Abstract
Chemoprevention is an important potential tool in reducing lung cancer incidence. Non-calcified nodules (NCNs) observed on low-dose computed tomography (LDCT) have been proposed as intermediate endpoints in chemoprevention trials, but whether NCNs represent cancer precursors is unclear. We analyzed data from subjects in the LDCT arm of the National Lung Screening Trial (NLST) to examine short and long-term lung cancer risks associated with NCNs and to elucidate whether some NCNS may be cancer precursors. NLST subjects received a baseline and two additional LDCT screens and were followed for a median of 6.5 years. We examined lung cancer incidence over three distinct periods from baseline – 0–23 months (short-term), 24–59 months (medium-term) and 60–84 months (long-term) – in relation to baseline NCN characteristics. Spatially, lung cancer incidence was analyzed at the person, lung and lobe levels relative to NCN location. 26,272 subjects received the baseline LDCT screen, with 468, 413, and 190 lung cancers observed in the three periods. The presence of an NCN gave significantly elevated long-term lung cancer risk ratios (RRs) of 1.8, 2.4 and 3.5 at the person, lung and lobe levels; corresponding short-term RRs were 10.3, 16.8 and 38.0. Ground glass attenuation was positively associated with long term-lung cancer risk but inversely associated with short-term risk; NCN size was positively associated with short-term risk but not significantly associated with long-term risk. That NCNs convey significantly elevated excess long-term of lung cancer lends evidence to the hypothesis that some NCNs may be cancer precursors.
Keywords: chemoprevention, lung cancer, CT screening, long-term risk, non-calcified nodules
Introduction
Chemoprevention is an important potential tool in the effort to reduce the burden of lung cancer. However, chemopreventive drug development has been complicated by the difficulty in identifying intermediate endpoints that could be used as surrogates for cancer incidence in early phase clinical trials. It has been proposed that a decrease in the size and number of lung nodules identified on low-dose computed tomography (LDCT) may be used to indicate preliminary efficacy of putative chemopreventive agents, based on the hypothesis that non-calcified lung nodules (NCNs) may represent precursors to lung adenocarcinoma (1). Case series of surgically resected NCNs with ground glass attenutation have demonstrated that some of these lesions represent a lung adencarcinoma precursor known as atypical adenomatous hyperplasia or actual early lung adenocarcinomas, although the data regarding the identity of small solid nodules are less clear (2,3).
There are convincing observations from numerous studies that some NCNs observed on LDCT represent active lung cancer (4–6). However, data from the National Lung Screening Trial (NLST) show that only about 4% of subjects with (4+mm) NCNs were diagnosed with lung cancer within one year (4). Thus, the vast majority of NCNs do not represent active cancer. Some NCNs noted on LDCT are clearly unrelated to lung cancer, for example, some represent residues of infectious processes. It is possible, though not unequivocally established, however, that some NCNs are on the cancer pathway but do not represent invasive cancer at the time of detection; these could represent cancer precursors. Whether such precursor NCNs actually exist, and if so, whether they have specific radiologic characteristics, such as ground glass versus solid attenuation, remains to be definitively determined.
The aim of this study was to assess, using data from the NLST, whether NCNs seen on baseline LDCT are associated with excess long-term risk of lung cancer and to compare such risk with the known excess short-term risk associated with NCNs. The reason to examine both short and long-term risk is that baseline NCNs that represent an actual active tumor would likely present clinically or be detected by screening in the short or medium-term, say within five years of initial detection, whereas baseline NCNs that represent cancer precursors would likely require longer time frames (e.g., more than five years) to present as cancer. We examined various NCN characteristics, such as size, attenuation and margins, to determine where any such characteristics are differentially associated with long term versus short-term risk. In addition, we attempted to ascertain whether NCNs are tumor precursors themselves or merely markers of increased lung cancer risk. To distinguish these possibilities, we correlated the location (at the lung and lobe level) of any eventual lung cancers with the location of NCNs to determine if any excess risk is specific to the NCN location, which would lend weight to the hypothesis that the NCNs do represent actual cancer precursors.
Materials and Methods
NLST Design
The NLST randomized subjects aged 55–74 to either a LDCT or chest-radiograph (CXR) lung screening arm. Eligibility criteria included a 30+ pack year smoking history and current smoking status or having quit within the past 15 years (4). Subjects were enrolled at 33 U.S. screening centers during 2002–2004 and received LDCT or CXR screens at baseline (T0) and annually for two more years (T1,T2).
LDCT screening results were reported on standardized forms. The NLST protocol defined a ≥ 4mm NCN as a positive screen. For each such NCN, radiologists reported the lobe location, greatest transverse and perpendicular diameter, margins and attenuation characteristics. For the T1/T2 screens, radiologists recorded whether any noted NCNs were pre-existing at the prior screen(s). Positive screens were tracked for follow-up diagnostic procedures and cancer diagnoses. In addition, subjects were followed through the end of 2009 with yearly status updates to ascertain incident cancers in the absence of a positive screen. All reported cancers were verified with medical records.
Analysis Plan
The cohort for this analysis consisted of all CT arm NLST subjects who received the baseline CT screen. We examined lung cancer incidence in relation to baseline screen findings in three distinct follow-up periods - a short-term (0–23 months from the baseline screen), medium-term (24–59 months from baseline) and a long-term period (60–84 months from baseline). Factor(s) assessed for association with lung cancer incidence in these periods were the presence and characteristics (size, attenuation, margins and persistence) of NCNs recorded on the baseline screen. In addition, demographic, smoking and medical history factors assessed at baseline were also examined.
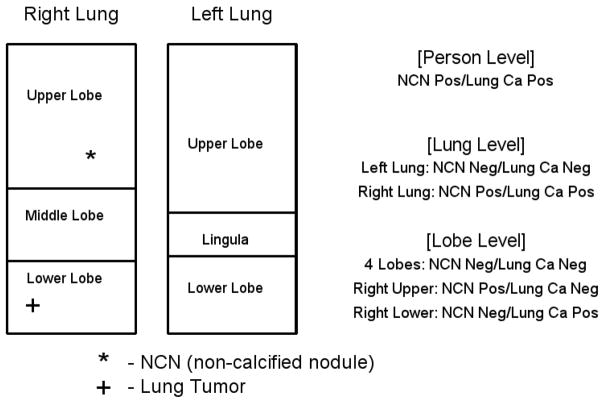
To ascertain whether any excess lung cancer risk was spatially correlated to the NCN location, we performed analyses on three levels - the person, lung, and lobe levels. At the person level, the question was whether having a baseline NCN anywhere was predictive of developing lung cancer anywhere. For the lung and lobe levels, the unit of analysis was the lung or lobe and the outcome was lung cancer diagnosis in that lung or lobe. Since the NLST did not attempt to link a subsequent cancer to a given NCN seen on screening CT, co-locating the cancer and an NCN in the same lobe is the closest one can come to determining that the NCN represented the cancer. Figure 1 displays how the three levels of analysis were performed in an example scenario.
Figure 1. Schematic of per-person, per-lung and per-lobe analyses.
For a hypothetical sample subject with a NCN in the right upper lobe and a subsequent cancer diagnosed in the right lower lobe, both NCN status and lung cancer status (Pos=positive, Neg=negative) at each spatial level of analysis are displayed. The lingula is counted as a lobe.
Statistical Methods
We computed lung cancer incidence rates by baseline NCN-related factors for all three levels of analysis (subject, lung, and lobe) and all three time periods. Incidence rates were computed using a denominator of person years of follow-up, and similarly using “lung years” or “lobe years” for the per-lung and per-lobe analyses; rate ratios were computed from the corresponding incidence rates. For the short-term period, follow-up began at the baseline screen and continued until lung cancer incidence, death, loss to follow-up, or the end of the 0–23 month interval, whichever came first. For the medium-term period, follow-up began at 24 months post-baseline and also continued until lung cancer incidence, death, loss to follow-up or the end of the 24–59 month period (whichever came first). Follow-up for the long-term period was computed similarly; the 84 month limit corresponds to the maximum NLST follow-up. Only those subjects who survived without a lung cancer diagnosis to 24 and to 60 months contributed person years to the medium and long-term periods, respectively. Therefore, the rate ratios for the medium and long-term periods are to be interpreted as conditional on surviving without a lung cancer diagnosis to the start of the period. Subjects were excluded from the per-lung or per-lobe analysis if the cancer location could not be pinpointed to a particular lung or lobe.
We utilized multivariate Cox proportional hazards models to control for possible confounders of the relationship between NCNs and lung cancer (7). In addition to NCN-related factors, we included in the models age, gender, smoking status (current/former), pack years and history of COPD. Separate models were run for each time period. For all 3 levels (person, lung, lobe) we fit a model with presence/absence of an NCN as the only NCN-related factor, though for the per-lung and per-lobe analysis we also included the presence of an NCN in the other lung or another lobe. For the per-lobe analysis, we also fit a model using only those lobes with at least one NCN; the model included the NCN characteristics of attenuation, margins, size and persistence. Because these nodule characteristics were often highly correlated with each other, we used a forward stepwise approach to select a final model, with p=0.05 entry/exit criterion. Additionally, since lobes (lungs) within the same subject are correlated, we used bootstrapping to derive confidence limits for both the univariate and multivariate analyses (8).
Reader Study
A critical question in assessing whether NCNs are cancer precursors is whether baseline NCNs associated with long-term lung cancer risk are persistent, at least through the 2 additional years of NLST screening. There was considerable variability among NLST radiologists in the average count of NCNs per exam (9). Therefore, in assessing NCN persistence, the fact that the NLST reading radiologist at T2 did not note an NCN does not necessarily mean that a baseline NCN, often noted by a different radiologist, was not persistent. To obtain a more definitive assessment of NCN persistence, we performed a reader study using a nested case-control design. Specifically, all long-term lung cancers with a NCN in the same lobe as the cancer were selected for the study, as were two controls per case, where controls had an NCN in some lobe and no cancer. Two readers, blinded to case-control status, evaluated each subject, examining only the lobe of interest. Readers were given the baseline NLST exam findings, and asked whether the noted NCNs were persistent through the T2 exam.
Results
A total of 26,272 subjects received the baseline CT screen and were thus included in the analysis cohort; all of these subjects contributed person time for the short-term period. For the medium and long-term periods, 25,097 and 24,105 subjects, respectively, contributed person time based on surviving without a lung cancer diagnosis through the start of the period. Average follow-up time during the three periods was 1.9, 2.9, and 1.6 years, respectively.
A total of 41% of the cohort were women, 27% were age 65 or over and 48% were current smokers. Slightly over a quarter (N=7084, or 26.5%) had at least one (4+mm) baseline NCN, for a total count of 11,779 (baseline) NCNs. NCN characteristics are displayed in Table 1. Approximately 15% had ground glass attenuation, 30% had spiculated or poorly defined margins and 16% were 10+ mm.
Table 1.
Characteristics of NCNs reported on the baseline CT screen
| All Nodules | N=11,779 | |
|---|---|---|
| % of NCNs | ||
| Nodule Attenuation | Soft Tissue | 74.8 |
| Ground Glass | 15.3 | |
| Mixed | 4.3 | |
| Other/Unknown | 5.6 | |
| Nodule Margins | Smooth | 65.9 |
| Spiculated | 10.0 | |
| Poorly Defined | 19.9 | |
| Unable to Determine | 4.3 | |
| Nodule Size (largest diameter) | 4–9 mm | 83.9 |
| 10+ mm | 16.1 | |
| Nodule Location | Right Upper Lobe | 23.6 |
| Right Middle Lobe | 13.7 | |
| Right Lower Lobe | 23.7 | |
| Left Upper Lobe | 14.4 | |
| Lingula | 4.3 | |
| Left Lower Lobe | 19.7 | |
| Other | 0.6 |
Note: All NCNs are 4+mm.
Univariate Analyses
Tables 2 and 3 show the results of univariate analyses at the person, lung and lobe level for the three time periods. There were 468, 413 and 190 lung cancers in the short, medium and long-term periods, respectively. For the per-lung and per-lobe analyses, fewer cancers could be evaluated due to missing lung or lobe location (449, 394 and 176, respectively, at the lung and 436, 367 and 161, respectively, at the lobe level).
Table 2.
Lung Cancer Incidence Rates by Time Period and NCN status
| Time Period | 0–23 Months | 24–59 Months | 60–84 Months | |||
|---|---|---|---|---|---|---|
| Spatial Level | ||||||
| Person Level | Lung Ca # (Rate 1) | Rate Ratio (95% CI) | Lung Ca # (Rate 1) | Rate Ratio (95% CI) | Lung Ca # (Rate 1) | Rate Ratio (95% CI) |
| No NCN | 100 (26.9) | Referent | 233 (42.6) | Referent | 117 (39.9) | Referent |
| Any NCN (4+mm) | 368 (276) | 10.3 (8–13) | 180 (94.0) | 2.2 (1.8–2.7) | 73 (72.0) | 1.8 (1.4–2.4) |
| All | 468 | 413 | 190 | |||
| Lung Level | ||||||
| No NCN in lung | 108 (12.7) | Referent | 252 (20.2) | Referent | 122 (18.3) | Referent |
| Any NCN (4+ mm) | 341 (213) | 16.8 (14–21) | 142 (61.7) | 3.0 (2.5–3.7) | 54 (44.5) | 2.4 (1.8–3.4) |
| Lobe Level | ||||||
| No NCN in lobe | 126 (4.4) | Referent | 269(6.5) | Referent | 132 (5.8) | Referent |
| Any NCN (4+mm) | 310 (166) | 38.0 (31–47) | 98 (36.6) | 5.7 (4.5–7.1) | 29 (21.0) | 3.5 (2.3–5.2) |
Rate is per 10,000 person (or lung or lobe) years.
Table 3.
Lung Cancer Incidence Rates by Time Period and NCN characteristics
| Time Period | 0–23 Months | 24–59 Months | 60–84 Months | |||
|---|---|---|---|---|---|---|
| Nodule Characteristic (Lobe Level) | Lung Ca # (Rate 1) | Rate Ratio (95% CI) | Lung Ca # (Rate 1) | Rate Ratio (95% CI) | Lung Ca # (Rate 1) | Rate Ratio (95% CI) |
| Only 4–9 mm NCNs | 62 (40.1) | Referent | 49 (21.6) | Referent | 22 (18.3) | Referent |
| ≥1 10+mm NCN | 248 (792) | 19.8 (15–26) | 49 (120.2) | 5.6 (3.7–8.3) | 7 (34.3) | 1.9 (0.8–4.3) |
| 1 NCN | 257 (162) | Referent | 73 (31.9) | Referent | 24 (19.9) | Referent |
| Multiple (2+) NCNs | 53 (197) | 1.2 (0.9–1.6) | 25 (64.8) | 2.0 (1.3–3.2) | 5 (25.2) | 1.3 (0.5–3.3) |
| No Persistent NCN | N/A | 16 (14.4) | Referent | 10 (16.8) | Referent | |
| 1+ Persistent NCN 2 | N/A | 79 (54.1) | 3.8 (2.2–6.4) | 18 (23.6) | 1.4 (0.7–3.0) | |
| Soft Tissue/Other Attenuation | 240 (164) | Referent | 61 (28.7) | Referent | 17 (15.2) | Referent |
| Ground Glass Attenuation 3 | 35 (115) | 0.7 (0.5– 0.99) | 29 (67.0) | 2.3 (1.5–3.6) | 11 (48.9) | 3.2 (1.5–6.8) |
| Mixed Attenuation | 35 (404) | 2.5 (1.7–3.5) | 8 (67.3) | 2.3 (1.1–4.8) | 1 (16.1) | 1.1 (0.2–6.3) |
| Smooth Margins (all NCNs) | 63 (49.1) | Referent | 41 (21.8) | Referent | 14 (14.0) | Referent |
| ≥1 NCN w/ Spiculated or Poorly Defined Margins | 247 (429) | 8.7 (6.6– 11.5) | 57 (71.8) | 3.3 (2.2–4.9) | 15 (36.6) | 2.6 (1.3–5.3) |
Rate is per 10,000 lobe years.
Persistence through last NLST screen (generally year 2). Subjects with no post-baseline screen excluded.
Lobes with both ground glass and mixed attenuation NCNs are grouped in the ground glass category.
Within each time period, rate ratios (RRs) for the presence of an NCN (compared to no NCN) increased with the spatial precision of the analysis, i.e., as the unit of analysis moved from the person to the lobe level (Table 2). Additionally, at each spatial level of analysis, RRs increased with temporal precision, i.e., as the follow-up period approached baseline. RRs were statistically significantly elevated at each spatial level and time period. For the long-term follow-up period, RRs (for 1+ NCN versus none) were 1.8 (95% CI: 1.4–2.5), 2.4 (95% CI: 1.8–3.4) and 3.5 (95% CI: 2.3–5.2) at the person, lung and lobe level, respectively, whereas for short-term follow-up, RRs were 10.3(95% CI: 8–13), 16.8 (95% CI: 14–21) and 38.0 (95% CI:31–47) at these three levels.
At the lobe level, RRs were computed for NCN characteristics (Table 3). It is noteworthy that the magnitude and/or direction of risk for some NCN characteristics varied over the time periods. For example, the RR for a 10+ mm NCN (as compared to 4–9mm NCNs) was not significantly elevated in the long-term (RR =1.9; 95% CI: 0.8–4.3) but was very substantially (and statistically significantly) elevated in the short-term (RR=19.8; 95% CI: 16–29) and moderately elevated in the medium-term (RR=5.6). The RR associated with an NCN with spiculated or poorly defined margins (as compared to smooth margins) was also attenuated in going from the short-term (RR=8.7) to the long-term (RR=2.6), though still statistically significant in all periods. The greatest difference in risk across time periods was for nodule attenuation. In the medium and long-term, risks for ground glass attenuation (as compared to soft tissue attenuation) were significantly elevated, with RRs of 2.3 (95% CI: 1.5–3.7) and 3.2 (95% CI: 1.5–6.8), respectively; in contrast, in the short-term the RR was borderline significantly below one (RR=0.7, 95% 0.5–0.99). Interestingly, the short-term RR for mixed attenuation nodules (compared to soft-tissue) was significantly elevated, with an RR of 2.5 (95% CI: 1.7–3.5); mixed attenuation nodules also showed significantly elevated risk as compared to ground glass nodules (RR=3.5, 95% CI: 2.2–5.6 – not shown in Table).
Persistence of NCNs was assessed at the medium and long-term periods; the RR for persistent NCNs (as compared to non-persistent NCNs) was significantly elevated in the medium-term (RR=3.8, 95% CI: 2.2–6.4) but not in the long-term (RR=1.4, 95% CI: 0.7–3.0).
Multivariate Analyses
The results of the multivariate Cox proportional hazards models are shown in Table 4. Across all time periods and spatial levels, the multivariate hazard ratios (HRs) were of generally similar magnitude as the univariate RRs for the excess risk associated with an NCN (Table 4). Having an NCN in another lung or lobe was not significantly associated with lung cancer risk in the medium and long term, and was inversely associated in the short-term (HR=0.6).
Table 4.
Multivariate Cox proportional hazards models for lung cancer risk associated with NCN characteristics for different time periods.
| Time Period | 0–23 Months | 24–59 Months | 60–84 Months | |
|---|---|---|---|---|
| Spatial Level | Presence/Absence of NCNs 1 | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Person | ≥1 NCN (vs. no NCNs) | 9.8 (7.8 –12.2) | 2.0 (1.6–2.5) | 1.6 (1.2–2.2) |
| Lung | ≥1 NCN (vs. no NCNs) | 17.1 (13.7– 21.3) | 2.9 (2.3–3.6) | 2.3 (1.6–3.2) |
| NCN in other lung (versus none) | 0.7 (0.6–0.9) | 0.9 (0.7–1.2) | 1.0 (0.8–1.2) | |
| Lobe | ≥1 NCN (vs no NCNs) | 40.4 (33–50) | 5.2 (4.1–6.6) | 3.0 (2.0–4.5) |
| NCN in another lobe (versus none) | 0.6 (0.5–0.8) | 1.0 (0.8–1.3) | 1.3 (0.9–1.9) | |
| Lobe | NCN Characteristics 2 | |||
| ≥1 10+ mm NCN (versus only 4–9 mm NCNs) | 12.8 (9.5–17.2) | 4.7 (2.9–7.5) | N.S. | |
| ≥1 NCN w/ Spiculated or Poorly Defined Margins (versus only NCNs with smooth margins) | 4.1 (3.0–5.5) | 2.3 (1.5–3.5) | N.S. | |
| ≥1 Persistent NCN (versus non-persistent NCNs) | N/A 3 | 4.8 (2.8–8.3) | N.S. | |
| All NCNs Soft Tissue Attenuation | Ref | Ref | Ref | |
| ≥1 NCN w/ Ground Glass Attenuation | 0.3 (0.2–0.4) | N.S. | 3.1 (1.4–6.6) | |
| ≥1 NCN w/ Mixed Attenuation | N.S. | N.S. | N.S. |
Model is for all lobes. Other factors in model were age, sex, smoking status, pack years, and COPD status.
Model is for only those lobes with at least one NCN. Forward step-wise approach was employed for variable selection for NCN characteristics; N.S. denotes that the factor was not selected for the final model in the step-wise process. The characteristic of multiple NCNs in a lobe was not selected for any time period. Other factors in model were age, sex, smoking status, pack years, and COPD status.
Not applicable because lung cancer assessments begins immediately with no lag.
For NCN characteristics, at the lobe level, in the short-term 10+mm NCN size (HR=12.8) and spiculated or poorly defined margins (HR=4.1) were significantly associated with increased lung cancer risk and ground glass attenuation was significantly associated with decreased risk (HR=0.3) (Table 4). Large size and spiculated or poorly defined margins were also significantly associated with increased risk in the medium-term. In contrast, in the long-term, ground glass attenuation gave a significantly elevated risk for lung cancer (HR=3.1), and NCN size and margins were not significantly associated with lung cancer risk.
Reader Study
Of 29 subjects with a NCN and cancer diagnosis in the same lobe in the long-term period, 28 had follow-up screens and 26 had images available for review. Also reviewed were 56 controls with a reported NCN in a lobe and no cancer. Of the 26 cases, 23 (88%) were determined on image review to have a persistent NCN in the cancer lobe through two years. Of controls, 7 of the 56 were determined not to have had NCNs at baseline; of the remaining 49, 44 (90%) were judged to have a persistent NCN. This contrasts with the original NLST reads, in which 18 of 28 (64%) of cancer-associated lobes (in the long-term period) had persistent NCNs.
Discussion
We found that NCNs (≥4 mm) were significantly associated with lung cancer risk for a period beginning five years after the NCN was originally noted. The association became stronger as the spatial connection between the NCN and the tumor was strengthened, with the RR increasing from 1.8 to 2.4 to 3.5 for NCNs examined at the person, lung, and lobe level. Further, our analysis showed that having an NCN in another lung or lobe did not significantly increase the long-term lung cancer risk in the given lobe or lung. These trends suggest that NCNs may not merely be markers of a state of increased risk for future lung cancer but may be actual cancer precursors themselves.
The RRs, as well as the absolute risks, associated with NCNs increased as the time period approached the (baseline) exam on which the NCN was found. This is consistent with the hypothesis that baseline NCNs represent actual cancers for subjects diagnosed earlier but represent cancer precursors or markers of locally increased risk for subjects diagnosed later; since not all cancer precursors advance to actual cancer, the long-term risk would be expected to be lower than the short-term risk.
A modeling exercise examined data from 6 lung CT screening studies to estimate a mean sojourn time of lung cancer of 2.07 years; sojourn time is the length of time that a cancer exists in the pre-clinical phase (10). Using this mean sojourn time, the probability that a preclinical cancer present at baseline would not have presented clinically for 5 years is around 10%. Further, since this was a screened population, some cancers that would not have presented clinically would have presumably been detected by the CT screening. Thus, the probability is low that an NCN that was an active cancer at baseline would not be diagnosed for at least 5 years. This lends support to the theory that the higher long-term risk associated with baseline NCNs is not due to the NCNs representing active cancer at baseline, but representing cancer precursors or markers of increased risk.
Interestingly, we found that various NCN characteristics displayed different strengths and/or directions of effect depending on the time period of lung cancer incidence assessed. Most strikingly, in the short-term of 0–23 months, large (≥10mm) NCN size was strongly related to cancer outcome (HR=12.8) and ground glass attenuation was inversely related to lung cancer outcome (HR=0.3 compared to soft tissue attenuation), whereas in the long-term (60–84 months) NCN size showed no significant association and ground glass attenuation was positively related to lung cancer outcome (HR=3.1). One explanation for these findings is that ground glass attenuation is a feature of the pre-cancerous state, whereas the transition to invasive malignancy results in a shift to soft tissue attenuation. Then in the short term ground glass attenuation would convey lower risk because it is correlated with being a cancer precursor but in the longer term the risk would increase as the transition to overt cancer occurs. With respect to size, an NCN on the cancer pathway may be able to grow to a certain large size only if it has already transitioned to active cancer. Thus a large NCN might not convey increased long-term lung cancer risk, since a large NCN on the cancer pathway would be likely to present clinically (or be screen detected) in a shorter time frame.
Note that although ground glass attenuation was significantly associated with long-term cancer risk, excess long-term risk was not confined to ground glass NCNs alone. Lobes containing NCNs with soft-tissue attenuation also showed elevated long-term risk compared to lobes without NCNs (multivariate HR= 2.2, 95% CI: 1.3–3.7), suggesting that solid nodules may also be cancer precursors or at least markers of increased risk.
Several studies have examined medium to longer-term risk associated with nodules. A Korean study followed 89 subjects with pure ground glass nodules detected through LDCT screening that had persisted (without a cancer diagnosis) for at least two years (11). After a median follow-up of 59 months 11 subjects (12%) were diagnosed with lung cancer arising from the nodules. Bellomi et al. followed 165 smokers with 238 NCNs of 2–5mm (mean 3.55mm) detected on a baseline screen (12). After four additional annual CT exams, 3 cancers were linked to the original NCNs, giving a per-nodule malignancy rate of 1.2% over four years. Silva et al. followed 56 subjects with 76 ground glass nodules for a mean of 50 months; 4 lung cancers were detected among these subjects (13). With respect to risk from ground glass nodules at different time periods, McWilliams et al. found that ground glass nodules had lower short-term (3 year) lung cancer risk than solid nodules, similar to our findings, although the difference was not statistically significant (14). Maisonneuve et al. found an increased risk for ground glass as compared to solid nodules in the period 12–48 months post-baseline (15).
The finding that NCNs do convey long-term lung cancer risk, and that some may be cancer precursors, lends support to the idea of using NCNs as surrogate outcomes for chemoprevention. However, some caveats are in order. First, since not all NCNs are cancer precursors, a chemopreventative agent could reduce the number or size of NCNs overall but have no effect on those NCNs that were actually cancer precursors. Additionally, an effective agent could regress a cancer precursor, leading to diminished size or resolution of the nodule, or alternatively it could prevent progression without affecting the lesion’s size or imaging characteristics. Nevertheless, the current study provides evidence that nodules, especially non-solid ones, may be precursors of lung cancer and thus deserve further study as intermediate endpoints for chemoprevention trials. Additionally, the fact that NCNs appear to convey increased long-term risk may have clinical implications in the management of subjects with NCNs noted on LDCT screening.
Acknowledgments
Funding: Contracts from the National Cancer Institute
Footnotes
Conflicts of interest: None
References
- 1.Veronesi G, Szabo E, DeCensi A, Guerrieri-Gonzaga A, Bellomi M, Radice D, et al. Randomized phase II trial of inhaled budesonide versus placebo in high-risk individuals with CT screen-detected lung nodules. Cancer Prev Res. 2011;4:34–42. doi: 10.1158/1940-6207.CAPR-10-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245:267–275. doi: 10.1148/radiol.2451061682. [DOI] [PubMed] [Google Scholar]
- 3.Ohtsuka T, Watanabe K, Kaji M, Naruke T, Suemasu K. A clinicopathological study of resected pulmonary nodules with focal pure ground-glass opacity. Eur J Cardiothorac Surg. 2006;30:160–163. doi: 10.1016/j.ejcts.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. New Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235:259–265. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, et al. The Pittsburgh lung screening study (PLuSS): Outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;178:956–961. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prentice RL, Kalbfleisch JD. Hazard rate models with covariates. Biometrics. 1979;35:25–39. [PubMed] [Google Scholar]
- 8.Efron B. The Jackknife, the Bootstrap and Other Resampling Plans. Philadelphia PA: Society for Industrial and Applied Mathematics; 1982. [Google Scholar]
- 9.Pinsky PF, Gierada D, Nath H, Kazerooni EA, Amorosa J. National Lung Screening Trial: variability in nodule detection rates in chest CT studies. Radiology. 2013;268:856–873. doi: 10.1148/radiol.13121530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien C, Chen TH. Mean sojourn time and effectiveness of mortality reduction for lung cancer screening with computed tomography. Int J Cancer. 2008;122:2594–2599. doi: 10.1002/ijc.23413. [DOI] [PubMed] [Google Scholar]
- 11.Chang B, Hwang JH, Choi Y, Chung MP, Kim H, Kwon OJ, et al. Natural history of pure ground glass opacity lung nodules detected by low-dose CT scan. Chest. 2013;143:172–178. doi: 10.1378/chest.11-2501. [DOI] [PubMed] [Google Scholar]
- 12.Bellomi M, Veronesi G, Rampinelli C, Ferretti S, De Fiori E, Miasonneuve P. Evolution of lung nodules ≤5mm detected with low-dose CT in asymptomatic smokers. Br J Radiol. 2007;80:708–712. doi: 10.1259/bjr/46019726. [DOI] [PubMed] [Google Scholar]
- 13.Silva M, Sverzellati N, Manna C, Negrini G, Marchiano A, Zompatori M, et al. Long-term surveillance of ground-glass nodules: Evidence from the MILD Trial. J Thorac Oncol. 2012;7(10):1541–1546. doi: 10.1097/JTO.0b013e3182641bba. [DOI] [PubMed] [Google Scholar]
- 14.McWilliams A, Tammemagi M, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. New Engl J Med. 2013;369:910–919. doi: 10.1056/NEJMoa1214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maisonneuve P, Bagnardi V, Bellomi M, Spaggiari L, Pelosi G, Rampinelli C, et al. Lung cancer risk prediction to select smokers for screening CT – a model based on the Italian COSMOS trial. Cancer Prev Res. 2011;4:1778–1789. doi: 10.1158/1940-6207.CAPR-11-0026. [DOI] [PubMed] [Google Scholar]