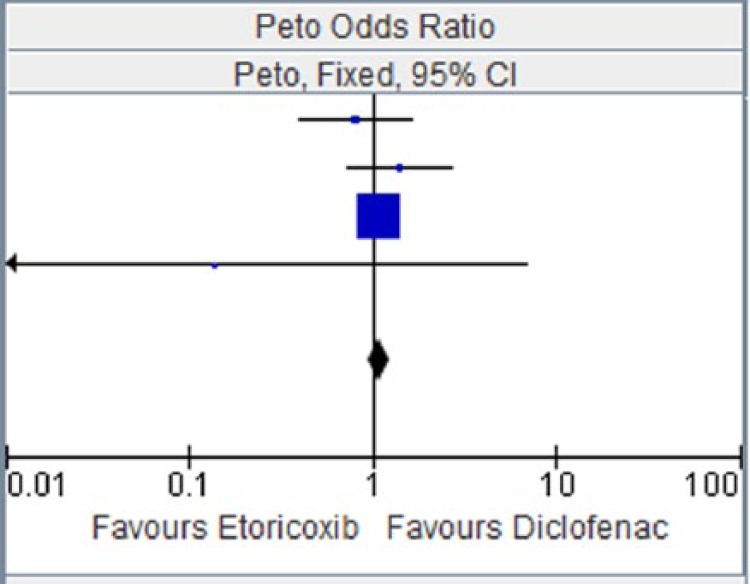
Etoricoxib versus diclofenac for major vascular events.
CI, confidence interval.
Figure 3.
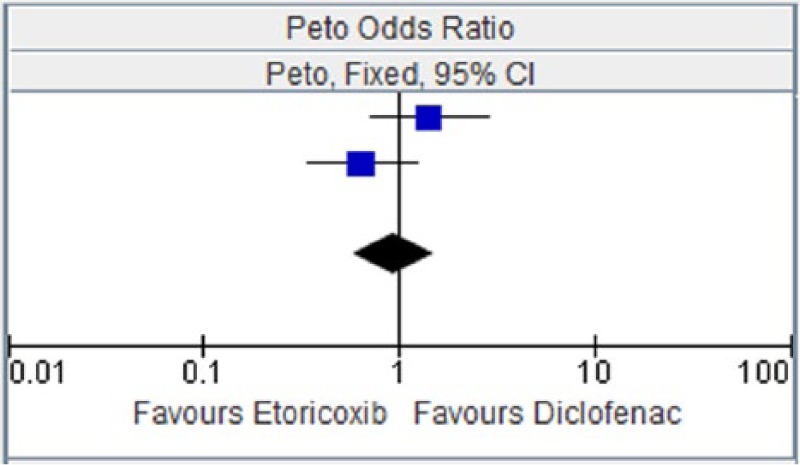
Etoricoxib versus diclofenac for myocardial infarction.
CI, confidence interval.
Figure 4.
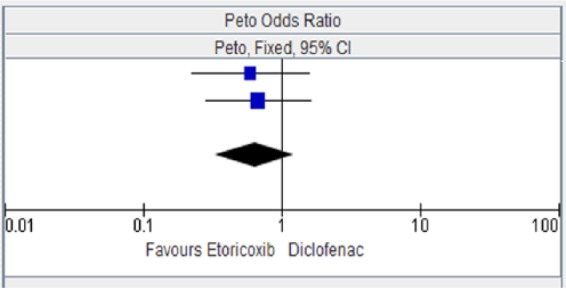
Etoricoxib versus diclofenac for stroke.
CI, confidence interval.
Figure 5.
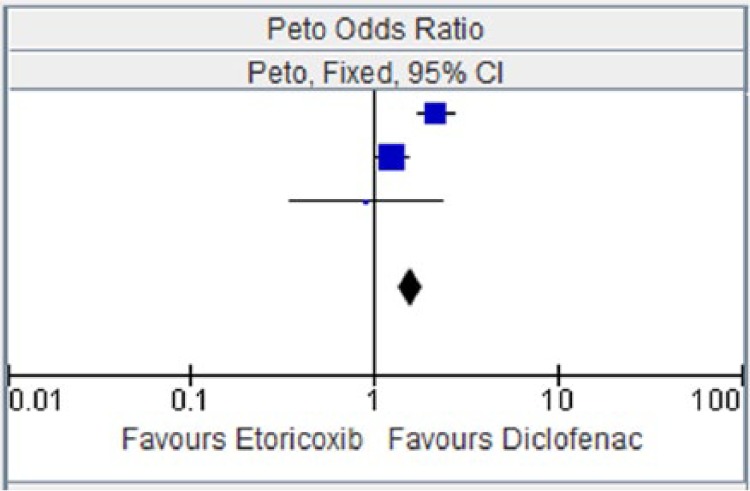
Etoricoxib versus diclofenac for hypertension.
CI, confidence interval.
Figure 6.
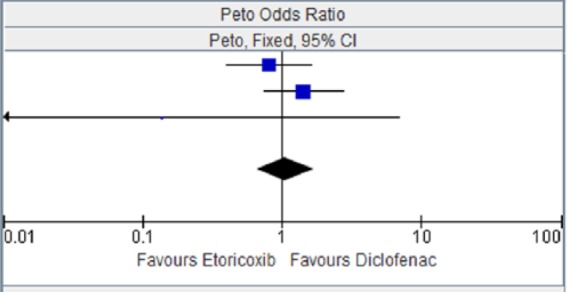
Etoricoxib versus diclofenac for congestive heart failure.
CI, confidence interval.
Table 7.
Celecoxib versus diclofenac for major vascular events.
| Study or subgroup | Celecoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| CAESAR 2009 [Dahlberg et al. 2009] | 3 | 458 | 8 | 458 | 41.4% | 0.40 [0.12, 1.31] |
| CONDOR 2010 [Chan et al. 2010] | 8 | 2238 | 1 | 2246 | 34.2% | 4.77 [1.29, 17.63] |
| McKenna et al. [2001] | 0 | 201 | 2 | 199 | 7.6% | 0.13 [0.01, 2.14] |
| SUCCESS-I [Singh et al. 2006] | 3 | 8800 | 2 | 4394 | 16.9% | 0.74 [0.12, 4.75] |
| Total (95% CI) | 11697 | 7297 | 100.00% | 0.95 [0.44, 2.04] | ||
| Total events | 14 | 13 | ||||
Heterogeneity: chi squared = 9.89, degrees of freedom = 3 (p = 0.02); I2 = 70%. Test for overall effect: Z = 0.13 (p = 0.90).
CI, confidence interval; OR, odds ratio.
Table 8.
Celecoxib versus diclofenac for myocardial infarction.
| Study or subgroup | Celecoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| CAESAR 2009 [Dahlberg et al. 2009] | 4 | 458 | 6 | 458 | 41.8% | 0.67 [0.19, 2.32] |
| CONDOR 2010 [Chan et al. 2010] | 2 | 2238 | 2 | 2246 | 34.2% | 1.00 [0.14, 7.13] |
| SUCCESS-I [Singh et al. 2006] | 10 | 8800 | 1 | 4394 | 16.9% | 2.98 [0.85, 10.44] |
| Total (95% CI) | 11,496 | 7098 | 100.00% | 1.33 [0.59, 2.97] | ||
| Total events | 16 | 9 | ||||
Heterogeneity: Chi2 = 2.84, df = 2 (p = 0.24); I2 = 30%. Test for overall effect: Z = 0.69 (p = 0.49).
CI, confidence interval; OR, odds ratio.
Table 9.
Celecoxib versus diclofenac for stroke.
| Study or Subgroup | Celecoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| CAESAR 2009 [Dahlberg et al. 2009] | 1 | 458 | 5 | 458 | 25.2% | 0.26 [0.05, 1.30] |
| CONDOR 2010 [Chan et al. 2010] | 3 | 2238 | 3 | 2246 | 25.3% | 1.00 [0.20,4.98] |
| McKenna et al. [2001] | 0 | 201 | 2 | 199 | 8.4% | 0.13 [0.01, 2.14] |
| SUCCESS-I [Singh et al. 2006] | 5 | 8800 | 6 | 4394 | 41.2% | 0.38 [0.11, 1.35] |
| Total (95% CI) | 11597 | 7297 | 100.00% | 0.41 [0.18, 0.91] | ||
| Total events | 9 | 16 | ||||
Heterogeneity: chi squared = 2.14, degrees of freedom = 3 (p = 0.54); I2 = 0%. Test for overall effect: Z = 2.19 (p = 0.03).
CI, confidence interval; OR, odds ratio.
Table 10.
Celecoxib versus diclofenac for hypertension.
| Study or subgroup | Celecoxib |
Diclofenac |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| CAESAR 2009 [Dahlberg et al. 2009] | 12 | 458 | 15 | 458 | 74.7% | 0.80 [0.37, 1.71] |
| Emery et al. [1999] | 4 | 326 | 5 | 329 | 25.3% | 0.81 [0.22, 3.00] |
| Total (95% CI) | 784 | 787 | 100.00% | 0.80 [0.41, 1.55] | ||
| Total events | 16 | 20 | ||||
Heterogeneity: chi squared = 0.00, degree of freedom = 1 (p = 0.99); I2 = 0%. Test for overall effect: Z = 0.67 (p = 0.50).
CI, confidence interval; OR, odds ratio.
Figure 7.
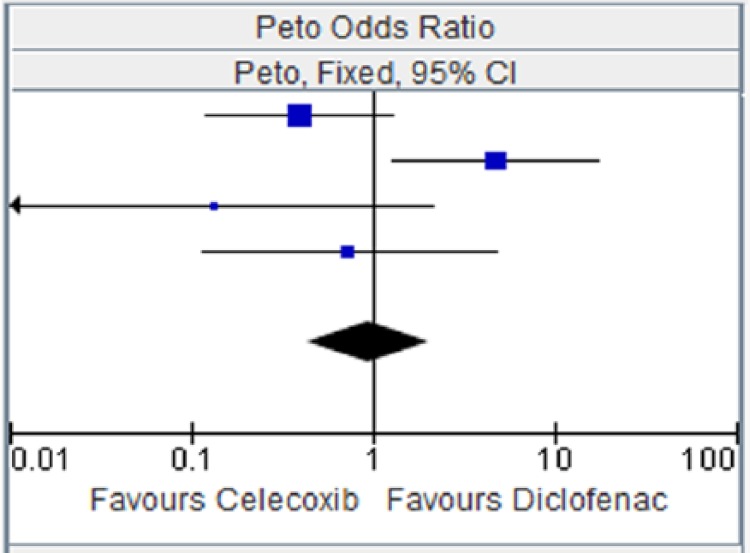
Celecoxib versus diclofenac for major vascular events.
CI, confidence interval.
Figure 8.
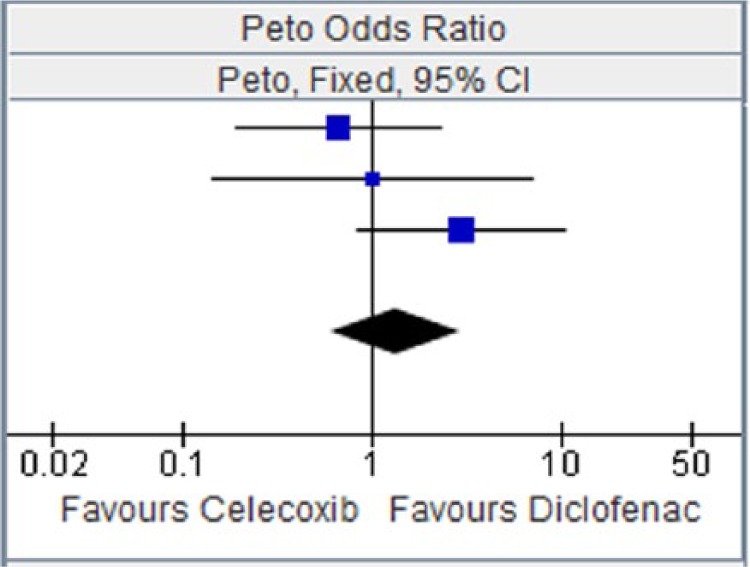
Celecoxib versus diclofenac for myocardial infarction.
CI, confidence interval.
Figure 9.
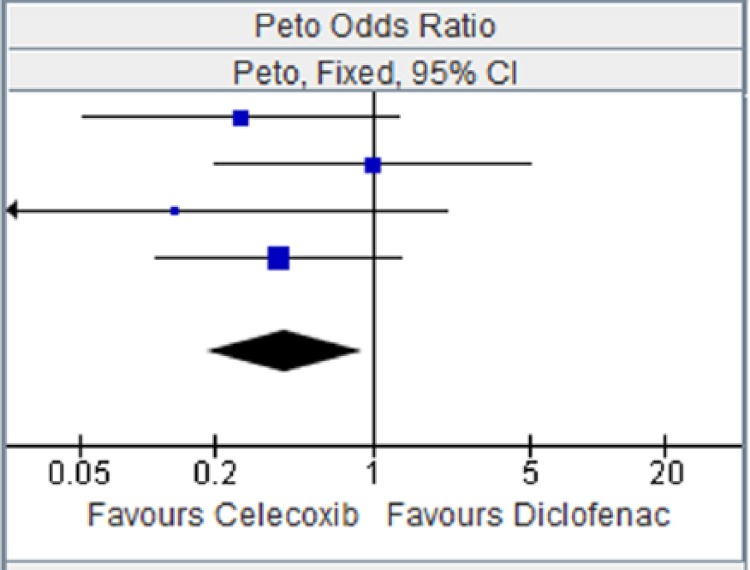
Celecoxib versus diclofenac for stroke.
CI, confidence interval.
Figure 10.
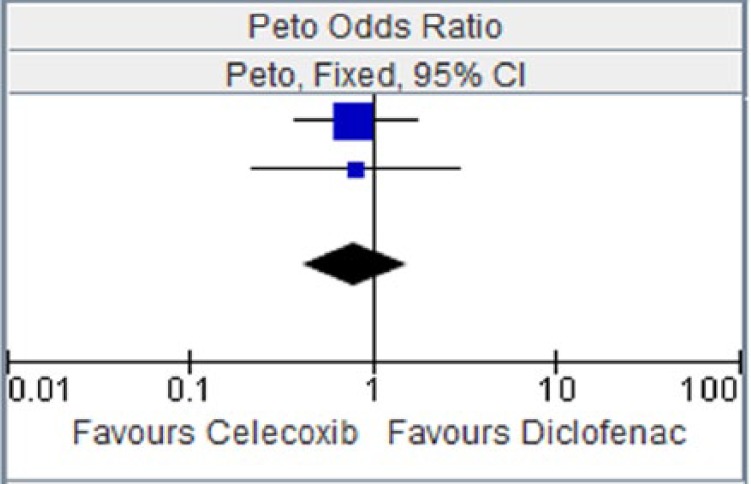
Celecoxib versus diclofenac for hypertension.
CI, confidence interval.
Table 11.
Etoricoxib versus naproxen for hypertension.
| Study or subgroup | Etoricoxib |
Naproxen |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| Collantes et al. [2002] | 12 | 353 | 5 | 181 | 62.5% | 1.23 [0.44, 3.41] |
| Matsumoto et al. [2002] | 7 | 323 | 3 | 170 | 37.5% | 1.22 [0.33, 4.56] |
| Total (95% CI) | 676 | 351 | 100.00% | 1.23 [0.55, 2.75] | ||
| Total events | 19 | 8 | ||||
Heterogeneity: chi squared = 0.00, degree of freedom = 1 (p = 1.00); I2 = 0%. Test for overall effect: Z = 0.50 (p = 0.62).
CI, confidence interval; OR, odds ratio.
Figure 11.
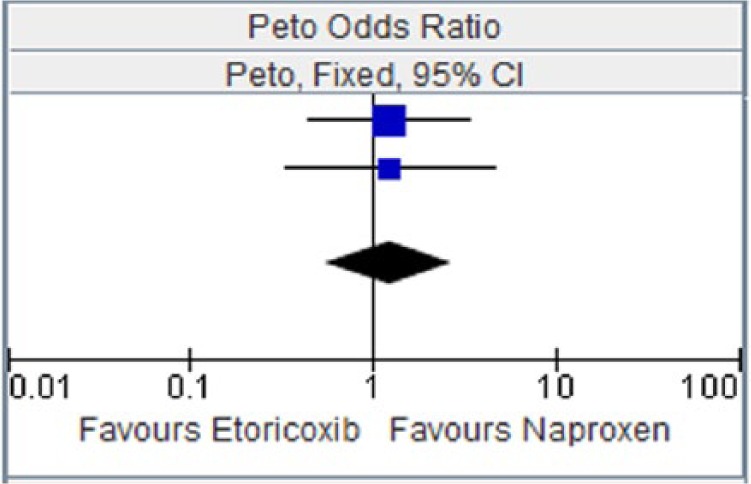
Etoricoxib versus naproxen for hypertension.
CI, confidence interval.
Table 12.
Rofecoxib versus naproxen for myocardial infarction.
| Study or subgroup | Rofecoxib |
Naproxen |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| ADVANTAGE 2003 [Lisse et al. 2003] | 5 | 2785 | 1 | 2772 | 22.3% | 3.78 [0.76, 18.74] |
| Schnitzer et al. [2005] | 0 | 104 | 1 | 121 | 3.7% | 0.16 [0.00, 7.94] |
| VIGOR 2000 [Bombardier et al. 2000] | 16 | 4047 | 4 | 4029 | 74.1% | 3.31 [1.38, 7.97] |
| Total (95% CI) | 6936 | 6922 | 100.00% | 3.05 [1.43, 6.49] | ||
| Total events | 21 | 6 | ||||
Heterogeneity: chi squared = 2.30, degree of freedom = 2 (p = 0.32); I2 = 13%. Test for overall effect: Z = 2.89 (p = 0.004).
CI, confidence interval; OR, odds ratio.
Table 13.
Rofecoxib versus naproxen for major vascular events.
| Study or subgroup | Rofecoxib |
Naproxen |
Weight | Peto OR, Fixed, 95% CI | ||
|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||
| ADVANTAGE 2003 [Lisse et al. 2003] | 14 | 2785 | 7 | 2772 | 95.5% | 1.94 [0.82, 4.58] |
| Schnitzer et al. [2005] | 0 | 104 | 1 | 121 | 4.5% | 0.16 [0.00, 7.94] |
| Total (95% CI) | 2889 | 2893 | 100.00% | 1.73 [0.75, 4.00] | ||
| Total events | 14 | 8 | ||||
Heterogeneity: chi squared = 1.51, degree of freedome = 1 (p = 0.22); I2 = 34%. Test for overall effect: Z = 1.29 (p = 0.20).
CI, confidence interval; OR, odds ratio.
Figure 12.
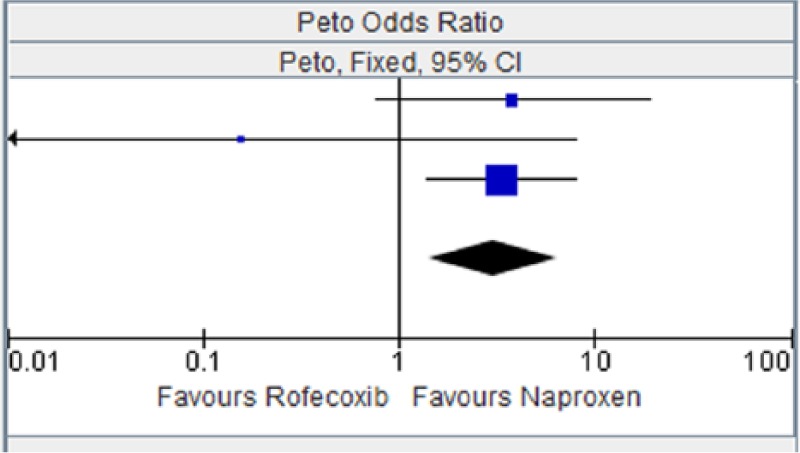
Rofecoxib versus naproxen for myocardial infarction.
CI, confidence interval.
Figure 13.
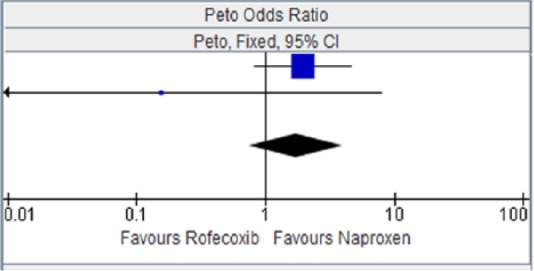
Rofecoxib versus naproxen for major vascular events.
CI, confidence interval.
Table 14.
Rofecoxib versus diclofenac for major vascular event.
| Study or subgroup | Rofecoxib |
Diclofenac |
Peto OR |
||
|---|---|---|---|---|---|
| Events | Total | Events | Total | Peto, Fixed 95% CI | |
| Saag et al. [2000] | 10 | 1209 | 3 | 230 | 0.59 (0.13, 2.60) |
CI, confidence interval; OR, odds ratio.
Table 15.
Rofecoxib versus diclofenac for myocardial infarction.
| Study or subgroup | Rofecoxib |
Diclofenac |
Peto OR |
||
|---|---|---|---|---|---|
| Events | Total | Events | Total | Peto, Fixed 95% CI | |
| Cannon et al. [2000] | 2 | 515 | 2 | 268 | 0.49 (0.06, 3.91) |
CI, confidence interval; OR, odds ratio.
Table 16.
Rofecoxib versus naproxen for stroke.
| Study or subgroup | Rofecoxib |
Naproxen |
Peto OR |
||
|---|---|---|---|---|---|
| Events | Total | Events | Total | Peto, Fixed 95% CI | |
| ADVANTAGE 2003 [Lisse et al. 2003] | 0 | 2785 | 6 | 2772 | 0.13 (0.03, 0.67) |
CI, confidence interval; OR, odds ratio.
Table 17.
Rofecoxib versus naproxen for hypertension.
| Study or subgroup | Rofecoxib |
Naproxen |
Peto OR |
||
|---|---|---|---|---|---|
| Events | Total | Events | Total | Peto, Fixed 95% CI | |
| Geusens et al. [2002] | 28 | 592 | 4 | 268 | 2.47 (1.15, 5.28) |
CI, confidence interval; OR, odds ratio.
Table 18.
Etoricoxib versus ibuprofen for hypertension.
| Study or subgroup | Etoricoxib |
Ibuprofen |
Peto OR |
||
|---|---|---|---|---|---|
| Events | Total | Events | Total | Peto, Fixed 95% CI | |
| Wiesenhutter et al. [2005] | 4 | 214 | 8 | 210 | 0.49 (0.16, 1.56) |
CI, confidence interval; OR, odds ratio.
Table 19.
Rofecoxib versus ibuprofen for major vascular events.
| Study or subgroup | Rofecoxib |
Ibufrofen |
Peto OR |
||
|---|---|---|---|---|---|
| Events | Total | Events | Total | Peto, Fixed 95% CI | |
| Saag et al. [2000] | 3 | 446 | 0 | 221 | 4.48 (0.40, 49.79) |
CI, confidence interval; OR, odds ratio.
Indirect comparison of interventions
When there is little or no direct evidence, the indirect method may be useful to estimate the relative efficacy or side effects attributed to competing interventions. Empirical evidence in the literature [Song, 2003] indicates that results obtained from adjusted indirect comparisons of treatments are not significantly different from those obtained from direct comparisons. Two commonly used indirect comparisons are naïve (unadjusted) indirect comparison and adjusted indirect comparison. In the naive indirect comparison, the data between individual treatments are compared as if they are from the same trial, whereas the adjusted indirect comparison compares the results from the individual trials. In this analysis, the naive comparison of results has been used.
Comparison of tNSAIDs with antiplatelet properties (naproxen) to NSAIDS without antiplatelet properties (ibuprofen and diclofenac)
We compared naproxen with ibuprofen and diclofenac using the endpoints, major vascular events (MVE) and hypertension (HT) which were common to all three drugs as well as using relevant studies where they had a common comparator.
MVE
The aim of the comparison between tNSAIDs with antiplatelet activity (naproxen) and tNSAIDs without antiplatelet activity (ibuprofen and diclofenac) was to determine if tNSAIDs with no antiplatelet activity would be associated with more MVE. From the results of the point estimate below (Table 20), both naproxen and ibuprofen showed decreased risk of having MVE compared with rofecoxib whereas diclofenac showed a higher risk. There was a considerable overlap in the confidence interval for both naproxen and ibuprofen comparison, so it is not possible to conclude from this result that one is better than the other with regards to increased risk of MVE. This might be due to the fact that the sample size is moderate and not large for the ibuprofen comparison (hence the fairly large confidence interval). There was only one study available for the diclofenac versus rofecoxib comparison and the ibuprofen versus rofecoxib comparison, so these results need to be interpreted with caution.
Table 20.
Comparison of naproxen to diclofenac and ibuprofen using rofecoxib as common comparator for major vascular events.
| Intervention | Comparator | OR (intervention versus comparator) | 95% CI (OR) |
|---|---|---|---|
| Naproxen | Rofecoxib | 0.71 | 0.27, 1.83 |
| Diclofenac | Rofecoxib | 1.70 | 0.38, 7.56 |
| Ibuprofen | Rofecoxib | 0.22 | 0.02, 2.48 |
CI, confidence interval; OR, odds ratio.
HT
HT was also used as an endpoint in various studies comparing diclofenac or naproxen with celecoxib. The point estimates from the results of this comparison (Table 21) shows that both naproxen and diclofenac had increased risk compared with celecoxib with regards to HT. However, both comparisons include unity and so the differences were not significant.
Table 21.
Comparison of naproxen to diclofenac using celecoxib as common comparator for hypertension.
| Intervention | Comparator | OR (intervention versus comparator) | 95% CI (OR) |
|---|---|---|---|
| Naproxen | Celecoxib | 1.23 | 0.66, 2.31 |
| Diclofenac | Celecoxib | 1.25 | 0.65, 2.43 |
[Sowers et al. 2005; CEASAR 2009; Emery et al. 1999]
CI, confidence interval; OR, odds ratio.
The comparison in Table 22 showed that both naproxen and diclofenac were better than etoricoxib with regards to risk of HT (with diclofenac being significantly better). Ibuprofen was worse off compared with etoricoxib when looking at the same endpoint; however, only one study was analysed for the ibuprofen versus etoricoxib comparison and so the result has to be interpreted with caution. The relatively wide confidence interval confirms the imprecision of that particular comparison.
Table 22.
Comparison of naproxen, diclofenac and ibuprofen using etoricoxib as common comparator for hypertension.
| Intervention | Comparator | OR (intervention versus comparator) | 95% CI (OR) |
|---|---|---|---|
| Naproxen | Etoricoxib | 0.81 | 0.32, 1.82 |
| Diclofenac | Etoricoxib | 0.64 | 0.55, 0.74 |
| Ibuprofen | Etoricoxib | 2.02 | 0.64, 6.36 |
CI, confidence interval; OR, odds ratio.
Comparison of the currently available COX-2 inhibitors
MVE/MI/stroke/HT as endpoint and diclofenac as comparator
The only two COX-2 inhibitors on the market in Europe are celecoxib and etoricoxib. There were no head to head RCTs comparing the two drugs in terms of safety. However, there was one head to head efficacy trial which met the inclusion criteria for this analysis [Bingham et al. 2007]. In this study, etoricoxib was found to have a higher risk of HT compared with celecoxib although this difference was not significant [Peto OR 1.77, 95% confidence interval (CI) 0.78, 4.02]. In the absence of head to head safety trials, we conducted an indirect comparison using studies with similar endpoints and where each drug was compared with a common comparator. The results (Table 23) showed that etoricoxib had a higher risk of CV events analysed in this project apart from the risk of MI where it was better than celecoxib. The increased risk for HT for etoricoxib was significant as was the decreased risk of celecoxib for stroke.
Table 23.
Comparison of etoricoxib to celecoxib using diclofenac as the common comparator for MVE, MI, stroke and HT [Baraf et al. 2007; Krueger et al. 2008; Zacher et al. 2003; Combe et al. 2009; Dahlberg et al. 2009; Chan et al. 2010; McKenna et al. 2001; Singh et al. 2006; Emery et al. 1999].
| Intervention | Comparator | MVE |
MI |
Stroke |
HT |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR ratio (95% CI) | ||
| Etoricoxib | Diclofenac | 1.08 (0.94, 1.24) | 0.94 (0.58, 1.51) | 0.64(0.33, 1.23) | 1.56 (1.35, 1.81) |
| Celecoxib | Diclofenac | 0.95 (0.44, 2.04) | 1.33 (0.59, 2.97) | 0.41 (0.18, 0.91) | 0.80 (0.41, 1.55) |
CI, confidence interval; HT, hypertension; MI, myocardial infarction; MVE, major vascular events;
OR, odds ratio.
Discussion
CV events occurred in all three vascular beds, with more cardiac than cerebrovascular or peripheral vascular events, irrespective of treatment group. It would have been interesting to look at the effect of baseline CV disease or risk on the primary and secondary outcomes in this analysis. However, this was not feasible because most of the studies provided little or no information on the baseline CV disease or risk for patients.
The evaluation of individual events indicates that the absolute number of any of these events were small. There were numeric differences between treatments for some event types. For example, some events occurred at a higher rate in the etoricoxib group (e.g. HT compared with diclofenac). Although, the CIs intervals include unity in most of the comparison, suggestive of no significant difference, there were also a few events with significance differences.
Where there were more than two different studies, we conducted a meta-analysis of the relevant studies using the Cochrane methodology. However, where there was just one relevant study for analysis these studies were highlighted and presented together.
Using the primary endpoint of MVE and a prespecified cutoff point of 1.30, diclofenac (versus 1 comparator) and rofecoxib (versus 2 comparators) had increased risk for MVE (OR >1.30). Using the same criteria, diclofenac (versus 1 comparator) had an increased risk for MI. Although celecoxib had a slightly increased risk for MI (OR 1.33, versus 1 comparator), the CI included 1 and was not significant.
For the secondary endpoints, etoricoxib and rofecoxib were significantly worse off for HT (versus 1 comparator) and naproxen was significantly worse off for stroke (versus 1 comparator). Although ibuprofen was worse off for HT (versus 1 comparator) the increased risk was not significant.
The findings of this analysis and other studies [Juni et al. 2004; Farkouh et al. 2004; Graham et al. 2005; Mamdani et al. 2004] suggest that NSAIDs have different CV risk profiles. Essential differences that may contribute to different CV profiles include differences in structure, plasma elimination halflife, metabolism and distribution. For example, lumiracoxib (withdrawn from market due to severe liver toxicity) was an acid, etoricoxib and rofecoxib are sulfones, and valdecoxib (also withdrawn due to concerns about severe skin reactions) and celecoxib are sulphonamides [Mangold et al. 2004]. A study found that the sulfone COX-2 inhibitors (etoricoxib and rofecoxib) increased the susceptibility of human low-density lipoprotein (LDL) and plasma to oxidative modification compared with nonsulfone COX-2 inhibitors (valdecoxib and celecoxib) and traditional NSAIDs (ibuprofen, naproxen and diclofenac). The authors of the study suggested that this finding may provide a mechanistic explanation (which is not related to the effect on COX enzymes) for the reported differences in CV risk for some COX-2 inhibitors [Walter et al. 2004].
In a preclinical study [Brueggemann et al. 2009], the authors suggested that there might be effects beyond COX-2 inhibition implicated in the differences seen in the CV risk associated with NSAIDs. The study showed that, unlike diclofenac or rofecoxib, celecoxib inhibited L-type calcium current and enhanced KCNQ potassium current in vascular smooth muscle cells (VSMCs), which resulted in a pronounced dilation of intact arteries. These actions, which were not dependent on COX-2, may offset what would otherwise be a detrimental increase in vasoconstriction mediated by COX-2 inhibition. In contrast, for diclofenac and rofecoxib, which do not exhibit this protective effect, the vasoconstrictor effects are unopposed, which can lead to increased CV complications. However, this remains an hypothesis alone at this time and is not yet confirmed in clinical trials.
The half-lives of different COX-2s and NSAIDs vary. For example, the halflife of etoricoxib is 22 hours, 17 hours for rofecoxib, 11 hours for celecoxib, about 8–11 hours for valdecoxib, 12–15 hours for naproxen, about 2 hours for diclofenac and 2 hours for ibuprofen [Campbell and Steed, 2004; Takemoto et al. 2008; Aurobindo Pharma, 2012; Novartis Pharmaceuticals UK, 2012; Boots Company, 2012; Pfizer, 2004]. For the COX-2 inhibitors, a long halflife may lead to a sustained inhibition of COX-2-dependent prostacyclin production over a 24-hour period and this may have significant clinical effect over the long term. HT is a known risk factor for CV disease and NSAIDs generally have been associated with a mean increase in systolic blood pressure (SBP) of about 5.0 mm Hg (with some having more effect on this endpoint than others). Such small changes in SBP (1–5 mm Hg increase) have also been associated with stroke and up to 35,700 additional ischemic heart diseases in patients with OA over a period of 1 year [Singh et al. 2003]. It is also known that HT and/or fluid and sodium retention can lead to oedema and CHF. According to the Framingham Study, HT accounts for about one quarter of heart failure cases [Kannel and Cobb, 1992]. In the elderly population, as many as 68% of heart failure cases are attributed to hypertension [Yamasaki et al. 2003].
This analysis suggests that, when NSAIDs are used within licensed/recommended doses and for the treatment of arthritis rather than prevention of conditions such as adenomatous polyposis or Alzheimer’s disease, the specific CV events associated with each compound tend to vary from molecule to molecule. What is not known is how each molecule compares with another in a trial specifically designed to detect CV events. The gold standard for confirming this hypothesis will obviously be a randomized head to head comparison of several molecules, but there has been a reluctance by many companies that manufacture NSAIDs to sponsor such a study, probably because of the amount of resources that will need to be put into such a large-scale study, the time it might take to recruit patients and the length of time to see the outcome of interest and not the least of all, the ethical considerations involved in undertaking such a trial.
The ongoing Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen or Naproxen (PRECISION) and the Standard Care versus Celecoxib Outcome Trial (SCOT) study (www.scottrial.co.uk) will shed more light on the relative risk of some commonly used NSAIDs when the results are available in 2014 [Becker et al. 2009]. The trial is investigating the CV safety of celecoxib, ibuprofen and naproxen in patients with symptomatic OA or RA at high risk for, or with, established CV disease. The primary endpoint is the Antiplatelet Trialists’ Collaboration (APTC) composite endpoint of CV death, nonfatal MI or nonfatal stroke. The trial is scheduled to continue until about 762 primary events occur with at least 18 months follow up.
Prior to the availability of results from studies which are specifically designed to investigate the CV risk associated with NSAIDs, it is important to help clinicians make informed decisions by looking to answer the question of whether the CV outcomes associated with NSAIDs are designated as ‘class effect’ or ‘compound dependent’. If analyses show they are likely to be ‘compound dependent’, it will also be helpful to identify the compound with the most likely or least likely risks in patients who are on symptomatic treatment for arthritis.
This analysis has several limitations. Firstly, although this analysis covered more than 50,000 patient years of follow up, the number of events for most outcomes was low and the estimates for the odd ratios were imprecise as indicated by the wide 95% CIs.
Secondly, the analysis did not consider all NSAIDs. This is because there is lack of well conducted RCTs on many of these NSAIDs. In addition there are even fewer studies which reported on CV side effects, or conducted a head to head trial with other NSAIDs. However, this study has included all of the most commonly prescribed COX-2 inhibitors and traditional NSAIDs in Europe.
Thirdly, the studies analysed were not designed or powered to detect CV side effects. One consequence of this is that, the CV endpoints were not adjudicated for most of the studies and hence there could be a misclassification or reporting bias in either direction of the treatment arms.
It has been suggested by some authors that the CV risk associated with NSAIDs depends on the dose used. We were not able to explore this in this analysis as the number of studies which looked at different doses of each of the drugs in question and the numbers of patients treated with low doses were very small. In addition, the number of such studies that met the inclusion and exclusion criteria for this analysis was even fewer. This makes it difficult for any meaningful analysis on the dose comparison to be performed.
Although there were many studies reviewed (and there will be some differences between these studies), the entry criteria for patient population were very similar (i.e. RCTs in adult patients with confirmed clinical and or radiological diagnosis of OA/RA who are on NSAIDs for symptom control). The assessment and confirmation of the diagnosis of OA and RA were clearly defined in the studies and used as one of the inclusion criteria for our analysis. As with all meta-analysis, one of the limitations is that the quality of the analysis will depend on the quality of studies included in the review. As such, the summary provided in this analysis is only as reliable as the methods used to estimate the effect in each of the primary studies. To minimize the risk of bias with the individual studies, we assessed the quality of each study (for example through checking for allocation bias, concealment bias and blinding of patients). Using these parameters, we found that the studies included were of sufficient quality to be included in the review.
In most of the studies reviewed, the adjudication of safety endpoints of interest were not carried out because the studies were primarily efficacy trials. This could have an effect on the results. However, it is unlikely that this might have had a huge impact on the results given the results are consistent with previously reported risk of CV outcomes associated with some of the NSAIDs.
One of the strengths of this analysis was that a comprehensive search strategy was used to identify all relevant studies and it is unlikely that any important trial was missed in this analysis.
All previous analysis on this topic have looked at, and included, supratherapeutic and unlicensed doses of the various NSAIDs in their analysis. This might not be relevant to physicians using these drugs in recommended doses and has the tendency to bias the study towards an increased risk of side effects.
In addition, several authors have also included indications where these NSAIDs were used for the prevention of conditions like Alzheimer’s disease and adenomatous polyposis. This might not provide clinicians with the most important information they need when using these drugs for licensed indications, where the benefits of treatment (pain relief) is clear and quantifiable compared with when the drug is used for preventive purposes.
In conclusion, the result of this analysis suggests that the risk of having a CV event varies considerably when arthritis patients use NSAIDs at licensed doses for symptom control, and more importantly, this risk appears to be compound dependent. Whilst some NSAIDs may have an effect on only one CV event such as stroke, others may have an effect on more than one CV event, thereby increasing the cumulative risk for the molecule considerably.
NSAIDs as a class are very important in the symptomatic treatment of musculoskeletal disorders. However, the potential risk of a CV event associated with these drugs coupled with the high profile withdrawal of some drugs in the class have made clinicians extremely cautious about their use for symptom control in arthritis patients, at times to the detriment of the patients who are told to persist with other analgesics for the control of their pain even when it is clear that the analgesia is no longer effective.
Some clinicians prefer to try different analgesics rather than prescribing NSAIDs appropriately for those patients who need them (following a risk benefit assessment for the patient).
The conflicting body of data on this topic is unhelpful. It is essential that large head to head RCTs are carried out to define the CV outcomes associated with these drugs as this will give clinicians the much needed data to make informed choices with their patients as to which of the NSAIDs is best for them.
Acknowledgments
The views expressed in this publication are those of the authors alone.
Appendix
Appendix I.
Study data.
| Trial | Indication | Dose (mg) | Duration | Sample size | MI | Stroke | MVE | CHF | HT |
|---|---|---|---|---|---|---|---|---|---|
| SUCCESS-I [Singh et al. 2006] | OA | C200 | 3 months | 4393 | 8 | 1 | 1 | - | - |
| C400 | 3 months | 4407 | 2 | 4 | 2 | - | - | ||
| Nap 1000 | 3 months | 4394 | 1 | 6 | 2 | - | - | ||
| Dic100 | |||||||||
| EDGE [Baraf et al. 2007] | OA | E90 | 9.3 months | 3593 | 19 | 6 | 14 | 5 | 210 |
| Dic150 | 8.9 months | 3518 | 13 | 10 | 17 | 4 | 95 | ||
| EDGE II [Krueger et al. 2008] | RA | E90 | 19.3 months | 2032 | 14 | 8 | 21 | 16 | 240 |
| Dic150 | 19.1 months | 2054 | 22 | 12 | 15 | 10 | 197 | ||
| MEDAL 2009 [Coombe et al. 2009] | OA/RA | E60 | 20.8 months | 11787 | - | - | 394 | - | - |
| E90 | 20.8 months | - | - | - | - | ||||
| Dic150 | 20.8 months | 11717 | - | - | 363 | - | - | ||
| VIGOR [Bombardier et al. 2000] | RA | R50 | 9 months | 4047 | 16 | - | - | - | - |
| Nap1000 | 9 months | 4029 | 4 | - | - | - | - | ||
| ADVANTAGE [Lisse et al. 2003] | OA | R25 | 3 months | 2785 | 5 | 1 | 14 | - | - |
| Nap1000 | 3 months | 2772 | 1 | 6 | 7 | - | - | ||
| Geusens et al. [2002] | RA | R25 | 3 months | 306 | - | - | - | - | 14 |
| Nap1000 | 3 months | 142 | - | - | - | - | 4 | ||
| Geusens et al. [2002] | RA | R50 | 3 months | 286 | - | - | - | - | 14 |
| Nap1000 | 3 months | 142 | - | - | - | - | 0 | ||
| CONDOR [Chan et al. 2010] | OA/RA | C200Dic150 | 6 months6 months | 22382246 | 3 2 | 3 3 | 8 1 | – | – |
| Cannon et al. [2000] | OA | R12.5 | 12 months | 259 | 1 | - | - | 1 | - |
| R25 | 12 months | 257 | 1 | - | - | 0 | - | ||
| Dic150 | 12 months | 268 | 2 | - | - | 3 | - | ||
| Collantes et al. [2002] | RA | E90 | 3 months | 353 | - | - | - | 0 | 12 |
| Nap1000 | 3 months | 181 | - | - | - | 0 | 5 | ||
| P | 3 months | 357 | - | - | - | 0 | 5 | ||
| Weisenhutter et al. [2005] | OA | E30 | 3 months | 214 | - | - | - | 0 | 4 |
| Ib2400 | 3 months | 210 | - | - | - | 0 | 8 | ||
| P | 3 months | 104 | - | - | - | 0 | 1 | ||
| Saag et al. [2000] | OA | R12.5 | 12 months | 231 | - | - | 3 | - | - |
| R25 | 12 months | 232 | - | - | 4 | - | - | ||
| Dic150 | 12 months | 230 | - | - | 3 | - | - | ||
| Saag et al. [2000] | OA | R12.5 | 6 weeks | 219 | - | - | 1 | - | - |
| R25 | 6 weeks | 227 | - | - | 2 | - | - | ||
| Ib2400 | 6 weeks | 221 | - | - | 0 | - | - | ||
| Zacher et al. [2003] | OA | E60 | 6 weeks | 256 | - | - | 0 | 0 | 8 |
| Dic150 | 6 weeks | 260 | - | - | 1 | 1 | 9 | ||
| McKenna et al. [2001] | OA | C200 | 6 weeks | 201 | - | - | 0 | - | - |
| D150 | 6 weeks | 199 | - | - | 2 | - | - | ||
| Matsumoto et al. [2002] | RA | E90 | 12 weeks | 323 | - | - | - | - | 7 |
| Nap1000 | 12 weeks | 170 | - | - | - | - | 3 | ||
| CAESAR [Dahlberg et al. 2009] | OA | C200 | 52 weeks | 458 | 4 | 1 | 3 | - | - |
| Dic150 | 52 weeks | 458 | 6 | 5 | 2 | - | - | ||
| Emery et al. [1999] | RA | C200 | 6 weeks | 326 | 0 | 0 | 0 | 0 | 4 |
| Dic75 | 6 weeks | 329 | 0 | 0 | 0 | 0 | 5 | ||
| Bingham et al. [2007] | OA | E30 | 26 weeks | 231 | - | - | - | - | 15 |
| C200 | 26 weeks | 241 | - | - | - | - | 9 | ||
| P | 26 weeks | 127 | - | - | - | - | 0 | ||
| Schnitzer et al. [2005] | OA | R25 | 6 weeks | 104 | 0 | 0 | 0 | - | - |
| P | 6 weeks | 107 | 1 | 0 | 1 | - | - | ||
| Nap1000 | 6 weeks | 121 | 1 | 0 | 1 | - | - |
C, celecoxib; CHF, congestive heart failure; Dic, diclofenac; E, etoricoxib; HT, hypertension; Ib, ibuprofen; MI, myocardial infarction; MVE, major vascular events; Nap, naproxen; OA, osteoarthritis; R, rofecoxib; RA, rheumatoid arthritis.
Footnotes
Funding: The research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: J.F. is an ex-employee of Pfizer Ltd and currently works at Astrazeneca.
Contributor Information
John Fabule, Astrazeneca – Global Medical Affairs, 2 Kingdom Street, London W2 6BD, UK.
Ade Adebajo, Academic Rheumatology Group, Faculty of Medicine, University of Sheffield and Barnsley Hospital NHS Foundation Trust, Barnsley, UK.
References
- Aurobindo Pharma (2012) Summary of product characteristics for naproxen. Available from: http://www.medicines.org.uk/emc/medicine/23037 (accessed 2 April 2012).
- Baraf H., Fuentealba C., Greenwald M., Brzezicki J., O’Brien K., Soffer B., et al. and EDGE Study Group (2007) Gastrointestinal side effects of etoricoxib in patients with osteoarthritis: results of the Etoricoxib versus Diclofenac Sodium Gastrointestinal Tolerability and Effectiveness (EDGE) trial. J Rheumatol 34:408–420 [PubMed] [Google Scholar]
- Becker M., Wang T., Wisniewski L., Wolski K., Libby P., Lüscher T., et al. (2009) Rationale, design, and governance of Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION), a cardiovascular end point trial of non-steroidal anti-inflammatory agents in patients with arthritis. Am Heart J 157: 606–612 [DOI] [PubMed] [Google Scholar]
- Bingham C., Sebba A., Rubin B., Ruoff G., Kremer J., Bird S., et al. (2007) Efficacy and safety of etoricoxib 30mg and celecoxib 200mg in the treatment of osteoarthritis in two identically designed, randomized, placebo controlled, non-inferiority studies. Rheumatology, 46, 496–507 [DOI] [PubMed] [Google Scholar]
- Bombardier C., Laine L., Reicin A., Shapiro D., Burgos-Vargas R., Davis B., et al. (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. New Engl J Med 343: 1520–1528 [DOI] [PubMed] [Google Scholar]
- Boots Company (2012) Summary of product characteristics for ibuprofen. Available from: http://www.medicines.org.uk/emc/medicine/11179 (accessed 2 April 2012).
- Bresalier R., Sandler R., Bolognese J., Quam H., Oxenius B., Joseph R. (2005) A randomized trial of rofecoxib to prevent colorectal adenomas: the APPROVe trial. Gastroenterology 128 (Suppl. 2): 200 [Google Scholar]
- Brueggemann L., Mackie A., Mani B., Cribbs L., Byron K. (2009) Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol Pharmacol 76: 1053–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke A., Smyth E., FitzGerald G. (2006) Analgesic-antipyretic agents: pharmacotherapy of gout. In: Brunton L., Lazo J., Parker K. (eds), Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th edn. New York: McGraw-Hill, pp. 673–715 [Google Scholar]
- Campbell R., Steed K. (2004) Acute congestive heart failure induced by rofecoxib. J Am Board Fam Pract 17: 131–135 [DOI] [PubMed] [Google Scholar]
- Cannon C., Curtis S., FitzGerald G., Krum H., Kaur A., Bolognese J., et al. (2006) Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 368: 1771–1781 [DOI] [PubMed] [Google Scholar]
- Cannon G., Caldwell J., Holt P., Mclean B., Seidenberg B., Bolognese J., et al. (2000), for the Rofecoxib Phase III Protocol 035 Study Group. Rofecoxib, a specific inhibitor of cyclooxygenase 2,with clinical efficacy comparable with that of diclofenac sodium. Arthritis Rheum 43: 978–987 [DOI] [PubMed] [Google Scholar]
- Chan F., Lanas A., Scheiman J., Berger J., Nguyen H., Goldstein J., et al. (2010) Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 276: 173–179 [DOI] [PubMed] [Google Scholar]
- Cipollone F., Cicolini G., Bucci M. (2008) Cyclooxygenase and prostaglandin synthases in atherosclerosis: recent insights and future perspectives. Pharmacol Therapeut 118: 161–180 [DOI] [PubMed] [Google Scholar]
- Collantes E., Curtis S., Lee K., Casas N., McCarthy T., Melian A., et al. (2002) A multinational randomized, controlled, clinical trial of etoricoxib in the treatment of rheumatoid arthritis [ISRCTN25142273]. BMC Fam Pract 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe B., Swergold G., McLay J., McCarthy T., Zerbini C., Emery P., et al. (2009) Cardiovascular safety and gastrointestinal tolerability of etoricoxib vs diclofenac in a randomized controlled clinical trial (The MEDAL study). Rheumatology 48: 425–432 [DOI] [PubMed] [Google Scholar]
- Dahlberg L., Holme I., Høye K., Ringertz B. (2009) A randomized, multicentre, double-blind, parallel-group study to assess the adverse event-related discontinuation rate with celecoxib and diclofenac in elderly patients with osteoarthritis. Scand J Rheumatol 38: 133–143 [DOI] [PubMed] [Google Scholar]
- EMA (2012a) European Medicines Agency finalizes review of recent published data on cardiovascular safety of NSAIDs, press release 19 October 2012, EMA/CHMP/667707/2012. London: European Medicines Agency [Google Scholar]
- EMA (2012b) Questions and answers on the review of non selective, non steroidal anti inflammatory drugs (NSAIDs) and cardiovascular risk. Outcome of a procedure under Article 5(3) of Regulation (EC) No 726/2004. EMA/653433/2012. London: European Medicines Agency [Google Scholar]
- Emery P., Zeidler H., Kvien T., Guslandi T., Naudin R., Stead H., et al. (1999) Celecoxib versus diclofenac in long-term management of rheumatoid arthritis: randomised double-blind comparison, Lancet, 354: 2106–2111 [DOI] [PubMed] [Google Scholar]
- Farkouh M., Kirshner H., Harrington R., Ruland S., Verheugt F., Schnitzer T., et al. (2004) Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: a randomised controlled trial. Lancet 364: 675–684 [DOI] [PubMed] [Google Scholar]
- FitzGerald G. (2004) Coxibs and cardiovascular disease. N Engl J Med 351: 1709–1711 [DOI] [PubMed] [Google Scholar]
- FitzGerald G., Patrono C. (2001) The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 345: 433–442 [DOI] [PubMed] [Google Scholar]
- Geusens P., Truitt K., Sfikakis P., Zhao P., Shingo D., Lau C., et al. (2002) A placebo and active comparator-controlled trial of rofecoxib for the treatment of rheumatoid arthritis. Scand J Rheumatol 31: 230–238 [DOI] [PubMed] [Google Scholar]
- Graham D., Campen D., Hui R., Spence M., Cheetham C., Levy G., et al. (2005) Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 365: 475–481 [DOI] [PubMed] [Google Scholar]
- Green S. (2008) Chapter 9: Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions, Vol. 5), Higgins J. (ed.). Chichester: Wiley-Blackwell. Available from: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Ch09_Analysing.pdf (accessed 20 October 2012). [Google Scholar]
- Hernández-Díaz S., Varas-Lorenzo C., García Rodríguez L. (2006) Non-steroidal anti-inflammatory drugs and the risk of acute myocardial infarction. Basic Clin Pharmacol Toxicol 98: 266–274 [DOI] [PubMed] [Google Scholar]
- Jenkins J., Seligman P. (2005) Analysis and recommendations for Agency action regarding non-steroidal anti-inflammatory drugs and cardiovascular risk. Memorandum. Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm106201.pdf (accessed 10 December 2012). [Google Scholar]
- Juni P., Nartey L., Reichenbach S., Sterchi R., Dieppe P., Egger P. (2004) Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet, 364: 2021–2029 [DOI] [PubMed] [Google Scholar]
- Kannel W., Cobb J. (1992) Left ventricular hypertrophy and mortality–results from the Framingham Study. Cardiology 81: 291–298 [DOI] [PubMed] [Google Scholar]
- Kearney P., Baigent C., Godwin J., Halls H., Emberson J., Patrono C. (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332: 1302–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby N., Lundberg M., Harrington L., Leadbeater P., Milne G., Potter C., et al. (2012) Cyclooxygenase-1, not cyclooxygenase-2, is responsible for physiological production of prostacyclin in the cardiovascular system. Proc Natl Acad Sci U S A 109: 17597–17602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger K., Lino L., Dore R., Radominski S., Zhang Y., Simpson R., et al. (2008) Ann Rheum Dis 67: 315–322 [DOI] [PubMed] [Google Scholar]
- Krum H., Liew D., Aw J., Haas S. (2004) Cardiovascular effects of selective cyclooxygenase-2 inhibitors. Expert Rev Cardiovasc Ther 2: 265–270 [DOI] [PubMed] [Google Scholar]
- Lisse J., Perlman M., Johansson G., Shoemaker J., Schechtman J., Skalky C., et al. for the ADVANTAGE Study Group (2003) Gastrointestinal tolerability and effectiveness of rofecoxib versus naproxen in the treatment of osteoarthritis: a randomized, controlled trial. Ann Intern Med 139: 539–546 [DOI] [PubMed] [Google Scholar]
- Mamdani M., Juurlink D., Lee D., Rochon P., Kopp A., Naglie G., et al. (2004) Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 363: 1751–1756 [DOI] [PubMed] [Google Scholar]
- Mangold J., Gu H., Rodriguez L., Bonner J., Dickson J., Rordorf C. (2004) Pharmacokinetics and metabolism of lumiracoxib in healthy male subjects. Drug Metab Dispos 32: 566–571 [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Melian A., Mandel D., McIlwain H., Borenstein D., Zhao P., et al. (2002) and Etoricoxib Rheumatoid Arthritis Study Group. A randomized, controlled, clinical trial of etoricoxib in the treatment of rheumatoid arthritis. J Rheumatol 29: 1623–1630 [PubMed] [Google Scholar]
- McAlister F., Laupacis A., Wells G., Sackett D. (1999) Users’ guides to the medical literature: XIX. Applying clinical trial results. Guidelines for determining if a drug is exerting (more than) a class effect. JAMA 282: 1371–1377 [DOI] [PubMed] [Google Scholar]
- McKenna F., Borenstein D., Wendt H., Wallemark C., Lefkowith J., Geis G. (2001) Celecoxib versus diclofenac in the management of osteoarthritis of the knee: a placebo-controlled, randomised, double-blind comparison. Scand J Rheumatol 30: 11–18 [DOI] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals UK (2012) Summary of product characteristics for diclofenac. Available from: http://www.medicines.org.uk/emc/medicine/1342 (accessed 2 April 2012).
- Nussmeier N., Whelton A., Brown M., Langford R., Hoeft A., Parlow J., et al. (2005) Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352: 1081–1091 [DOI] [PubMed] [Google Scholar]
- Ott E., Nussmeier N., Duke P., Feneck R., Alston R., Snabes M., et al. (2003) Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 125: 1481–1492 [DOI] [PubMed] [Google Scholar]
- Pahor M., Psaty B., Alderman M., Applegate W., Williamson J., Cavazzini C., et al. (2000) Health outcomes associated with calcium antagonists compared with other first-line antihypertensive therapies: a meta-analysis of randomised controlled trials. Lancet 356: 1949–1954 [DOI] [PubMed] [Google Scholar]
- Pfizer (2004) BEXTRA® valdecoxib tablets. New York: Pfizer. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21341lbl.pdf (accessed 25 November 2012). [Google Scholar]
- Saag K., van der Heijde D., Fisher C., Samara A., DeTora L., Bolognese J., et al. (2000) Rofecoxib, a new cyclooxygenase 2 inhibitor, shows sustained efficacy, comparable with other nonsteroidal anti-inflammatory drugs: a 6-week and a 1-year trial in patients with osteoarthritis. Osteoarthritis Studies Group. Arch Fam Med 9: 1124–1134 [DOI] [PubMed] [Google Scholar]
- Schnitzer T., Kivitz A., Lipetz R., Sanders N., Hee A. (2005) Comparison of the COX-inhibiting nitric oxide donator AZD3582 and rofecoxib in treating the signs and symptoms of Osteoarthritis of the knee. Arthritis Care Res 53: 827–837 [DOI] [PubMed] [Google Scholar]
- Singh G., Fort J., Goldstein J., Levy R., Hanrahan P., Bello A., et al. (2006) Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I Study. Am J Med 119: 255–266 [DOI] [PubMed] [Google Scholar]
- Singh G., Miller J., Huse D., Pettitt D., D’Agostino R., Russell M. (2003) Consequences of increased systolic blood pressure in patients with osteoarthritis and rheumatoid arthritis. J Rheumatol 30: 714–719 [PubMed] [Google Scholar]
- Solomon S., Wittes J., Finn P., Fowler R., Viner J., Bertagnolli M., et al. (2008) Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials. Circulation 117: 2104–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F. (2003) Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 326: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooriakumaran P. (2006) COX-2 inhibitors and the heart: are all coxibs the same? Postgrad Med J 82: 242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers J., White W., Pitt B., Whelton A., Simon L., Winer N., et al. (2005) The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med 165: 161–168 [DOI] [PubMed] [Google Scholar]
- Takemoto J., Reynolds J., Remsberg C., Vega-Villa K., Davies N. (2008) Clinical pharmacokinetic and pharmacodynamic profile of etoricoxib. Clin Pharmacokinet 47: 703–720 [DOI] [PubMed] [Google Scholar]
- Trelle S., Reichenbach S., Wandel S., Hildebrand P., Tschannen B., Villiger P., et al. (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecken A., Schwartz J., Depré M., De Lepeleire I., Dallob A., Tanaka W., et al. (2000) Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol 40: 1109–1120 [PubMed] [Google Scholar]
- Walter M. F., Jacob R. F., Day C. A., Dahlborg R., Weng Y., Mason R. P. (2004). Sulfone COX-2 inhibitors increase susceptibility of human LDL and plasma to oxidative modification: comparison to sulfonamide COX-2 inhibitors and NSAIDs. Atherosclerosis 177(2): 235–243 [DOI] [PubMed] [Google Scholar]
- White W., West C., Borer J., Gorelick P., Lavange L., Pan S., et al. (2007) Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol 99: 91–98 [DOI] [PubMed] [Google Scholar]
- Wiesenhutter C., Boice J., Ko A., Sheldon E., Murphy F., Wittmer B., et al. (2005) Evaluation of the comparative efficacy of etoricoxib and ibuprofen for treatment of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. Mayo Clin Proc 80: 470–479 [DOI] [PubMed] [Google Scholar]
- Yamasaki N., Kitaoka H., Matsumura Y., Furuno T., Nishinaga M., Doi Y. (2003) Heart failure in the elderly. Intern Med 42: 383–388 [DOI] [PubMed] [Google Scholar]
- Zacher J., Feldman D., Gerli R., Scott D., Hou S., Uebelhart D., et al. (2003) A comparison of the therapeutic efficacy and tolerability of etoricoxib and diclofenac in patients with osteoarthritis. Curr Med Res Opin 19: 725–736 [DOI] [PubMed] [Google Scholar]