Abstract
Phenytoin is a widely used antiepileptic drug with a narrow therapeutic index and large interpatient variability, partly due to genetic variations in the gene encoding cytochrome P450 (CYP)2C9 (CYP2C9). Furthermore, the variant allele HLA‐B*15:02, encoding human leukocyte antigen, is associated with an increased risk of Stevens–Johnson syndrome and toxic epidermal necrolysis in response to phenytoin treatment. We summarize evidence from the published literature supporting these associations and provide recommendations for the use of phenytoin based on CYP2C9 and/or HLA‐B genotype (also available on PharmGKB: http://www.pharmgkb.org). The purpose of this guideline is to provide information for the interpretation of HLA‐B and/or CYP2C9 genotype tests so that the results can guide dosing and/or use of phenytoin. Detailed guidelines for the use of phenytoin as well as analyses of cost‐effectiveness are out of scope. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines are periodically updated at http://www.pharmgkb.org.
Clinical Pharmacology & Therapeutics (2014); 96 5, 542–548. doi:10.1038/clpt.2014.159
FOCUSED LITERATURE REVIEW
A literature review focused on CYP2C9 and HLA‐B*15:02 genotypes and phenytoin use (see Supplementary Material online) was conducted. Reviews were included to summarize the available literature.
GENES: HLA‐B AND CYP2C9
Background
In this guideline, (i) the gene encoding human leukocyte antigen B (HLA‐B) will be discussed as it relates to phenytoin‐induced cutaneous adverse drug reactions (ADRs) of Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), and (ii) the gene encoding hepatic cytochrome P450 (CYP)2C9 (CYP2C9) and its alleles are discussed as they relate to phenytoin metabolism and dosing.
HLA‐B. HLA‐B is part of a gene cluster designated as the human major histocompatibility complex, located on the short arm of chromosome 6. The cluster contains three classes (I, II, and III). Major histocompatibility complex class I contains three genes: HLA‐B, HLA‐A, and HLA‐C. HLA‐B encodes a cell surface protein that binds peptides generated by proteolysis and extruded from proteasomes. The presentation of these peptides on the cell surface enables the immune system to distinguish self‐proteins from foreign proteins typically introduced by infectious organisms (e.g., viruses and bacteria) (see Supplementary Material online for further discussion).
HLA genes, specifically HLA‐B, are among the most highly polymorphic genes in the human genome. HLA polymorphisms were previously ascertained serologically, but genotyping and DNA sequencing methods reveal much greater genetic complexity. More than 2,000 HLA‐B alleles, many of which differ by more than one nucleotide from each other, were deposited into the World Health Organization Nomenclature Committee for Factors of the HLA System (http://hla.alleles.org). Each allele is designated by the gene name, which is followed by an asterisk and up to an eight‐digit (four pairs) identifier giving information about the allele type (designated by the first two digits) and specific protein subtypes (second set of digits). For more information and a diagram outlining the description of the current HLA allele nomenclature, see http://hla.alleles.org/nomenclature/naming.html. The details of HLA nomenclature are also described in a previous CPIC guideline. 1 The current guideline specifically discusses only the HLA‐B*15:02 allele as it relates to the phenytoin‐induced cutaneous ADRs of SJS and TEN.
CYP2C9. The hepatic CYP2C9 enzyme contributes to the metabolism of many clinically relevant drugs, including phenytoin (http://www.pharmgkb.org/pathway/PA145011115). The CYP2C9 gene is highly polymorphic, having more than 50 known variant alleles (http://www.cypalleles.ki.se/cyp2c9.htm, Supplementary Tables S1 and S2 online). Individuals homozygous for the reference CYP2C9 allele (CYP2C9*1) have the “normal metabolizer” phenotype. Each named CYP2C9 star (*) allele is defined by a genotype at one or more specific single‐nucleotide polymorphisms (SNPs) with variable enzyme activity. The two most common variants with decreased enzyme function in Europeans are CYP2C9*2 (rs1799853) and CYP2C9*3 (rs1057910). 2
Genetic test interpretation
HLA‐B. Clinical genotyping test results for HLA‐B*15:02 are interpreted as “positive” if one or two copies of HLA‐B*15:02 are present or as “negative” if no copies of HLA‐B*15:02 are present. Phenotype assignments for HLA‐B*15:02 genotypes are summarized in Table 1 . The allele frequencies of HLA‐B vary greatly among populations. Specifically, HLA‐B*15:02 is most prevalent in Oceania and in East Asian and South/Central Asian populations, ranging from 1% to more than 10%. It is less frequent in European populations (0–1%) and apparently absent in several African populations ( Supplementary Tables S3 and S4 online). The global average derived from more than 46,000 individuals is 1.37%.
Table 1.
Assignment of likely phenotype based on genotypes
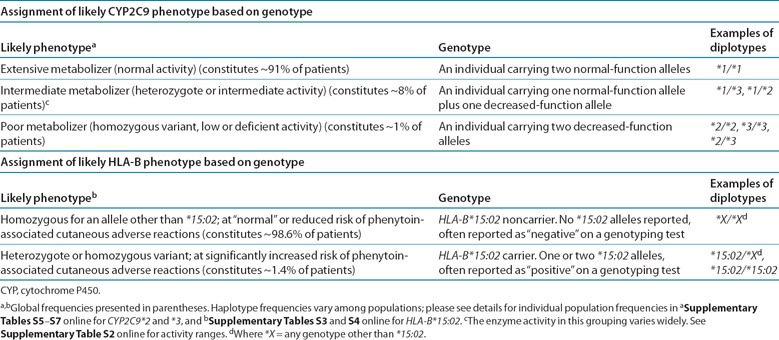
CYP2C9. Most clinical laboratories reporting CYP2C9 genotype use the star allele nomenclature and may interpret the patient's predicted metabolizer phenotype ( Table 1 , Supplementary Table S1 online). The combination of alleles is used to determine a patient's diplotype. Not all CYP2C9 allelic variants may be tested, influencing the accuracy of the genotype‐based dose prediction, primarily in individuals of Asian or African ancestry who carry other common functionally decreased function CYP2C9 variant alleles ( Supplementary Table S5 online). The frequencies of the CYP2C9*2 and *3 alleles and diplotypes derived from these and other alleles differ among racial/ethnic groups ( Supplementary Tables S5 – S7 online). 2 CYP2C9 alleles are typically characterized as wild‐type (normal function) or decreased‐function alleles depending on the reported activity of the enzyme that they encode.
Available genetic test options
Several methods of CYP2C9 and HLA‐B genotyping are commercially available. The Supplementary Material online and the website http://www.pharmgkb.org contain more information on available clinical testing options.
Incidental findings
HLA‐B alleles are associated with hypersensitivity reactions to other drugs. CPIC guidelines are available for HLA‐B*57:01 and abacavir‐induced hypersensitivity reactions, HLA‐B*58:01 and allopurinol‐induced severe cutaneous adverse reactions, and HLA‐B*15:02 and carbamazepine‐induced SJS and TEN. 1 , 3 , 4
No studies have linked genetic variations in CYP2C9 with any disease, except for a small study that linked CYP2C9*2 and *3 variants and phenytoin use with a higher frequency of cerebellar atrophy. 5 CYP2C9 poor metabolizers may be predisposed to serious bleeding during warfarin therapy. 6
Other considerations
Not applicable.
DRUGS: PHENYTOIN AND FOSPHENYTOIN
Phenytoin and its prodrug fosphenytoin constitute one of the mainstays of treatment for both focal and generalized convulsive status epilepticus. Dosing is complex owing to the highly unusual pharmacokinetics of phenytoin, requiring adjustments to be made in line with patient weight, sex, and age (description of phenytoin and fosphenytoin metabolism available in Supplementary Material online and Supplementary Figures S1 and S2 online). Outpatient therapy is generally initiated at 5–7 mg/kg/day in adults (slightly higher in children) and may be given once daily (or twice daily in children). The starting dose must be lower in the setting of hepatic impairment. Careful dose adjustments must then be made—generally 30–40 mg at a time in 2‐week intervals in adults—to stabilize the level within the typical therapeutic range (10–20 µg/dl). In urgent situations such as status epilepticus, i.v. loading doses of 15–20 mg/kg are given, followed by maintenance doses, i.v. or oral, as above. Acute dose‐related side effects include sedation, ataxia, dizziness, nystagmus, nausea, and cognitive impairment. The drug is highly allergenic, and rashes ranging from mild eruptions to life‐threatening hypersensitivity reactions may be seen. HLA‐B*15:02 is associated with phenytoin‐induced SJS and TEN. SJS is characterized by epidermal detachment involving up to 10% of body surface area, whereas TEN usually affects more than 30% of the body surface area. Subacutely, hematologic and hepatic toxicity can occur; the latter is probably a hypersensitivity reaction itself, as it is usually accompanied by rash, 7 whereas the former may consist of leukopenia or pancytopenia.
Because of the acute dose‐related side effects, initial maintenance dose selection is important. Higher plasma concentrations increase the probability of these toxicities. However, nonlinear saturable pharmacokinetics, autoinductive effects with maintenance dosing, and CYP2C9 pharmacogenetic polymorphisms complicate dose selection. CYP2C9 poor‐metabolizer phenotype and CYP2C9 drug interactions, such as those produced by voriconazole, can significantly augment phenytoin exposure. 8 Variability in protein binding, primarily related to changes in albumin concentrations, can confound the relationship between therapeutic drug monitoring and pharmacodynamic expectations. Further discussion of the metabolism of phenytoin and fosphenytoin can be found in the Supplementary Material online.
Linking genetic variability to variability in drug‐related phenotypes
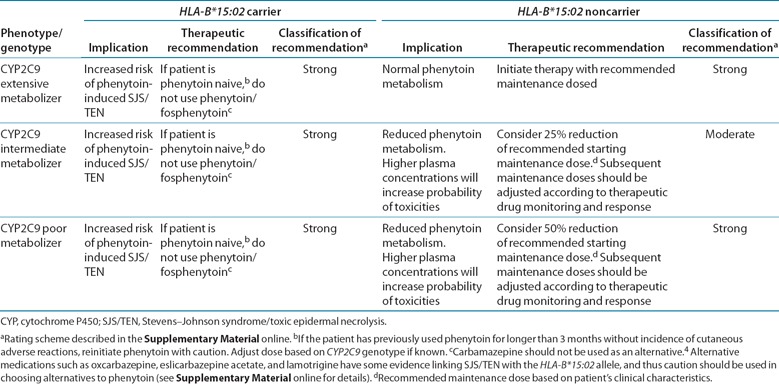
Substantial evidence links CYP2C9 and HLA‐B*15:02 genotypes with phenotypic variability (see Supplementary Tables S8 and S9 online). Application of a grading system to evidence linking genotypic with phenotypic variability indicates a high quality of evidence in the majority of cases ( Supplementary Tables S8 and S9 online). The evidence presented here and in Supplementary Tables S8 and S9 provides the basis for the dosing recommendations in Table 2 .
Table 2.
Recommended dosing of phenytoin/fosphenytoin based on HLA‐B*15:02 and CYP2C9 phenotype/genotype
HLA‐B. An increased risk of SJS/TEN has been associated with the HLA‐B*15:02 allele in Han Chinese and other Asian groups (see Supplementary Material online and Supplementary Table S8 online). Cheung et al. conducted a meta‐analysis of two studies in Taiwan 9 and Hong Kong, 10 comprising a total of 41 cases and 188 controls, and showed a positive association of HLA‐B*15:02 with phenytoin‐induced SJS/TEN (P < 3 × 10–4; odds ratio = 4.26; 95% confidence interval (CI) = 1.93–9.39) under a fixed‐effect model with statistically insignificant heterogeneity. By pooling data directly, the association had a sensitivity of 36.6% (95% CI = 23.6–51.9) and specificity of 87.2% (95% CI = 81.7–91.3). Therefore, the absence of these variants does not rule out the possibility of a patient developing phenytoin‐induced SJS/TEN. The strength of the association between phenytoin use and SJS/TEN is weaker than that of the association between carbamazepine use and SJS/TEN due to the limited number of studies and observations with phenytoin or fosphenytoin in the literature. However, taken together with the known association of carbamazepine and SJS/TEN in carriers of HLA‐B*15:02, the association supports the US Food and Drug Administration recommendations to avoid these agents as substitutes for carbamazepine in individuals who test positive for HLA‐B*15:02. 4
CYP2C9. Available model estimates predict that variant CYP2C9 alleles lower phenytoin intrinsic clearance based on the allele and number of variants. Several studies indicate that individuals with CYP2C9*1/*3 and CYP2C9*1/*2 genotypes have mild to moderately reduced clearance values ( Supplementary Table S9 online); these individuals are classified as intermediate metabolizers. Individuals with two decreased‐activity alleles (CYP2C9*2/*2, CYP2C9*3/*3) have reduced clearance of several drugs and are classified as CYP2C9 poor metabolizers. Phenytoin maintenance doses were reported to be reduced by 23–38% in heterozygous individuals with one decreased function allele 11 , 12 , 13 and by 31–52% in carriers with two decreased function CYP2C9 alleles vs. the doses for individuals homozygous for CYP2C9*1. 12 , 13 Furthermore, case reports indicate that poor metabolizers appear to be at higher risk for exposure‐related toxicities than patients homozygous for the wild‐type alleles. 14 , 15 , 16 , 17
Therapeutic recommendations
HLA‐B*15:02 recommendations. The Food and Drug Administration warning for phenytoin states, “Consideration should be given to avoiding phenytoin as an alternative for carbamazepine in patients positive for HLA‐B*15:02” due to the increased risk of SJS/TEN in patients of Asian ancestry. The evidence linking HLA‐B*15:02 to phenytoin‐induced SJS/TEN was generated in individuals of Asian ancestry because the frequency of HLA‐B*15:02 is very low in other populations (see Supplementary Tables S3 and S4 online for frequency information) that have been studied. However, HLA‐B*15:02 may also occur in other populations throughout the world yet to be studied, and patients may be unaware of or fail to disclose more distant Asian ancestry in their families. Furthermore, much of the evidence (summarized in Supplementary Table S8 online) linking HLA‐B*15:02 to phenytoin‐induced SJS/TEN was generated in both children and adults. Therefore, regardless of the CYP2C9 genotype and the individual's ancestry or age, if the HLA‐B*15:02 test result is positive, the recommendation is to consider using an anticonvulsant other than carbamazepine and phenytoin, unless the benefits of treating the underlying disease clearly outweigh the risks (see Table 2 ). Some evidence exists linking SJS/TEN with the HLA‐B*15:02 allele in association with the use of alternative medications such as oxcarbazepine, eslicarbazepine acetate, and lamotrigine, and thus caution should be used in choosing alternatives to phenytoin (see Supplementary Material online for details).
CYP2C9 recommendations. Phenytoin and fosphenytoin dose should first be adjusted according to a patient's clinical characteristics. Table 2 summarizes the gene‐based dosing recommendations for phenytoin based on CYP2C9 phenotype. The recommended phenytoin maintenance dose does not need adjustment based on genotype for CYP2C9 extensive metabolizers. Available evidence does not clearly indicate the amount of dose reduction needed to prevent phenytoin‐related toxicities in CYP2C9 intermediate and poor metabolizers; thus, our recommendations should be considered conservative estimates, given the variability surrounding phenytoin dosing in an individual. On the basis of the doses reported in the pharmacokinetic and pharmacogenetic studies mentioned above 11 , 12 , 13 and in Supplementary Table S9 online, at least a 25% reduction of the recommended starting maintenance dose may be considered for CYP2C9 intermediate metabolizers, with subsequent maintenance doses adjusted based on therapeutic drug monitoring and response. For CYP2C9 poor metabolizers, consider at least a 50% reduction of starting maintenance dose, with subsequent maintenance doses adjusted based on therapeutic drug monitoring or response.
Furthermore, although in vitro data suggest that the degree of reduction of catalytic activity is greater for the CYP2C9*3 variant than for the CYP2C9*2 variant, 18 clinical pharmacokinetic studies indicate similar dose reductions and pharmacokinetic parameters (e.g., trough levels, and serum 5‐(4′‐hydroxyphenyl)‐5‐phenylhydantoin/phenytoin ratio) for these variants as compared with the wild‐type alleles. 12 , 19 , 20 Therefore, our recommendation is to start with at least the above‐recommended reduction of the maintenance dose, followed by an adjustment of dose based on therapeutic drug monitoring.
Pediatrics. Special consideration should be given to the pediatric population. Phenytoin is used in the treatment of neonatal seizures and, subsequently, after discharge from the neonatal intensive care unit. Maintaining therapeutic levels can be particularly problematic in this population. This may be due to the developmental expression of hepatic CYP2C. CYP expression and functional activities have been shown to develop at different rates within subfamilies. 21 It has been found that activity levels of CYP2C9 are at 1–2% of adult values in the fetus during the first trimester. These levels gradually increase to 30% of adult values at term. There is a high variability in these levels during the first 5 months of life, with levels eventually approaching adult values somewhere between 5 months and 2 years of age. 22 Other considerations include the fact that clearance of phenytoin is twice that of adult values in children younger than 6 years of age. This is attributed to the finding that the maximal rate of phenytoin metabolism is inversely related to age. However, this varies significantly within age subgroups. 23 For these reasons, phenytoin therapeutic recommendations based on CYP2C9 genotype in this population are difficult. There is only one published report describing a 2‐year‐old patient (CYP2C9*2/*2 and CYP2C19*1/*4) presenting with phenytoin toxicity 2 h after a 15‐mg/kg phenytoin loading dose with symptoms lasting 122 h. 24 The half‐life was much higher than expected (112 h vs 46.7 h), which could be explained by the influence of CYP2C9 and CYP2C19 genetic polymorphisms (other predisposing factors such as malnourishment, renal failure, hepatic dysfunction, and inhibition of phenytoin metabolism by other drugs were ruled out). Therefore, for pediatric patients who are CYP2C9 intermediate or poor metabolizers, dose adjustment is recommended with close therapeutic drug monitoring.
HLA‐B*15:02 and CYP2C9 dosing recommendation. If both HLA‐B*15:02 and CYP2C9 genotypes are known, consider the HLA‐B*15:02 genotype first, then the CYP2C9 genotype ( Figure 1 ; Table 2 ).
Figure 1.
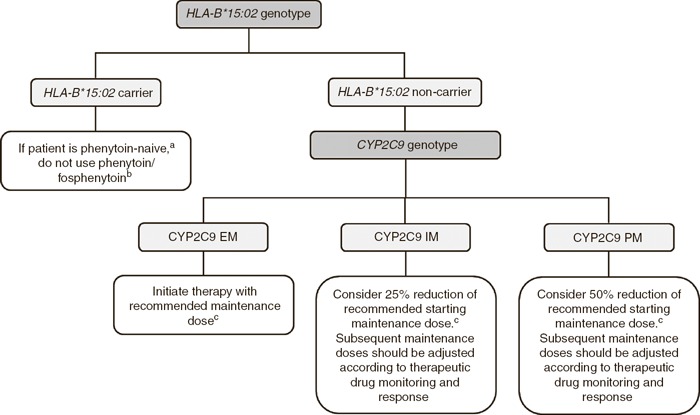
Algorithm for suggested clinical actions based on HLA‐B*15:02 and CYP2C9 genotypes. aIf patient has previously used phenytoin for longer than 3 months without incidence of cutaneous adverse reactions, reinitiate phenytoin with caution. Adjust dose based on CYP2C9 genotype if known. bCarbamazepine should not be used as an alternative. 4 Alternative medications such as oxcarbazepine, eslicarbazepine acetate, and lamotrigine have some evidence linking SJS/TEN with the HLA‐B*15:02 allele, and thus caution should be used in choosing alternatives to phenytoin (see Supplementary Material online for details). cRecommended maintenance dose based on patient's clinical characteristics. EM, extensive metabolizer; IM, intermediate metabolizer; PM, poor metabolizer; SJS/ TEN, Stevens–Johnson syndrome/toxic epidermal necrolysis.
The Supplementary Material online contains example clinical decision support (CDS) tools that can be used within electronic health records (EHRs) which assist clinicians to use genetic information to optimize drug therapy. Clinical implementation resources include cross‐references for drug and gene names to widely used terminologies and standardized nomenclature systems ( Supplementary Tables S10 and S11 online), workflow diagrams ( Supplementary Figures S3 and S4 online), tables that translate genotype test results into an interpreted phenotype ( Supplementary Table S12 online), and example text for documentation in the EHR and point‐of‐care alerts ( Supplementary Tables S13 and S14 online).
Other considerations
HLA‐B. HLA‐B*15:02 is linked to SJS and TEN but not to a predisposition for other phenytoin‐induced cutaneous adverse reactions such as mild maculopapular eruptions or drug hypersensitivity syndrome. 25
CYP2C9. Because of its potent CYP‐inducing properties, phenytoin is involved in a very large number of drug interactions, especially those involving increased metabolism of other agents, with subsequent decreases in their levels. 26 A full discussion of these is beyond the scope of this guideline, but agents prominently and significantly affected include antineoplastic and immunosuppressive agents, lipid‐lowering agents, psychotropics, oral contraceptives, and warfarin, to name just a few. Furthermore, inhibitors of CYP2C9 can generate phenytoin overexposure and toxicity. Although fluconazole and amiodarone are recognized as potent CYP2C9 enzyme inhibitors, other less‐potent drugs can produce significant elevations in phenytoin plasma concentrations. Therefore, it is important to interpret the results of genetic testing in the context of other coadministered drugs.
CYP2C9 genetic variation does not account for all of the pharmacogenetic variability in phenytoin metabolism. Some studies have implicated variants in other genes associated with phenytoin metabolism (e.g., CPY2C19, CYP1A1, and EPHX1; see ref. 27 for a review), and combined genetic analysis might improve the predictability of CYP2C9 alone. 11 , 13 However, this has not been consistently replicated, and there are limited studies evaluating the effect of multiple‐gene variation and phenytoin dose adjustment requirement. Consequently, this guideline on genotype‐directed phenytoin dosing is limited to CYP2C9 variant alleles.
Recommendations for incidental findings
Several drugs structurally and therapeutically similar to phenytoin, such as oxcarbazepine and carbamazepine, have also been associated with SJS/TEN and HLA‐B*15:02 in Asian populations 28 , 29 , 30 , 31 , 32 , 33 , 34 (see Supplementary Material online). The drug‐specific evidence linking HLA‐B*15:02 and SJS/TEN is discussed in the CPIC guideline for HLA‐B genotype and carbamazepine dosing 4 and may have implications for choosing alternatives to phenytoin in those who carry the HLA‐B*15:02 allele. Case reports have identified cross‐reactions to lamotrigine and other antiepileptic drugs in the presence of HLA‐B*15:02 (see Supplementary Material online for further discussion). However, larger studies appear to be needed for confirmation.
CYP2C9 metabolism includes substrates from several drug classes, including nonsteroidal anti‐inflammatory drugs, oral hypoglycemics/sulfonylureas, and a miscellaneous group of drugs. Reports support that patients with enhanced sensitivity to warfarin are likely to have a decreased capacity to metabolize phenytoin. 35
POTENTIAL BENEFITS AND RISKS FOR THE PATIENT
The potential benefit for patients with existing CYP2C9 and/or HLA‐B*15:02 genotyping information is in avoiding adverse effects in those patients who are CYP2C9 poor metabolizers by making significant reductions in their starting maintenance dose or by selecting alternative agents for those who are HLA‐B*15:02 carriers. For HLA‐B*15:02 carriers, a potential risk is that phenytoin therapy may have been needlessly avoided in patients who may not have developed SJS/TEN; however, this risk is mitigated because alternatives to phenytoin with comparable effectiveness exist. Another potential risk would be an error in genotyping. Furthermore, many commercially available genotyping tests do not detect alleles that are rare or de novo variants. Other alleles are not well characterized, resulting in uncertainty when predicting the phenotype for some genetic test results. Due to the fact that the absence of HLA‐B*15:02 does not rule out the possibility of a patient developing phenytoin‐induced SJS/TEN 36 or in the event of a rare variant that is not detected by the genetic test, a high‐risk patient could be prescribed phenytoin or prescribed a higher dose than needed. Moreover, because not all phenytoin‐induced adverse events are attributable to HLA‐B*15:02 or CYP2C9 metabolizer status, clinicians should carefully monitor all patients according to standard practices.
CAVEATS: APPROPRIATE USE AND/OR POTENTIAL MISUSE OF GENETIC TESTS
The application of genotype‐based dosing is most appropriate when initiating phenytoin therapy. Obtaining genetic information after months of drug therapy is less helpful, given that the drug dose may have already been adjusted based on plasma concentrations, response, or side effects. As with all diagnostic tests, genetic tests constitute only one of several pieces of clinical information that should be considered before initiating drug therapy.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
ACKNOWLEDGMENTS
We acknowledge the critical input of members of the Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network, particularly Mary V. Relling (St Jude Children's Research Hospital), funded by the National Institutes of Health (NIH). This work was funded by NIH grants R24 GM61374, U01 GM092666, GM32165, UO1 GM092676, and U01 HL0105918.
CPIC guidelines reflect expert consensus based on clinical evidence and peer‐reviewed literature available at the time they are written and are intended only to assist clinicians in decision making, in addition to identifying questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variations among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health‐care provider to determine the best course of treatment for the patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application to be solely made by the clinician and the patient. The CPIC assumes no responsibility for any injury to persons or damage to property related to any use of CPIC guidelines, or for any errors or omissions.
CONFLICT OF INTEREST
T.E.K. is a consultant for Personalis. The other authors declared no conflict of interest.
Supporting information
Supplementary Material
References
- 1. Martin, M.A. , Klein, T.E. , Dong, B.J. , Pirmohamed, M. , Haas, D.W. & Kroetz, D.L. ; Clinical Pharmacogenetics Implementation Consortium . Clinical Pharmacogenetics Implementation Consortium guidelines for HLA‐B genotype and abacavir dosing. Clin. Pharmacol. Ther. 91, 734–738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee, C.R. , Goldstein, J.A. & Pieper, J.A. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in‐vitro and human data. Pharmacogenetics 12, 251–263 (2002). [DOI] [PubMed] [Google Scholar]
- 3. Hershfield, M.S. et al Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen‐B genotype and allopurinol dosing. Clin. Pharmacol. Ther. 93, 153–158 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leckband, S.G. et al; Clinical Pharmacogenetics Implementation Consortium . Clinical Pharmacogenetics Implementation Consortium guidelines for HLA‐B genotype and carbamazepine dosing. Clin. Pharmacol. Ther. 94, 324–328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Twardowschy, C.A. , Werneck, L.C. , Scola, R.H. , Borgio, J.G. , De Paola, L. & Silvado, C. The role of CYP2C9 polymorphisms in phenytoin‐related cerebellar atrophy. Seizure 22, 194–197 (2013). [DOI] [PubMed] [Google Scholar]
- 6. Johnson, J.A. et al; Clinical Pharmacogenetics Implementation Consortium . Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 90, 625–629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker, W.A. & Shearer, C.A. Phenytoin hepatotoxicity: a case report and review. Neurology 29, 175–178 (1979). [DOI] [PubMed] [Google Scholar]
- 8. Purkins, L. , Wood, N. , Ghahramani, P. , Love, E.R. , Eve, M.D. & Fielding, A. Coadministration of voriconazole and phenytoin: pharmacokinetic interaction, safety, and toleration. Br. J. Clin. Pharmacol. 56 (suppl. 1), 37–44 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hung, S.I. et al Common risk allele in aromatic antiepileptic‐drug induced Stevens‐Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics 11, 349–356 (2010). [DOI] [PubMed] [Google Scholar]
- 10. Cheung, Y.K. , Cheng, S.H. , Chan, E.J. , Lo, S.V. , Ng, M.H. & Kwan, P. HLA‐B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia 54, 1307–1314 (2013). [DOI] [PubMed] [Google Scholar]
- 11. Hung, C.C. , Lin, C.J. , Chen, C.C. , Chang, C.J. & Liou, H.H. Dosage recommendation of phenytoin for patients with epilepsy with different CYP2C9/CYP2C19 polymorphisms. Ther. Drug Monit. 26, 534–540 (2004). [DOI] [PubMed] [Google Scholar]
- 12. van der Weide, J. , Steijns, L.S. , van Weelden, M.J. & de Haan, K. The effect of genetic polymorphism of cytochrome P450 CYP2C9 on phenytoin dose requirement. Pharmacogenetics 11, 287–291 (2001). [DOI] [PubMed] [Google Scholar]
- 13. Hung, C.C. et al Effects of polymorphisms in six candidate genes on phenytoin maintenance therapy in Han Chinese patients. Pharmacogenomics 13, 1339–1349 (2012). [DOI] [PubMed] [Google Scholar]
- 14. Brandolese, R. , Scordo, M.G. , Spina, E. , Gusella, M. & Padrini, R. Severe phenytoin intoxication in a subject homozygous for CYP2C9*3. Clin. Pharmacol. Ther. 70, 391–394 (2001). [PubMed] [Google Scholar]
- 15. Dorado, P. , Lopez‐Torres, E. , Peñas‐Lledó, E.M. , Martinez‐Anton, J. & Llerena, A. Neurological toxicity after phenytoin infusion in a pediatric patient with epilepsy: influence of CYP2C9, CYP2C19 and ABCB1 genetic polymorphisms. Pharmacogenomics J. 13, 359–361 (2013). [DOI] [PubMed] [Google Scholar]
- 16. Hennessy, S. et al CYP2C9, CYP2C19, and ABCB1 genotype and hospitalization for phenytoin toxicity. J. Clin. Pharmacol. 49, 1483–1487 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ramasamy, K. , Narayan, S.K. , Chanolean, S. & Chandrasekaran, A. Severe phenytoin toxicity in a CYP2C9*3*3 homozygous mutant from India. Neurol. India 55, 408–409 (2007). [DOI] [PubMed] [Google Scholar]
- 18. Rettie, A.E. , Haining, R.L. , Bajpai, M. & Levy, R.H. A common genetic basis for idiosyncratic toxicity of warfarin and phenytoin. Epilepsy Res. 35, 253–255 (1999). [DOI] [PubMed] [Google Scholar]
- 19. Aynacioglu, A.S. et al Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br. J. Clin. Pharmacol. 48, 409–415 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kerb, R. et al The predictive value of MDR1, CYP2C9, and CYP2C19 polymorphisms for phenytoin plasma levels. Pharmacogenomics J. 1, 204–210 (2001). [DOI] [PubMed] [Google Scholar]
- 21. Cresteil, T. , Beaune, P. , Kremers, P. , Celier, C. , Guengerich, F.P. & Leroux, J.P. Immunoquantification of epoxide hydrolase and cytochrome P‐450 isozymes in fetal and adult human liver microsomes. Eur. J. Biochem. 151, 345–350 (1985). [DOI] [PubMed] [Google Scholar]
- 22. Koukouritaki, S.B. et al Developmental expression of human hepatic CYP2C9 and CYP2C19. J. Pharmacol. Exp. Ther. 308, 965–974 (2004). [DOI] [PubMed] [Google Scholar]
- 23. Suzuki, Y. , Mimaki, T. , Cox, S. , Koepke, J. , Hayes, J. & Walson, P.D. Phenytoin age‐dose‐concentration relationship in children. Ther. Drug Monit. 16, 145–150 (1994). [DOI] [PubMed] [Google Scholar]
- 24. Dorado, P. , López‐Torres, E. , Peñas‐Lledó, E.M. , Martínez‐Antón, J. & Llerena, A. Neurological toxicity after phenytoin infusion in a pediatric patient with epilepsy: influence of CYP2C9, CYP2C19 and ABCB1 genetic polymorphisms. Pharmacogenomics J. 13, 359–361 (2013). [DOI] [PubMed] [Google Scholar]
- 25. Yip, V.L. , Marson, A.G. , Jorgensen, A.L. , Pirmohamed, M. & Alfirevic, A. HLA genotype and carbamazepine‐induced cutaneous adverse drug reactions: a systematic review. Clin. Pharmacol. Ther. 92, 757–765 (2012). [DOI] [PubMed] [Google Scholar]
- 26. Mintzer, S. et al Effects of antiepileptic drugs on lipids, homocysteine, and C‐reactive protein. Ann. Neurol. 65, 448–456 (2009). [DOI] [PubMed] [Google Scholar]
- 27. Thorn, C.F. , Whirl‐Carrillo, M. , Leeder, J.S. , Klein, T.E. & Altman, R.B. PharmGKB summary: phenytoin pathway. Pharmacogenet. Genomics 22, 466–470 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shankarkumar, U. , Shah, K.N. & Ghosh, K. Letter: HLA B*1502 allele association with oxcarbamazepine‐induced skin reactions in epilepsy patient from India. Epilepsia 50, 1837–1838 (2009). [DOI] [PubMed] [Google Scholar]
- 29. Lin, L.C. , Lai, P.C. , Yang, S.F. & Yang, R.C. Oxcarbazepine‐induced Stevens‐Johnson syndrome: a case report. Kaohsiung J. Med. Sci. 25, 82–86 (2009). [DOI] [PubMed] [Google Scholar]
- 30. Chen, Y.C. , Chu, C.Y. & Hsiao, C.H. Oxcarbazepine‐induced Stevens‐Johnson syndrome in a patient with HLA‐B*1502 genotype. J. Eur. Acad. Dermatol. Venereol. 23, 702–703 (2009). [DOI] [PubMed] [Google Scholar]
- 31. Locharernkul, C. et al Carbamazepine and phenytoin induced Stevens‐Johnson syndrome is associated with HLA‐B*1502 allele in Thai population. Epilepsia 49, 2087–2091 (2008). [DOI] [PubMed] [Google Scholar]
- 32. Whirl‐Carrillo, M. et al Pharmacogenomics knowledge for personalized medicine. Clin. Pharmacol. Ther. 92, 414–417 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu, F.Y. , Wu, X.T. , An, D.M. , Yan, B. , Stefan, H. & Zhou, D. Pilot association study of oxcarbazepine‐induced mild cutaneous adverse reactions with HLA‐B*1502 allele in Chinese Han population. Seizure 20, 160–162 (2011). [DOI] [PubMed] [Google Scholar]
- 34. Hu, F.Y. , Wu, X.T. , An, D.M. , Yan, B. , Stefan, H. & Zhou, D. Phenytoin‐induced Stevens‐Johnson syndrome with negative HLA‐B*1502 allele in mainland China: two cases. Seizure 20, 431–432 (2011). [DOI] [PubMed] [Google Scholar]
- 35. Bochner, F. , Hooper, W.D. , Eadie, M.J. & Tyrer, J.H. Decreased capacity to metabolize diphenylhydantoin in a patient with hypersensitivity to warfarin. Aust. N. Z. J. Med. 5, 462–466 (1975). [DOI] [PubMed] [Google Scholar]
- 36. Dong, D. , Sung, C. & Finkelstein, E.A. Cost‐effectiveness of HLA‐B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 79, 1259–1267 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


