Abstract
A recent wave of studies—over 100 conducted over the last decade—shows that exerting effort at controlling impulses or behavioral tendencies leaves a person depleted and less able to engage in subsequent rounds of regulation. Regulatory depletion is thought to play an important role in everyday problems (e.g., excessive spending, overeating) as well as psychiatric conditions, but its neurophysiological basis is poorly understood. Using a placebo-controlled, double-blind design, we demonstrate that the psychostimulant methylphenidate (commonly known as ‘Ritalin’), a catecholamine reuptake blocker that increases dopamine and norepinephrine at the synaptic cleft, fully blocks effort-induced depletion of regulatory control. Spectral analysis of trial-by-trial reaction times found specificity of methylphenidate effects on regulatory depletion in the slow-4 frequency band. This band is associated with the operation of resting state brain networks that produce mind wandering, raising potential connections between our results and recent brain network-based models of control over attention.
Keywords: methylphenidate, cognitive control, regulation, ego depletion, reaction time variability, spectral analysis, slow-4
Humans have a remarkable ability to regulate their thoughts, motives, and behavioral tendencies. Yet it is an all too familiar fact that attempts at self-control often end in failure. Indeed, frequent regulation failures are the hallmark of a number of everyday problems (e.g., excessive spending, overeating) as well as psychiatric conditions [e.g., attention-deficit/hyperactivity disorder (ADHD)]. Why is it that we have unparalleled abilities for self-regulation, and yet our attempts at regulation are so often unsuccessful?
A potentially important contributing factor is suggested in a recently proposed ‘strength’ model of self-control (Baumeister, Bratslavsky, Muraven, & Tice, 1998). This model holds that the capacity to exercise sustained regulatory control is—akin to the tiring of a muscle—limited and depletable. Over the last decade, over 100 studies have provided support for this model using the dual-task paradigm (Hagger, Wood, Stiff, & Chatzisarantis, 2010). During Phase 1 of this paradigm, participants perform one of two versions of a task that are matched in all respects except that one version requires the sustained use of regulatory control while the other does not. During Phase 2 that immediately follows, all participants are given a second task (differing from the Phase 1 task) that also demands use of regulatory processing. These studies reliably find that engaging in effortful regulation during Phase 1 tasks diminishes regulatory control during Phase 2.
Independently, neurobiological investigations have revealed important roles the catecholamine neurotransmitters dopamine and norepinephrine in regulatory processing (Robbins, 2005; Arnsten & Pliszka, 2011). This view is based on multiple lines of evidence. For example, ADHD, a serious psychiatric disorder that involves prominent deficits in regulatory control, is associated with distributed disturbances in the brain’s catecholamine system (Bellgrove, Hawi, Kirley, Gill, & Robertson, 2005). Additionally, catecholamine-boosting psychotropic drugs reliably enhance regulatory processing (Pietrzak, Mollica, Maruff, & Snyder, 2006; Robbins, 2005). It is not currently known, however, whether acute pharmacological manipulation of brain catecholamine levels specifically affects the aforementioned phenomenon of regulatory depletion, i.e., the impairment of regulatory control due to prior effortful regulation.
In this study, we investigate whether the depletion of regulatory control is affected by pretreatment with methylphenidate, a psychostimulant medication that reliably increases brain dopamine and norepinephrine levels (Volkow, Fowler, Wang, Ding, & Gatley, 2002). We also wanted to determine whether the effects of regulatory depletion and methylphenidate are associated with specific ‘spectral profiles’, i.e., with specific patterns of oscillatory variation in task performance over time. Our interest in this issue was based on recent findings regarding the default network, a brain network that is implicated in mind wandering and task-irrelevant thought (Weissman, Roberts, Visscher, & Woldorff, 2006; Mason et al., 2007). The default network exhibits very low frequency spontaneous oscillations. The oscillatory activity of the network is hypothesized to manifest as variability in trial-to-trial reaction times in the so-called ‘slow-4’ frequency band (Castellanos et al., 2005)—a band that represents oscillations in the 13-37s range. According to recent ‘network regulation’ models of attention control (Sonuga-Barke & Castellanos, 2007; Castellanos & Proal, 2012), in individuals or in states associated with poor attention control, there is insufficient regulation of the default network, which leads to elevated variability in the slow-4 band. We reasoned that if these network models are correct, then depletion of regulatory control due to prior effortful regulation would be associated with elevated variability specifically in the slow-4 band (due to insufficient regulation of the default network), and that methylphenidate’s effects on the depletion of regulatory control would specifically modulate this band.
Method
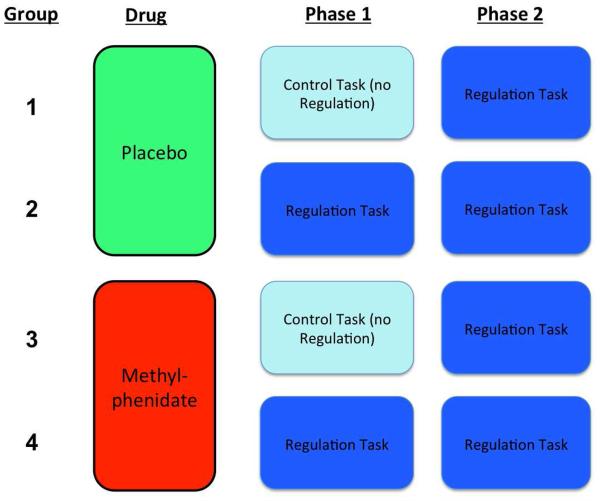
The experiment consisted of a 2×2 design in which the dual-task paradigm was crossed with pharmacological manipulation using methylphenidate. Guidelines suggest that for ANOVA designs in which factors have moderate effects, a sample size in the range of 30 participants per cell yields roughly 80% power (Van Voorhis & Morgan, 2007). We expected a large effect size for the effect of depletion with the letter E task (Cohen’s d=0.8-1.0) based on prior studies (Hagger et al., 2010) and pilot testing (without drug pretreatment), and recruited 108 participants, 27 per group. Participants were recruited using a University of Michigan sponsored online recruitment website, and were eligible if they were ages 18-35, were not actively using any psychoactive medications, and did not have any medical disorders or health symptoms (assessed through two health questionnaires) that might raise concerns for adverse effects with methylphenidate (Participant Characteristics: Age: 22.5±4.8(sd); Female=68; Caucasian=81, Asian=12, African-American=5, more than one race/other=7, no answer=3; University Students or Faculty=89).
Using a double-blind procedure, placebo or methylphenidate capsules identical in appearance were administered 60 minutes prior to the experiment to coincide with the window in which it was expected that methylphenidate would exert its cognitive effects (Swanson & Volkow, 2003). Participants next completed a brief practice session for the tasks while waiting for the 60-minute period to elapse. Phase 1 effortful regulation was manipulated with a modified version of the ‘Letter E Task’ (Baumeister et al., 1998) lasting 6 minutes 15 seconds and consisting of 150 trials. In the Regulation version of the task, participants press a button when a word with the letter ‘E’ is shown, but must withhold the response if the ‘E’ is next to or one letter away from another vowel (Figure 2 top). The No Regulation version is matched in all respects except participants press a button whenever a word with the letter ‘E’ is shown, and no suppression of prepotent tendencies is required (Figure 2 bottom). Of note, this task was selected because it was previously shown (Hagger et al., 2010) to have among the highest effect sizes in inducing regulatory depletion. Regulatory control was then tested at Phase 2 with a multi-source interference task (Bush, Shin, Holmes, Rosen, & Vogt, 2003) (MSIT; Figure 2 bottom) lasting 8 minutes 20 seconds and consisting of 200 trials, 100 congruent and 100 incongruent, presented in an interspersed, pseudorandom order. During incongruent trials, irrelevant size, position, and stimulus-related cues must be suppressed. For both tasks, trials began with a stimulus presented for 0.5s, followed by a 2.5s response period, after which the next trial immediately began.
Figure 2. Behavioral Tasks.
Participants completed a dual-task paradigm consisting of a Phase 1 task immediately followed by a Phase 2 task. Instructions and structure of trials for the Phase 1 and Phase 2 tasks are shown above.
The primary dependent measure was reaction time variability (RTV) during the Phase 2 task. RTV has been hypothesized to reflect levels of attention control (Douglas, 1999; Castellanos et al., 2005). Control over attention is required to maintain task-directed focus and prevent the emergence of task-irrelevant spontaneous thoughts (Weissman et al., 2006). When attention control is reduced, this leads to more frequent waning or lapsing of attention, which in turn produces variability in trial-to-trial reaction times (Douglas, 1999). On a pure Gaussian model, the distribution of reaction times can be characterized in terms of two parameters representing the mean (mu) and standard deviation (sigma). In this model, sigma represents the variability of reaction times across trials. Observation of actual reaction time distributions, however, shows that they are significantly positively skewed. They have sharper left boundaries, due to relatively few very short reaction times, and long right tails, due to relatively more very long reaction times. This skewed shape has been found to be well fit with ex-Gaussian models (Dawson, 1988), derived from summing a Gaussian and an exponential distribution. This model is parameterized with Gaussian mean mu and standard deviation sigma. In addition, there is a second variability parameter, tau, that is associated with the exponential distribution and which captures the longer right tail. Since we were interested in how fatigue and methylphenidate affected the overall variability of reaction times across trials, RTV was calculated as the sum of the two variability parameters, sigma and tau.
Participants were included in the analysis according to accuracy and outlier criteria commonly applied to studies employing RTV as the dependent measure (Karalunas, Huang-Pollock, & Nigg, 2013): >80% accuracy (on both Phase 1 and Phase 2 tasks) and within 2 standard deviations for reaction time and RTV measures for the Phase 2 task, which left 94 participants to enter analysis (23 in two cells and 24 in two cells). RTV was calculated for accurate trials only; reaction times for inaccurate responses and non-responses were not included.
Fast Fourier Transform (FFT), a spectral decomposition method, was used to identify patterns of temporal variation in the sequence of trial-by-trial reaction times. In intuitive terms, if a participant has spikes in her reaction times that occur every 20 seconds during the task, then FFT will register that there is greater power at the 20s frequency. We were interested in particular at power at neurophysiologically validated frequency bands (Penttonen & Buzsaki, 2003), which have been investigated in prior research on reaction times (Karalunas et al., 2013). We had an a priori hypothesis about the slow-4 band, which represents oscillations in the 13-37s range (see Introduction). The slow-3 band, which represents oscillations in the 5-13s range, was chosen as a comparison band because this band plausibly contains variation related to cognitive processing of tasks, has been used as the comparison band in most previous studies (see Karalunas et al., 2013 for a review), and other frequency bands (e.g., slow-2) contain oscillations too rapid to detect with standard tasks.
FFT was performed with subsequent trapezoidal integration of the power spectrum within slow-4 and slow-3 bands. In accordance with the procedures described elsewhere (Karalunas et al., 2013), prior to FFT analysis, reaction times were log transformed and the standard deviation of the reaction times was normalized to one for every subject to account for inequality of variances across conditions and to provide centering for tests of interaction.
Results
For incongruent trials, we observed a significant Drug-by-Prior Regulation interaction (F(1,90)=4.64, p=0.03, η2=0.045), indicating that prior regulation (i.e., during Phase 1) produced statistically different effects in participants receiving placebo versus methylphenidate (Figure 3A). In participants receiving placebo, prior regulation produced significantly greater RTV at Phase 2 [t(45)=2.38, p=0.02, d=.69], with no change in mean reaction time (p=0.65). In participants receiving methylphenidate, however, prior regulation did not affect Phase 2 RTV (p=0.57).
Figure 3. Effects of Methylphenidate and Prior Regulation on Reaction Time Variability.
A. With placebo pretreatment, engaging in prior sustained regulation increased reaction time variability—thought to reflect diminished control over attentional distractions and ‘mind wandering’—during trials requiring regulatory control in a second task. This effect was abolished with methylphenidate pretreatment. B. The effects of prior regulation and methylphenidate were quite similar in the sigma (Gaussian variability) and tau (exponential variability) parameters. C. Histogram showing the reaction time profile for the average subject in the Placebo/Post-Regulation condition versus the Methylphenidate/Post-Regulation condition. Relative to the Methylphenidate-Post-Regulation profile, the Placebo-Post-Regulation profile has a similar mean, but greater mass to the right of the mean and a longer tail. @=p<0.1. Error bars represent standard error of the mean.
For congruent trials, neither methylphenidate, prior regulation, nor their interaction significantly impacted RTV (all ps>0.23). A test of the three-way Trial Type-by-Drug-by-Prior Regulation interaction found a significant effect (trend level) for the sigma variability parameter [F(1,90)=3.00, p=0.09, η2=0.009). This interaction was driven by the fact that in participants receiving placebo, prior effortful regulation elevated both sigma and tau for incongruent trials, while it elevated only tau for congruent trials; for both kinds of trails, methylphenidate blocked these effects of regulation. This three-way interaction was not significant for tau (p=0.47). Importantly, reduced RTV during incongruent trials was not achieved by compromising accuracy, as there was a small but statistically significant improvement in mean accuracy among participants receiving methylphenidate [main effect of Drug: F(1,90)=7.07, p<0.01, η2=0.045], and this improvement did not differ as a function of prior regulation (Drug-by-Prior Regulation interaction: p=0.87).
In spectral analysis (Figure 4), we observed a three-way Band-by-Drug-by-Prior Regulation interaction [F(1,90)=4.20, p=0.04, η2=0.012). In the slow-4 band, there was a significant Drug-by-Prior Regulation interaction [F(1,90)=5.43, p=0.02, η2=0.057). With placebo, prior regulation increased slow-4 power [t(45=1.69, p=0.10, d=.49], but with methylphenidate, slow-4 power decreased [t(45)=-2.38, p=0.11, d=.47]. In the slow-3 band, these effects were not observed (main effect of Drug, main effect of Prior Regulation, and Drug-by-Prior Regulation interaction: all ps>0.29).
Figure 4. Normalized power by spectral bands.

A Drug-by-Prior Regulation interaction was found specifically in the slow-4 band, which encompasses oscillations in the 13-37s range. In this band, prior regulation increased power after placebo administration, but this effect was reversed after methylphenidate administration. @=p<0.1. Error bars represent standard error of the mean.
Discussion
This study advances our knowledge of the neurobiological basis of the depletion of regulatory control in two ways. First, we provide evidence for the first time that regulatory depletion that arises in the widely used dual-task paradigm can be pharmacologically manipulated: Pretreatment with the catecholamine-boosting agent methylphenidate blocks the depletion of regulatory control. Second, we utilized spectral decomposition methods, which have not previously been applied to investigate regulatory depletion. We found prior regulation increased spectral power specifically in the slow-4 frequency band, a band associated with oscillations of the brain’s default network, while methylphenidate reversed this effect. This finding is intriguing because it draws novel connections between regulatory depletion and emerging brain network models of attention control.
One hypothesis for explaining why methylphenidate blocks the depletion of regulatory control appeals to the drug’s more general effects in increasing alertness and arousal. There is a large literature in psychology and ergonomic research examining the effects of methylphenidate, and related compounds such as amphetamine, on ‘vigilant’ attention (Langner & Eickhoff, 2013). These studies nearly always used protracted, simple, monotonous tasks and showed that participants receiving these drugs were more alert and made fewer errors (see Koelega, 1993 for a review). We believe, however, that this ‘general arousal’ hypothesis is unlikely to explain the results of the current study. First, in contrast to extended tasks used in prior research, we used relatively brief tasks that do not normally produce significant decrements in alertness and arousal due to time on task. Second, in contrast to prior studies that used task accuracy as the primary dependent measure, we used variability of reaction time, which is hypothesized to reflect one’s level of attention control specifically rather than arousal generally (Douglas, 1999). Third, and perhaps most importantly, we manipulated the exertion of regulatory control during phase 1 of the dual-task paradigm, and found that methylphenidate’s effects on variability of reaction times during phase 2 were observed only in participants who had previously engaged in effortful regulation. Fourth, there was evidence for specificity of methylphenidate effects in incongruent trials that require cognitive regulation compared to congruent trials that do not. In particular, after prior regulation, methylphenidate reduced both variability parameters of the ex-Gaussian distribution, sigma and tau. However, it decreased sigma in incongruent trials more than in congruent trials (while it decreased tau on both types of trials). These observations taken together suggest that methylphenidate had at least some specific effects on the depletion of regulatory control due to prior regulatory exertion, and are hard to explain on the hypothesis that methylphenidate had only general, non-specific effects on alertness and arousal.
Of note, it is often claimed that the individual parameters of the ex-Gaussian distribution have specific psychological interpretations. For example, it has been proposed that the Gaussian parameters (mu and sigma) represent the perceptual phase of task processing, while tau represents the decision/action phase (Gordon & Carson, 1990). This interpretation, however, is based on limited evidence and remains controversial (Matzke & Wagenmakers, 2009). Further investigation into the psychological factors that drive sigma versus tau variability will help to further clarify methylphenidate’s apparent protective effects against regulatory depletion.
In contrast to the general arousal hypothesis, an alternative hypothesis proposes relatively specific effects of methylphenidate on top-down regulatory processing. Effortful regulation of automatic responses is thought to be implemented by a set of relatively discrete prefrontal circuits, including regions in the dorsal and lateral prefrontal cortex and anterior cingulate (Heatherton & Wagner, 2011). In previous studies, regulatory depletion has been found to reduce activation of these prefrontal circuits (Richeson et al., 2003; Wagner & Heatherton, 2013). Catecholamine boosting agents, on the other hand, are known to enhance the effectiveness of these control circuits (Solanto, 2002), consistent with a large body of neuroimaging evidence (Costa et al., 2013; Rubia et al., 2011). If this picture is correct, then augmentation of brain catecholamines by methylphenidate might block the depletion of regulatory control by delivering a performance boost to the specific prefrontal control circuits whose functioning is normally compromised by prior regulatory exertion.
This study also draws links between regulatory depletion and recent network models of attention control (Sonuga-Barke & Castellanos, 2007; Castellanos & Proal, 2012). According to these models, an important job for regulatory control circuits is to suppress the default network (Anticevic et al., 2012), a brain network involved in task-irrelevant spontaneous thoughts and which exhibits spontaneous oscillations in activity in the slow-4 frequency band. Our finding that depletion of regulatory control increases power in the slow-4 band might thus be interpreted in terms of enhanced default network oscillatory activity, which in turn might be explained by diminished regulatory control over this network. Moreover, our finding that methylphenidate reverses this effect suggests a potential role for brain catecholamines in enhancing regulatory control over the default network. These hypotheses linking regulatory depletion with increased default network activity warrant direct investigation in future pharmaco-imaging studies.
In sum, effort-induced depletion of regulatory control is potentially important in explaining self-control failures in day-to-day life and in psychiatric disorders. Our results provide new insight into the physiological mechanisms by which effort-induced depletion occurs, and could serve as a starting point for new lines of brain-based investigations into regulatory control failures.
Figure 1. 2×2 Experimental Design.
The dual-task paradigm, consisting of a Phase 1 task and Phase 2 task, was crossed with pharmacological manipulation with placebo or methylphenidate administered 60 minutes prior to task performance.
Acknowledgements
C.S.’s research was supported by NIH grant K23-AA-020297 and the John Templeton Foundation.
References
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Pliszka SR. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacology Biochemistry and Behavior. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RE, Bratslavsky E, Muraven M, Tice DM. Ego Depletion: Is the Active Self a Limited Resource? Journal of Personality and Social Psychology. 1998;74:1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: Sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends in Cognitive Sciences. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Riedel M, Pogarell O, Menzel-Zelnitschek F, Schwarz M, Reiser M, Ettinger U. Methylphenidate effects on neural activity during response inhibition in healthy humans. Cerebral cortex (New York, N.Y.: 1991) 2013;23:1179–1189. doi: 10.1093/cercor/bhs107. [DOI] [PubMed] [Google Scholar]
- Dawson MRW. Fitting the ex-Gaussian equation to reaction time distributions. Behavior Research Methods, Instruments, & Computers. 1988;20:54–57. [Google Scholar]
- Douglas VI. Cognitive control processes in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. Kluwer Academic Publishers; Dordrecht, Netherlands: 1999. pp. 105–138. [Google Scholar]
- Gordon B, Carson K. The basis for choice reaction time slowing in Alzheimer’s disease. Brain and Cognition. 1990;13:148–166. doi: 10.1016/0278-2626(90)90047-r. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL, Nigg JT. Is reaction time variability in ADHD mainly at low frequencies? Journal of Child Psychology and Psychiatry. 2013;54:536–544. doi: 10.1111/jcpp.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology. 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychological bulletin. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Horn JDV, Wegner DM, Grafton ST, Macrae CN. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D, Wagenmakers E-J. Psychological interpretation of the ex-Gaussian and shifted Wald parameters: A diffusion model analysis. Psychonomic Bulletin & Review. 2009;16:798–817. doi: 10.3758/PBR.16.5.798. [DOI] [PubMed] [Google Scholar]
- Penttonen M, Buzsaki G. Natural logarithmic relationship between brain oscillators. Thalamus & Related Systems. 2003;2:145–152. [Google Scholar]
- Pietrzak RH, Mollica CM, Maruff P, Snyder PJ. Cognitive effects of immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 2006;30:1225–45. doi: 10.1016/j.neubiorev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, Shelton JN. An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience. 2003;6:1323–1328. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- Robbins T. Chemistry of the mind: Neurochemical modulation of prefrontal cortical function. The Journal of Comparative Neurology. 2005;493:140–146. doi: 10.1002/cne.20717. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Smith AB, Mohammad A-M, Brammer M, Taylor E. Methylphenidate Normalizes Fronto-Striatal Underactivation During Interference Inhibition in Medication-Naïve Boys with Attention-Deficit Hyperactivity Disorder. Neuropsychopharmacology. 2011;36:1575–1586. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behavioural Brain Research. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neuroscience & Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Swanson J, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neuroscience & Biobehavioral Reviews. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Van Voorhis C, Morgan B. Understanding power and rules of thumb for determning sample size. Tutorials in Quantitative Methods for Psychology. 2007;3:43–50. [Google Scholar]
- Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. Journal of Attention Disorders. 2002;6(Suppl 1):S31–43. doi: 10.1177/070674370200601s05. [DOI] [PubMed] [Google Scholar]
- Wagner DD, Heatherton TF. Self-regulatory depletion increases emotional reactivity in the amygdala. Social Cognitive and Affective Neuroscience. 2013;8:410–417. doi: 10.1093/scan/nss082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]