Abstract
Critically ill patients often suffer from multiple organ failures involving lung, kidney, liver or brain. Genomic, proteomic and metabolomic approaches highlight common injury mechanisms leading to acute organ failure. This underlines the need to focus on therapeutic strategies affecting multiple injury pathways. The use of adult stem cells such as mesenchymal stem or stromal cells (MSC) may represent a promising new therapeutic approach as increasing evidence shows that MSC can exert protective effects following injury through the release of pro-mitotic, anti-apoptotic, anti-inflammatory and immunomodulatory soluble factors. Furthermore, they can mitigate metabolomic and oxidative stress imbalance. In this work, we review the biological capabilities of MSC and the results of clinical trials using MSC as therapy in acute organ injuries. Although preliminary results are encouraging, more studies concerning safety and efficacy of MSC therapy are needed to determine their optimal clinical use.
INTRODUCTION
In the intensive care unit (ICU), the care of patients with acute organ injuries leading to organ failure remains challenging. Organ failure was defined by the 1991 Consensus Conference of the American College of Chest Physicians and the Society of Critical Care Medicine as “the presence of altered organ functions in an acutely ill patient such that homeostasis cannot be maintained without intervention1.” This disorder represents a dynamic continuum of change over time2. Multiple organ dysfunction syndrome (MODS) can lead to a mortality rate of 60% after severe trauma, 40% in sepsis, 50% in pancreatitis, 30% in burn injury and 30% in patients admitted post-cardiac arrest3. The higher the number of failed organs, the higher the mortality4. In the context of single organ injury without MODS, acute kidney injury (AKI)5, acute respiratory distress syndrome (ARDS)6 and acute liver failure (ALF)7 are responsible for up to 60%, 40% and 30% of mortality respectively.
The underlying mechanisms leading to cell death in organ injury are diverse: the pro-inflammatory nuclear factor-kappa B pathway, endothelial activation with coagulation disorders, lipid mediators, microcirculatory dysfunction, and ischemia-reperfusion (I/R) injury including oxydative stress (OS)-, metabolomic disruption- and pro-apoptotic-induced injuries. Aside from the diversity, many mechanisms are also dependent on the sequence in time of injury and/or are organ specific. For instance, nuclear factor-kappa B pathway can be either damaging in the acute phase of sepsis, and/or can be involved in the repair process during the resolution phase of injury. Similarly, the function of phagocytes is dual-faced. Although beneficial in sepsis by clearing pathogens, macrophages can also generate neuron damage through phagocytosis and apoptosis.
This complexity probably explains in part why treatment strategies geared toward a single pathway and/or during a specific timepoint have failed, highlighting the limited therapeutic strategies available to clinicians to target the multi-organ injuries which may result, aside from the treatment of the initial cause of injury. Clinical management currently focuses on supporting failed organs until they recover, a period where patients may be exposed to new iatrogenic complications3. Consequently, innovative therapies are needed. Therapeutic use of adult stem cells may be one of them. Stem cells are undifferentiated precursor cells capable of self-renewal and multi-lineage differentiation. They are classified by their potency (pluri-potent vs multi-potent) and origin (adult vs embryonic). Adult stem cells include hematopoietic stem cells, mesenchymal stem cells (MSC), endothelial progenitor cells, and organ specific stem cells. Although originally the beneficial effect of adult stem cells was thought to be through engraftment and regeneration8, subsequent studies demonstrated the main therapeutic effects were mediated primarily through the secretion of soluble factors.
In this review, we focused on the potential therapeutic use of human MSC for acute organ injury, specifically in ARDS, AKI, ALF, acute brain injury encompassing stroke and traumatic brain injury (TBI), sepsis and MODS. To accomplish this goal, we searched PubMed for relevant studies published over the past ten years (2003–2013) and the proceedings of major relevant conferences, clinical trial databases, the reference lists of identified trials and major reviews. In this work, we decided to use the term “organ failure” and “organ injury” to define respectively the altered functional outcomes and the tissue lesions leading to this alteration in the corresponding organ.
DEFINITION OF MESENCHYMAL STEM CELLS
MSC are adult non-hematopoietic precursor cells derived from a variety of tissues such as the bone marrow, adipose tissue and placenta. The definition of MSC by the International Society of Cellular Therapy in 2006 is based on three criteria: (1) MSC must be adherent to plastic under standard tissue culture conditions; (2) MSC must express certain cell surface markers such as CD73, CD90, and CD105, but must not express CD45, CD34, CD14, or CD11b; and (3) MSC must have the capacity to differentiate into mesenchymal lineages including osteoblasts, adipocytes, and chondroblasts under in vitro conditions9.
Engraftment Versus Paracrine Effects
Therapeutic properties of MSC were originally thought to derive from their engraftment in the organ of injury and regeneration. However, subsequent in vivo studies demonstrated limited replacement of damaged tissue by transdifferentiated stem cells (<5%). Thus, the role of paracrine soluble factors with its endocrine actions were studied as potential mechanisms mediating the therapeutic effects10–13. Despite the transient presence of MSC in the injured organ, ranging from several hours to several days14,15, MSC are able to exert complex paracrine and endocrine actions, through the secretion of growth factors and cytokines12. Moreover, recent in vivo studies also underscore the new potential role of microvesicles, small (50–200 nm) anuclear membrane bound particles released from MSC as a paracrine vehicle to deliver messenger RNA (mRNA), micro RNA or proteins that may reprogram the injured cells or induce secretion of cytoprotective factors16–21. All these effects have been demonstrated in multiple organ injury models: acute lung injury (ALI)22–24, AKI14,15,25–27, ALF28–30 and acute brain injury31–33.
Mesenchymal Stem Cells Homing Capacity
The ability of stem cell to preferentially traffick to inflammatory sites is thought to play a crucial role in the success of cellular therapy for organ injury. Intravenous or intra-arterial infusion of MSC often initially result in the entrapment of the administered cells in organ capillary beds, especially in the lung and liver34. In non-injured states, intravenous MSC tend to migrate to the bone marrow35,36. However, following injury, MSC preferentially home to the site of inflammation where they migrate across the inflammed endothelium and enter the injured tissue bed37–41. MSC trafficking have been shown to be driven by different interactions between chemokines released from the injured tissue and chemokine receptors expressed by MSC. For instance, stromal cell-derived factor-1/CXCR4 pathway, which is upregulated under ischemic or hypoxic conditions, can mediate the localization of injected MSC into the injured brain or kidneys42–46. Interaction between CD44 expressed by MSC and hyaluronic acid in the injured tissue, expressed when the extra-cellular matrix is exposed47,48, is another major pathway38.
ORGAN INJURY PATHWAYS SPECIFICALLY IMPACTED BY MESENCHYMAL STEM CELLS
The multiple mechanisms involved in organ injury are diverse. Although organ injuries do not fit into a single common combination of pathways, we will highlight those impacted by MSC.
Acute Pro-inflammatory Pathway
In addition to “septic” inflammation, a severe inflammatory response can be triggered by non-infectious sources, such as danger associated molecular patterns49,50. In the acute phase of organ injury, multiple cells express pattern recognition receptors that can recognize either pathogen or danger associated molecular patterns. Pattern recognition receptors sense endogenous and exogenous danger signals and induce pro-inflammatory cytokines and type I interferons49 (Figure 1). Monocytes-macrophages and polymorphonuclear neutrophils migrate quickly to sites of injury and secrete reactive oxygen species (ROS) and pro-inflammatory cytokines/chemokines. Antigen-presenting cells also migrate to the site of injury and internalize and process either pathogen or danger associated molecular patterns and initiate the adaptive immune response. Adaptive immune cells such as natural killer cells, natural killer T cells, mast cells, T-lymphocytes and B-lymphocytes then converge, participating in the pro or anti-inflammatory response. T cells are essential players in the acute and intermediate inflammatory phase of organ injury, bridging together innate and adaptive immunity. CD4+ T helpers (Th) cells lead to polarization of the immune response in multiple pathways (Th1, Th2, Th17, Th22, Th3, T-regulatory), and CD8+ T cells are dramatically involved in the cytotoxic response leading to the lysis of the targeted cells. Rather than a patchwork process, acute organ injury is a continuum of responses from innate to adaptive immune cells.
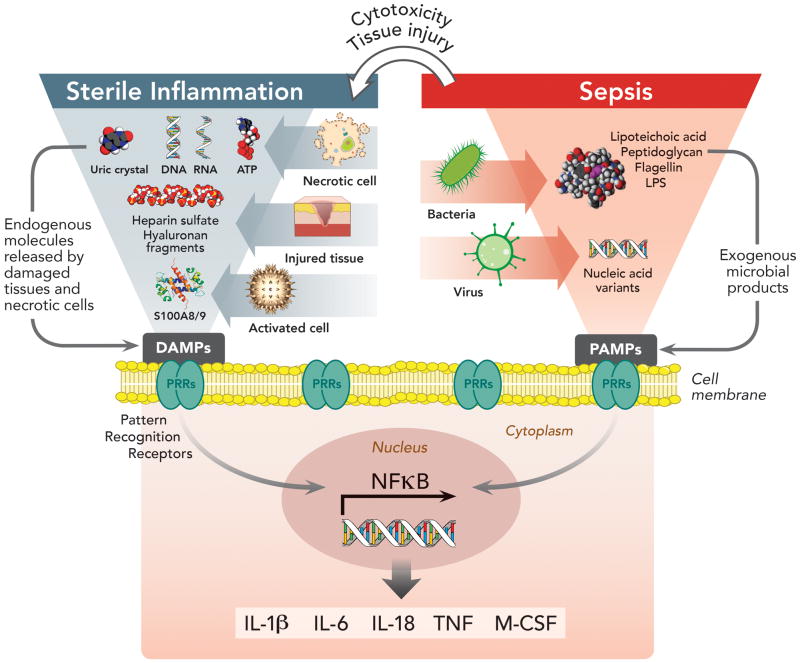
Figure 1. Pattern Recognition Receptors in Immunity and Their Involvement in Sterile and Sepsis-Related Inflammation.
Pattern recognition receptors (PRRs) expressed by antigen presenting cells (dendritic cells, monocytes, macrophages) constitute the first interaction between the extra-cellular environment and innate immunity. They are proteins, which include membrane-bound and cytoplasmic receptors, that bind either pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) derived from exogenous microorganisms (i.e. sepsis from infection) or endogenous molecules (i.e. sterile inflammation). Interaction of PRRs with PAMPs/DAMPs induces nuclear factor-kappa B signaling pathways, resulting in the secretion of pro-inflammatory cytokines and co-stimulatory molecules. In sepsis, the initial immune response triggered by PAMPs/PRRs interaction can lead to tissue damage and the release of DAMPs, which may act synergistically with PAMPs to enhance inflammation. Nevertheless, even without microorganism involvement, DAMPs released from dead or dying cells in response to injury or stress, are able to induce similar pro-inflammatory cytokine production from tissues, driving “sterile inflammation.”
ATP = adenosine triphosphate; DAMPs = damage-associated molecular patterns; IL-1β = interleukin-1 beta; IL-6 = interleukin 6; IL-18 = interleukin 18; LPS = lipopolysaccharide; M-CSF = macrophage colony-stimulating factor; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells; PAMPs = pathogen-associated molecular patterns; PRRs = pattern recognition receptors; S100A8/9 = (also known as calgranulins A and B, or MRP8 and MRP14 respectively) are members of the S100 multigene subfamily of cytoplasmic EF-hand Ca2+-binding proteins which are endogenous activators of Toll-like receptor 4; TNF = tumor necrosis factor.
Ischemia-Reperfusion Pathways: Oxydative Stress Injury, Metabolomic Disorders and Apoptosis
Oxidative stress is caused by increased production of reactive oxygen and nitrogen species or by depletion of protective antioxidants. Resulting oxidative products can damage DNA, promoting cell death/apoptosis and cause end-organ tissue damage. OS is present in many pathological situations, such as during reperfusion after ischemia or following toxic exposures. Whether through low regional blood flow or hypoxemia or both, ischemia is responsible for a dramatic shift in cell metabolism. The lack of oxygen to drive oxidative phosphorylation and other oxygen dependant metabolic reactions (aerobic glycolysis, fatty acid beta oxidation) results in inefficient anaerobic glycolysis as the major source of adenosine triphosphate (ATP) production and leads to ATP deficit51–54. Proteomic profiling indicate that during ischemia, metabolic key enzymes are decreased53. The resultant ATP-dependant metabolic reaction shutdown then produces deep imbalance in cellular homeostasis eventually leading to cell death53,55–57. Furthermore, any reduction in organ perfusion in terms of oxygen delivery51 can lead to organ damage by generating I/R injuries58. I/R injury is present in most clinical conditions leading to acute organ injury such as shock, hypoxemia, sepsis, cardiac arrest, trauma, burn injuries or following certain surgeries (cardiac, aortic and organ transplantation surgeries). Although ischemia-induced tissue hypoxia can lead to irreversible tissue injury if the period of ischemia is prolonged, much of the tissue damage occurs following restoration of perfusion59,60. While reperfusion can induce mitochondria to generate ATP and restore cell metabolism in less damaged tissue, it can also paradoxically exacerbate ischemia-induced inury in severely ischemic cells leading to release ROS generated by damaged mitochondria and nicotinamide adenine dinucleotide phosphate oxidase58,61–64. Proteomic profiling show that reperfusion can lead to pro-glycolytic enzyme depletion, pro-apoptotic proteome shift and mitochondrial dysfunction inducing OS65. These I/R-induced pathways can lead to cell death and organ failure (Figure 2).
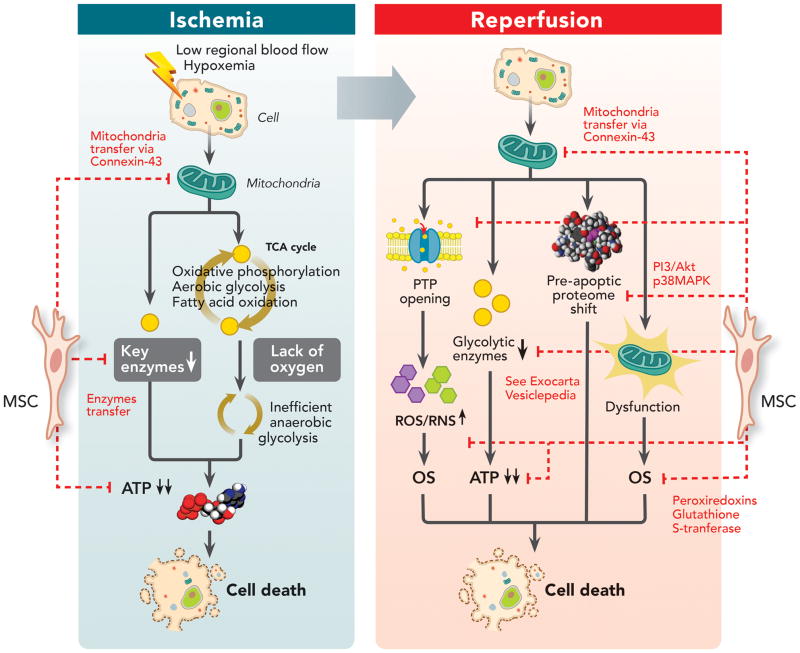
Figure 2. Impact of Mesenchymal Stem Cells on Ischemia-Reperfusion Injury Pathways.
Ischemia is a significant cause of acute organ injury that results from a decrease in regional oxygen delivery (such as low blood flow or hypoxemia), leading to inefficient anaerobic glycolysis as the major source of ATP production and ATP deficit. However, much of the tissue damage occurs during the reperfusion phase, leading to mitochondrial permeability transition pore opening, pro-glycolytic enzyme depletion, pro-apoptotic proteome shift and mitochondrial dysfunction inducing oxidative stress. MSC can decrease ischemia-reperfusion induced injury by: (1) Restoring ATP levels by possibly mitochondrial transfer through connexin-43 channels and replenishing depleted glycolytic enzymes; (2) Decreasing reactive oxygen species/reactive nitrogen species generated during oxidative stress by either preventing their release, circumventing the depletion of key enzymes or by transferring reactive oxygen species scavengers (such as peroxiredoxins and glutathione S-transferase) into injured cells; (3) And restoring proteomic alterations by activating pro-survival phosphatidylinositide 3-kinases/protein kinase B pathway via cluster of differentiation 73 or inhibiting p38 MAPK-caspase 3 pathway.
ATP = adenosine triphosphate; CD73 = cluster of differentiation 73; MAPK = mitogen-activated protein kinases; MSC = mesenchymal stem cell; OS = oxidative stress; PI3/Akt = phosphatidylinositide 3-kinases/protein kinase B; PTP = permeability transition pore; ROS = reactive oxygen species; RNS = reactive nitrogen species; TCA = tricarboxylic acid cycle.
PROPERTIES OF MESENCHYMAL STEM CELLS
Immunomodulatory Properties
MSC can modulate innate and adaptive immune cells, by enhancing anti-inflammatory pathways in the injured organ milieu66–68. This immunomodulation is mediated by cell-contact-dependant and independant mechanisms through the release of soluble factors such as tumor necrosis factor-stimulated gene 669, prostaglandin E270, interleukin (IL)-1070,71, IL-1 receptor antagonist72, transforming growth factor (TGF)-β73, hepatocyte growth factor73 or indolamine 2,3-dioxygenase67. Both decrease in pro-inflammatory mediators (IL-1β, tumor necrosis factor (TNF)-α, interferon-γ, IL-6) and increase in anti-inflammatory cytokines (IL-10, basic fibroblast growth factor, TGF-α, TGF-β) have been also pointed out as a key factor in preventing cell damage in acute kidney15,26 and liver30,74–76 injury models. Similar findings have been reported in acute stroke77 and sepsis78,79 animal models (Figure 3A).
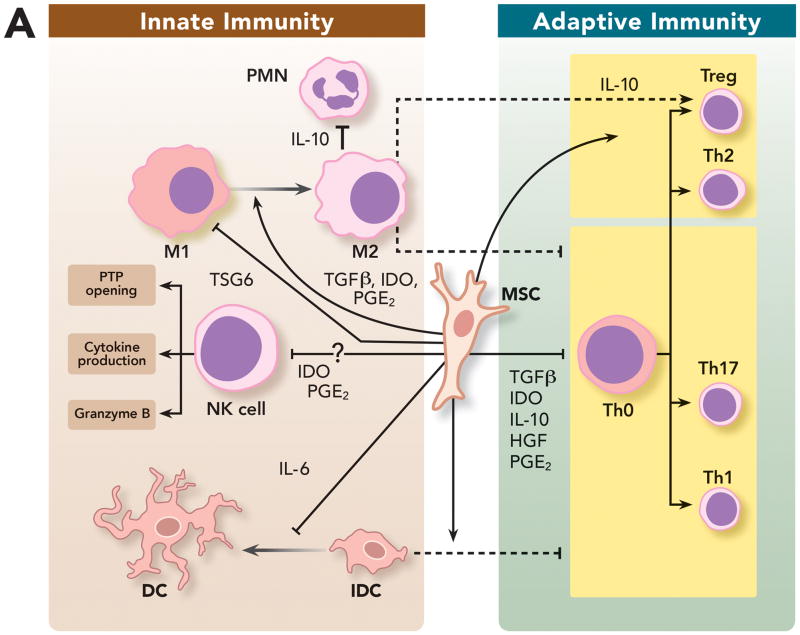
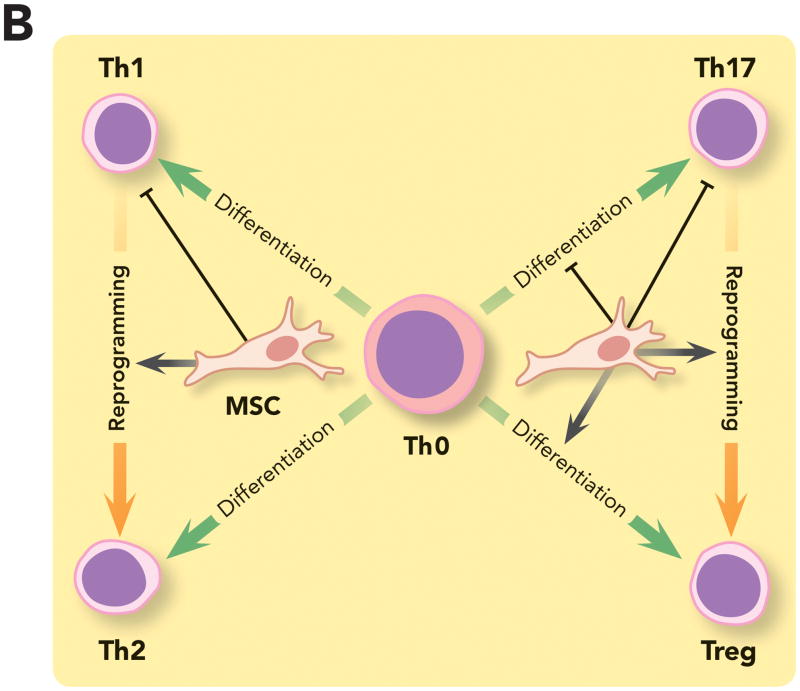
Figure 3. Immunomodulatory Properties of Mesenchymal Stem Cells on Innate and Adaptive Immunity.
(A) MSC can modulate innate and adaptive immune cells by: (1) Promoting repolarization of macrophages from type 1 to type 2 phenotype characterized by high levels of interleukin-10 secretion, which can block polymorphonuclear neutrophil influx into the injured tissue and prevent further damage; (2) Interfering with dendritic cells differentiation, maturation and function, skewing them toward a regulatory phenotype and decreasing their capacity to induce activation of T cells; (3) And impairing natural killer cells cytotoxic activity, cytokine production and granzyme B release. However, recent studies suggest that the complex interplay between MSC and natural killer cells may depend on the surrounding milieu. (B) MSC can suppress T cell activation and proliferation and also decrease their response by shifting them from a T helper 1 to a T helper 2 immune response. MSC have been shown to (1) inhibit the differentiation of naive T cells into T helper 17 cells and prevent the secretion of pro-inflammatory cytokines by T helper 17 cells; (2) And promote induction of immunosuppressive T regulatory cells in part by reprogramming T helper 17 cells into T regulatory cells.
DC = dendritic cell; HGF = hepatocyte growth factor; iDC = immature dendritic cell; IDO = indolamine 2,3-dioxygenase; IL-6 = interleukin-6; IL-10 = interleukin-10; M1 = type 1 phenotype; M2 = type 2 phenotype; MSC = mesenchymal stem cell; NK cell = natural killer cell; PGE2 = prostaglandin E2; PMN = polymorphonuclear neutrophil; TGFβ = transforming growth factor beta; Th = T helpers cell; Treg = T regulatory cell; TSG6 = tumor necrosis factor-stimulated gene 6.
Human MSC promote repolarization of monocytes and/or macrophages from a type 1 (pro-inflammatory) to a type 2 (anti-inflammatory) monocyte phenotype characterized by high levels of IL-10 secretion, increased phagocytosis and low levels of TNF-α and interferon-γ production and major histocompatibility class II expression80–82. This ability of MSC to reprogram monocytes/macrophages has been demonstrated in vivo in different models of sepsis70,83–85, endotoxin86 or live E.coli bacteria-induced ALI87,88, ischemia89 and regenerative medicine82,90. Often in these injury models, MSC reprogrammed type 2 monocytes produced large quantities of IL-10, which blocked polymorphonuclear neutrophil influx into the injured tissue and prevented further damage (Figures 3A). However, in a mouse model of TBI, intracerebral administred MSC modulated the inflammatory response through decreasing the phagocytic capability of microglia macrophages91. In this specific context, the reduction of phagocytosis by macrophages was beneficial, leading to better outcomes. These findings revealed the complexity of the crosstalk between MSC and macrophages, that may be organ specific and influenced by the injury milieu.
MSC can interfere with dendritic cells differentiation, maturation and function, skewing them toward a regulatory phenotype92,93. Dentritic cells generated in the presence of MSC have decreased capacity to induce activation of T cells, and exhibit an altered cytokine production pattern with lower pro-inflammatory and higher anti-inflammatory cytokines66,92 (Figure 3A).
MSC also modulate natural killer cells, which are involved in both the elimination of virus-infected and damaged cells and the secretion of an array of pro-inflammatory cytokines such as interferon-γ. Several studies clearly show that MSC, when co-cultured with natural killer cells, impair their cytotoxic activity, cytokine production and granzyme B release94–96 (Figure 3A). However, other studies have shown that MSC could enhance their pro-inflammatory phenotype depending on the culture conditions. Thus, the complex interplay between MSC and natural killer cells could result either in a pro-inflammatory or an anti-inflammatory phenotype depending on the type of the activation state of both cells and on the surrounding milieu67.
In addition, MSC are able to suppress T cell activation and proliferation and decrease their response by shifting them from a T helper (Th)1 to a Th2 immune phenotype72,73,97,98. MSC have been shown to 1) inhibit the differentiation of naive T cells into Th17 cells99–101, 2) inhibit secretion of pro-inflammatory cytokines by differentiated Th17 cells, 3) promote induction of immunosuppresive FoxP3+ T-regulatory cells100,102, and 4) drive reprogramming of Th17 cells into FoxP3+ T-regulatory cells100 (Figure 3B). MSC also potentially inhibit cytotoxic effect of antigen-primed cytotoxic T cells98 and induce T cell anergy67,73,103. This T regulatory- skewed response has been also demonstrated in vivo. In an ALI model, Sun et al. showed that MSC could upregulate T-regulatory cells, reducing some key Th1 cytokines (interferon-γ, TNF-α, macrophage inflammatory protein-2) and increasing Th2 cytokines (IL-10). Others have also demonstrated that MSC decreased pro-inflammatory cytokines/chemokines such as macrophage inflammatory protein-1, B-lymphocyte chemoattractant, and IL-12, with subsequent decrease in Th cells104.
Overall, an emerging body of data demonstrates at multiple levels the impact of MSC upon key cells involved in the continuum between innate and adaptive immunity, modulating inflammation in acute organ injury.
Antimicrobial Properties
Studies using bacteria-induced acute organ injury models demonstrated that MSC could exert direct and indirect antimicrobial properties. In E.coli pneumonia in mice, we demonstrated that MSC secreted antibacterial proteins/peptides such as LL-37105 and lipocalin-287, leading to improved bacterial clearance. Other anti-bacterial mechanisms of MSC include tryptophan catabolism by indolamine 2,3-dioxygenase 106 or increased pathogen phagocytosis which inhibit overall bacterial growth79,107–109. Using different in vivo and ex vivo models of sepsis or pneumonia, MSC were found to increase phagocytosis of bacteria by macrophages by switching from a type 1 to type 2 monocyte phenotype79,87,88,110. In a mouse model of Pseudomonas aeruginosa-induced peritonitis, Krasnodembskaya et al. demonstrated that MSC reduced the number of colony-forming units of Pseudomonas aeruginosa in the blood by increasing the monocyte phagocytic potency110. The authors highlighted two potential underlying mechanisms: 1) the upregulation of phagocytosis receptor CD11b on monocytes and 2) the increase in CD163 and CD206-positive activated monocytes/macrophages in the spleen110. In a cecal ligation and puncture mice model of sepsis, Nemeth et al. showed a decrease in blood bacteria counts in the MSC treated group. The authors speculated that this increase in blood bacteria clearance could be explained by IL-10-mediated neutrophil retention within the vascular compartment70. Recently, toll like receptor 3-triggered human MSC were shown to promote polymorphonuclear neutrophil activity, viability and improve its respiratory burst, increasing ROS release which is bactericidal108 (Figure 4).
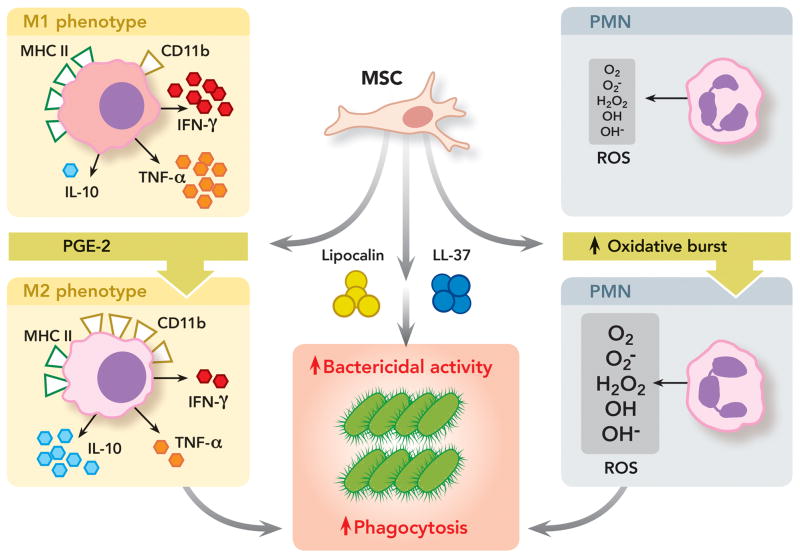
Figure 4. Antimicrobial Properties of Mesenchymal Stem Cells.
MSC can exert direct and indirect anti-microbial activity by: (1) Secreting anti-bacterial proteins/peptides such as cathelicidin-related antimicrobial peptides and lipocalin-2, leading to improved bacterial clearance; (2) Promoting repolarization of monocytes and/or macrophages from a pro-inflammatory to an anti-inflammatory phenotype characterized by high levels of interleukin-10 secretion and phagocytosis receptor cluster of differentiation 11b expression, low levels of tumor necrosis factor-α and interferon-γ production and major histocompatibility class II expression. Type 2 monocytes-macrophages have increased phagocytosis capability against bacteria; (3) And promoting neutrophil activity and viability with improved respiratory burst and increased reactive oxygen species release, which are bactericidal.
CD11b = cluster of differentiation molecule 11b; H2O2 = hydrogen peroxide; IFN-γ = interferon gamma; IL-10 = interleukin-10; LL-37 = Cathelicidin-related antimicrobial peptides; M1 = type 1 phenotype; M2 = type 2 phenotype; MHC II = major histocompatibility class II; MSC = mesenchymal stem cell; O2− = superoxide anion radical; O2 = oxygen; OH = hydroxide; OH− = hydroxyl radical; PGE2 = prostaglandin E2; PMN = polymorphonuclear neutrophil; ROS = reactive oxygen species; TNF-α = tumor necrosis factor alpha.
Anti-oxidative Effect
Recent studies of organ injuries involving the heart57, brain111,112, kidneys113–115 and liver116–119 demonstrated that MSC could exert an antioxidative effect leading to a decrease in the severity of organ injury56. This anti-oxidative property has been best exemplified in sepsis-induced organ failure models. In this context, authors have shown that MSC can reduce neutrophil-mediated oxidative injury in lungs, liver and kidneys70,78. This effect was primarily mediated through secretion of soluble factors, which prevent ROS accumulation through enhanced scavenging and antioxidant upregulation57,120. Interestingly, many of these studies focused on the adoptive transfer of anti-oxidant effects from exosomes by stem cells59,60,121. Similar to microvesicles, exosomes are bi-lipid membrane vesicles with a diameter < 50 nm They can carry a complex cargo of proteins, lipids, DNA, mRNA or microRNA which could be delivered into targeted cells and impact multiple cellular pathways16,122. MSC release a large quantity of exosomes in their environment upon diverse stimuli120. Both in vitro and in vivo studies have shown that MSC derived exosomes can decrease OS-induced injury by reversing the depletion of key enzymes in ROS metabolism and the resultant accumulation of toxic products from the electron transport chain59,60,65,121,123. For example, the transfer of peroxiredoxins and glutathione S-transferase by MSC derived exosomes into injured cells has been shown65. In addition, Zhou et al. recently demonstrated that the anti-oxidant effect of exosomes derived from human umbilical cord MSC in a cisplatin-induced AKI model may involve the inhibition of the p38 mitogen-activated protein kinases-caspase 3 pathway121 (Figure 2).
Metabolomics
Any potential treatment aimed at reversing the metabolomic disorders in acute organ injury should ideally overcome ATP deficit, compensate the proteomic alteration and repair the mitochondrial electron transport chain. Several sudies demonstrate some direct beneficial effects from MSC on metabolomics disorders. Beiral et al. demonstrated in a rat kidney I/R model that MSC could restore ATP synthesis124. In addition, proteomic and genomic profiling of MSC-derived exosomes (Exocarta125, Vesiclepedia126) showed that they contain key enzymes involved in the ATP-generating stage of glycolysis so that they could potentially restore proteomic alterations in injured tissue. Lai et al. showed in injured rat cardiomyoblast, that MSC-derived exosomes increased intracellular ATP levels by 75 and 55% after 15 and 30 minutes respectively60. In an ex vivo myocardial I/R injury model, MSC-derived exosomes increased ATP production in reperfused myocardium59. And in a model of lipopolysaccharide-induced ALI, Islam et al. demonstrated that mitochondrial transfer through connexin-43 may be involved in the restoration of ATP levels127 (Figure 2).
Pro-mitotic/Anti-apoptotic Effects
Multiple groups have studied the underlying mechanisms of MSC anti-apoptotic effects in various organ injury models. Two main mechanisms have been proposed. 1) MSC secretion of growth factors. In animal models of AKI27,128–131, acute stroke132–135 and traumatic brain injury91,136,137, a wide array of secreted growth factors such as insuline growth factor-1128,131,133,134, vascular endothelial growth factor27,130,135, hepatocyte growth factor129, brain-derived neutrophic factor91,132,136,137, nerve growth factor91,133,134,136,137 and neurotrophin-391,136,137, have been linked to the pro-regenerative effects mediated by MSC. 2) And increased expression of pro-regenerative/anti-apoptotic genes and/or possibly mRNA transfer to injured cells by MSC or MSC derived microvesicles or exosomes. In ALF, MSC induced over-expression of genes involved in hepatocellular regeneration such as hepatocyte growth factor, epidermal growth factor, transforming growth factor-β, stem cell factor and tissue metalloproteinase 374. In AKI, Bruno et al. showed that MSC released microvesicles could transfer mRNAs or microRNAs involved in cell proliferation to damaged renal cells18,19,138. In a glycerol-induced AKI model in immunocompromised mice, MSC microvesicles had a proliferative effect in tubular epithelial cells 19. RNAse pre-treated microvesicles lost their therapeutic potencies, suggesting a RNA-dependent effect. The underlying mechanisms were mainly attributed to a microvesicle induced up-regulation of anti-apoptotic genes (Bcl-xL, Bcl2) and to a down-regulation of apoptotic genes (caspase-1, caspase-8, lymphotoxin-α) in tubular epithelial cells. A similar decrease in apoptotic genes expression (caspase-3 pathway) and up-regulation of phosphorylated protein kinase B pro-survival pathway leading to new neuron generation139,140 were found in TBI treated with MSC136,141. Finally, the over expression of genes involved in the anti-apoptotic pathways (such as growth hormone and insulin growth factor-1 signaling) also played a therapeutic role in a model of sepsis treated with MSC78,79 (Figure 5).
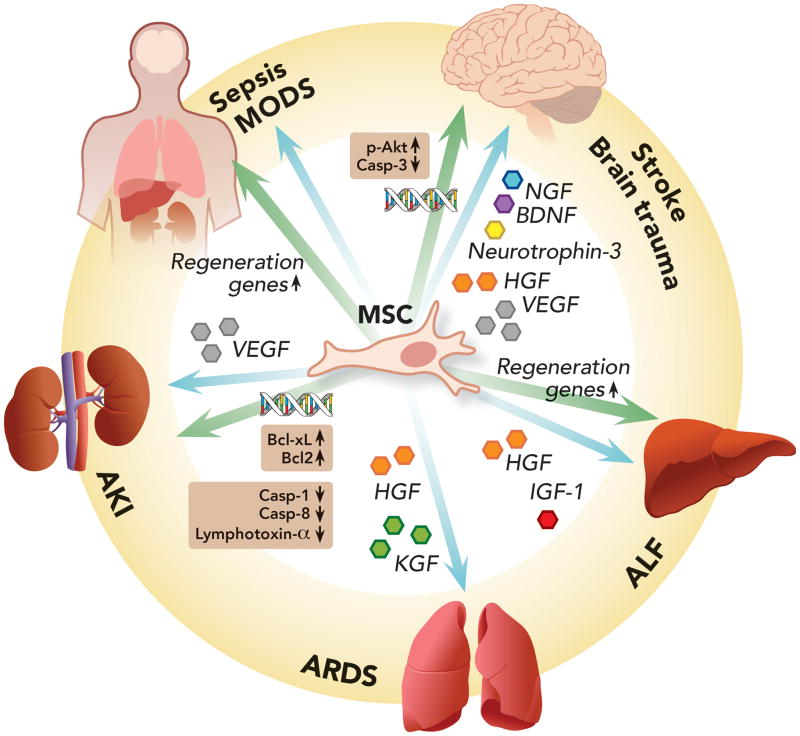
Figure 5. Pro-mitotic/Anti-apoptotic Properties of Mesenchymal Stem Cells.
Mesenchymal stem cells can exert anti-apoptotic effects in different organs through two main mechanisms: (1) Secretion of a wide array of growth factors promoting cell regeneration and tissue repair; (2) And promotion of pro-regenerative/anti-apoptotic gene expression by either inducing their transcription or transferring mRNA or microRNA involved with cell proliferation to damaged cells.
AKI = acute kidney injury; ALF = acute liver failure; ARDS = acute respiratory distress syndrome; Bcl2 = B-cell lymphoma 2; Bcl-xL = B-cell lymphoma-extra large; BDNF = brain-derived neutrophic factor; Casp-1 = caspase 1; Casp-3 = caspase 3; Casp-8 = caspase 8; HGF = hepatocyte growth factor; IGF-1 = insulin growth factor 1; KGF = keratinocyte growth factor; MODS = multiple organ dysfunction syndrome; NGF = nerve growth factor; p-Akt = phosphorylated protein kinase B; VEGF = vascular endothelial growth factor.
Ischemia-Reperfusion Injury
Several in vivo studies have pointed out the beneficial effects of MSC with respect to I/R of the heart142, lungs71,104,143–145 brain146, kidney15,147,148 and gut149,150. More specifically, studies focused in I/R-induced ALI model, showed some beneficial effects through a combination of immunomodulation71,143,145, anti-oxidant71,143,145 or anti-apoptotic143 properties. Others demonstrated that MSC could increase the activity of anti-oxidant enzymes in I/R151. Interestingly in a gut I/R model, MSC reduced rat intestinal I/R injury by increasing the expression of the intestinal tight junction protein zona occludens-1 and reducing tight junction disruption by suppressing the action of TNF-α150. The proteomic alteration in I/R injury65 can be supplemented by the cellular contents of MSC-derived exosomes60,123. By replenishing depleted glycolytic enzymes, supplementing damaged cells with additional protein components of the cellular antioxidant system, and activating pro-survival phosphatidylinositide 3-kinases/phosphorylated protein kinase B pathway via cluster of differentiation 73, MSC exosomes can increase ATP level and decrease OS and cell death59 (Figure 2).
Given the diversity of mechanisms involved in the potential therapeutic effect of MSC in various organ injuries (Figure 6), we will review the current literature underlying the rationale for the use of MSC in ARDS, AKI, ALF, acute brain injury and sepsis.
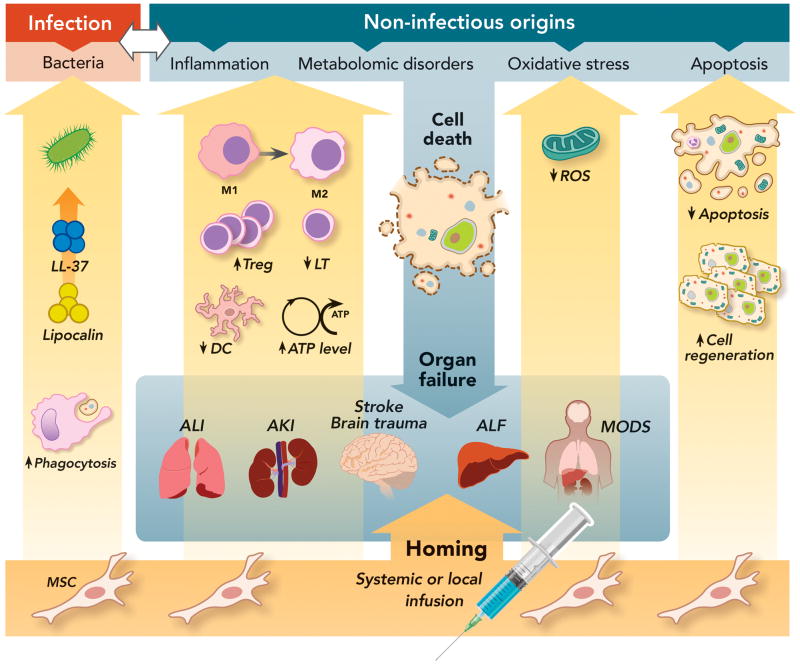
Figure 6. Therapeutic Effects of Mesenchymal Stem Cells on Multiple Signaling Pathways Leading to Acute Organ Injury.
Both infection and non-infectious causes can trigger organ damage through the activation of diverse cell signaling pathways such as inflammation, metabolomic disorders, oxidative stress and apoptosis, eventually leading to organ injury and failure. MSC can exert pleiotropic therapeutic effects through the secretion of a wide array of soluble factors, which lead to: (1) Anti-microbial activity with secretion of cathelicidin-related antimicrobial peptides and Lipocalin and increased phagocytosis by monocytes and macrophages; (2) Anti-Inflammatory activity by switching the phenotype of monocytes or macrophages from a M1 to a M2 phenotype, which is characterized by an enhanced phagocytosis capacity and increased anti-inflammatory cytokine secretion; Inhibition of T-lymphocyte and dendritic cell activation and increase in T regulatory cells; (3) Increase in ATP cellular levels and decrease in ROS accumulation, reducing oxidative stress; (4) And switch from a pro-apoptotic to a pro-mitotic phenotype.
AKI = acute kidney injury; ALF = acute liver failure; ALI = acute lung injury; DC = dendritic cell; LL-37 = cathelicidin-related antimicrobial peptides; LT = T lymphocyte; M1 = type 1 monocyte/macrophage; MODS = multiple organ dysfunction syndrome; MSC = mesenchymal stem cells; ROS = reactive oxygen species; Treg = T regulatory cell.
MESENCHYMAL STEM CELLS IN ACUTE RESPIRATORY DISTRESS SYNDROME
ARDS is major cause of acute respiratory failure in critically ill patients. Despite improvements in supportive care, mortality associated with ARDS remains high, up to 40%, depending on the etiology152,153. Current treatments remain focused on supportive care such as lung protective ventilation, fluid conservative strategy and prone positioning154–156. No pharmacological therapies from pre-clinical models have yet been translated to effective clinical treatment options. Past studies showed that focusing on either anti-inflammatory or anti-fibrotic pathways were too simplistic as a therapy. Pathophysiology of ARDS involves complex crosstalks between the immune system and the alveolocapillary barrier leading to an excess of pro-inflammatory Th1 polarized responses, increase in lung protein permeability and formation of pulmonary edema. Pulmonary edema results in impaired gas exchange and eventual hypoxemia153.
1. Mesenchymal Stem Cells Lung Specific Mechanism of Action
Aside from their immunomodulatory, anti-bacterial, anti-oxidant and anti-I/R injury properties, MSC can also display some lung specific functional effects.
Alveolar Fluid Clearance
ARDS is characterized by impaired alveolar fluid clearance, i.e. inability to decrease pulmonary edema, induced by excessive inflammation in the injured alveolar milieu157. Several studies have demonstrated that MSC secrete keratinocyte growth factor, which increases alveolar fluid clearance by upregulating key epithelial sodium channel gene expression and Na-K-ATPase activity, or by increasing trafficking of epithelial sodium channel proteins to the apical membrane158. These keratinocyte growth factor mediated effects were shown in animal models83,159,160 as well as in an ex vivo perfused human88,161 preparation. Most recently, we demonstrated that MSC-derived microvesicles could protect against lipopolysaccharide-induced ALI through delivery of the keratinocyte growth factor mRNA with subsequent expression of the protein in the injured alveolus21.
Lung Permeability
In ARDS, the injured lung capillary endothelium leads to protein leakage from the vascular bed into the alveolar space. This phenomenon aggravates the ability of the lung epithelium to reduce pulmonary edema. Recently, MSC have been shown to secrete angiopoietin-1, a soluble factor capable of reducing endothelial permeability through enhanced endothelial survival and vascular stabilization, through the preservation of cell adhesion molecules and cell junctions and the prevention of actin “stress fiber” formation162. We and others have demonstrated that angiopoietin-1 secreted by human MSC was essential to prevent an increase in lung protein permeability163–165.
2. Pre-clinical Acute Lung Injury Studies
A recent review reported the benefits of administering MSC in pre-clinical small animal lung injury models6. More than half of experimental studies concerned intra-tracheal lipopolysaccharide-induced ALI in rodents and intra-tracheal administration of MSC. Whereas, the intravenous route of deliver of MSC was prefered in bleomycin-induced, I/R or ventilator-induced lung injury. The beneficial effects of MSC were also been reported in bacterial-induced ALI models84,87,88,105, such as pneumonia, peritonitis and sepsis from cecal ligation and puncture, highlighting the antibacterial properties of MSC. Gupta et al. found a survival advantage from syngeneic mouse MSC in an E.coli bacterial pneumonia-induced ALI model87. Lee et al. also showed beneficial effects of MSC in E.coli bacterial-induced ALI in an ex vivo perfused human lung preparation88. Although this model excluded other systemic organs, which may generate an inflammatory response, it replicated many of the injury patterns seen in patients with ARDS. Aside from bone marrow, other sources of MSC have been studied. Human umbilical cord-derived MSC is currently being investigated in clinical trials, due to their accessibility (from the placenta), lack of ethical concerns and their faster population doubling time84,102,166. Although promising, adipose derived human MSC104,145, require further studies to clarify their potential therapeutic effects in ALI.
In ALI models in rodents, the mean dose of MSC typically was 20–30 × 106 cells/kg, and the timing of administration was within 6 hours following ALI. The maximum therapeutic effect of MSC was found 2 to 3 days following administration. One study using an ALI model in mice with a large dose of MSC (889 × 106 cells/kg) showed a delayed effect on day 28167. However, no dose response study has been yet published. Thus, it is still unclear whether there is a therapeutic ceiling or if a second dose of MSC is needed, especially during the resolution phase of ALI. Aside from the role of paracrine soluble factors, the role of MSC microvesicles or exosomes has been recently studied. Lee et al. found that murine MSC derived exosomes could prevent hypoxic pulmonary hypertension by reducing vascular remodeling, pulmonary influx of macrophages, and pro-inflammatory and proliferative mediators20. More recently, we demonstrated that human MSC microvesicles can reduce the severity of E.coli endotoxin-induced ALI in mice through the transfer of keratinocyte growth factor mRNA to the injured lung epithelium21. These recent findings shed first lights on a new stem cell-free therapy in ALI, circumventing caveats of MSC use such as genetic instability and potential malignant transformation.
3. Clinical Trials
Despite these multiple encouraging pre-clinical studies, translation into human clinical trials remains limited. Currently, two phase I/II clinical trials are underway (See table, Supplemental Digital Content 1, which lists the ongoing clinical trials). One Phase I/II study (NCT01775774) uses human bone marrow-derived MSC (BM-MSC) in ARDS patients. The aim of this multi-center, single group assignment study is to assess the safety and then the feasability of using escalating intravenous doses (1 to 10 × 106 cells/kg) of allogeneic human BM-MSC in patients with moderate or severe ARDS. Another randomized, double blind, placebo-controlled trial (NCT01902082), targets not only safety but also efficacy outcomes, using allogeneic adipose-derived MSC. In both studies, inclusion criteria are similar, the intravenous route is used and MSC therapeutic doses vary from 1 × 106 to 10 × 106 cells/kg. Both trials are still recruiting.
MESENCHYMAL STEM CELLS IN ACUTE KIDNEY INJURY
AKI is a clinical syndrome characterized by rapid loss of excretory function leading to accumulation of products of nitrogen metabolism and metabolic acids, increased potassium and phosphate serum concentration and decreased urine output. Incidence varies from 5000 cases per million people per year for non-dialysis-requiring AKI to 295 cases per million people per year for dialysis-requiring disease168. In critically ill patients, the AKI prevalence reachs 40% at admission to the ICU if sepsis is present169 and 60% during ICU stay170. No pharmacological therapies are available. Treatment is essentially supportive, including renal replacement therapy if needed. Mortality from AKI ranges from 44.7 to 53% in critically ill patients171. Most patients who survive recover their renal function ad integrum after a few weeks. However, some remain in chronic renal failure requiring definitive renal replacement therapy.
Etiology of clinical AKI is often multifactorial involving diverse triggers such as hypovolemia, ischemia, I/R, sepsis and toxic injuries. Most of the AKI seen in the ICU, occur within 72 hours from a combination of pre renal and renal injuries171. Most existing pre-clinical animal AKI models use ischemia induced by acute occlusion of the renal artery172–174. Although not wholly clinically relevant, these ischemic AKI models do imitate several activated pathways involved in AKI, such as coagulation system activation175, leukocyte infiltration176, endothelium injury177 with over-expression of adhesion molecules178, cytokines release179, Toll-Like Receptors induction180, intrarenal vasoconstriction pathway and apoptosis181. In addition, in septic and hepatorenal pre-clinical AKI models, triggered by a decrease in blood pressure secondary to a systemic or hepatosplanchnic vasodilation182, the renal sympathetic system183, the renin-angiotensin-aldosterone system184 and the tubuloglomerular feedback system184 are all activated. Depending on the intensity and the period of time of their association, these different factors contribute to a continuum ranging from tubular injuries to apoptosis/necrosis to renal failure171.
1. Pre-clinical Acute Kidney Injury Studies
MSC therapy is effective in reducing AKI in diverse experimental models including those induced by cisplatin19,25,38,128,130,185–190, glycerol19,38 and I/R injury14,15,26,129,191–193. Systemic route of administration is widely used via intravenous or intra-peritoneal injection, except for I/R model where MSC are infused intra-arterial14,15,26,129,191–193. Delivered doses range from 8 × 106 187 to 2 × 108 186 cells/kg. In cisplatin-induced AKI models, MSC prevented renal function impairment, improved renal function and preserved tubular integrity25,128, leading to an increase in the survival rate of mice following cisplatin injection188–190 compared to saline control. Interestingly, Morigi et al. found that, in the cisplatin-AKI model, cord blood derived MSC189 were more effective than BM-MSC188 in terms of renal function improvement and survival, whereas MSC derived from human adipose tissue did not improve renal function194. In addition, mice treated by human adipose tissue-derived MSC showed some tubular alterations such as casts, nuclear fragmentations and necrosis. However, because these histological alterations are similar to those observed in a cisplatin-induced AKI, these lesions could not be interpreted as being harmful effects of MSC.
In a lethal AKI model induced by cisplatin administration, Bruno et al. showed MSC microvesicles could enhance survival in immunnocompromised mice18. In this model, a single administration of microvesicles increased survival rate and ameliorated renal failure but did not prevent chronic tubular injury. However, multiple injections of microvesicles not only improved survival but also normalized histology and renal function at day 21.
2. Clinical Trials
Despite strong pre-clinical evidence of the therapeutic effect of MSC in AKI, only three Phase I/II clinical trials have been carried out12,195,196 (See table, Supplemental Digital Content 1, which lists the ongoing clinical trials). One on-going trial (NCT00733876)12,196 aims to investigate safety and efficacy of allogeneic MSC in preventing and treating AKI following on-pump coronary artery bypass surgery, using suprarenal aortic MSC infusion. Patients at high risk of post-operative acute renal failure patients are included. Preliminary data from the trial indicates that MSC infusion is safe and feasible. Moreover, MSC infusion prevented any post-operative renal failure (0% versus 20% AKI incidence compared to case control) and reduced by 40% the length of hospital stay and readmission rates12,196. A double-blind, placebo controlled, multicenter phase II trial is planned by the same investigators. Another clinical trial used allogeneic human BM-MSC in a multi-center, double-blind, placebo-controlled phase II study (NCT01602328) in patients with post cardio-pulmonary bypass-induced AKI. Safety and also efficacy outcomes such as time to kidney recovery and dialysis were the primary aims. The third ongoing pilot study (NCT01275612)195 investigates the safety and the feasability of systemic infusion of donor ex vivo-expanded MSC in cisplatin-induced acute renal failure in chemotherapy treated patients with solid organ cancer. Preliminary data from these clinical trials are pending.
MESENCHYAML STEM CELLS IN ACUTE LIVER FAILURE
ALF still remains a leading cause of death in 30% of the cases7. Principal etiologies include acetaminophen-induced injury, idiosyncratic drug induced liver injury, viral hepatitis, autoimmune hepatitis, Budd-Chiari syndrome and Wilson disease. Up to 15% of the etiology of ALF are indeterminate. Depending on the cause, spontaneous recovery may vary from 30 to 60%7. However, supportive therapies in ALF are dramatically limited and liver transplantation remains the gold standard for treating end-stage liver failure7,197.
In ALF, innate immunity with its resultant inflammatory cascade is activated. Uncontrolled hepatic inflammation with clinically high serum levels of pro-inflammatory cytokines such as IL-1, TNF-α, IL-6, IL-8 have been reported198–200 with resultant hepatic cytotoxicity201. Necrosis and/or apoptosis may also take an important part in the loss of hepatic function, overwhelming hepatocyte regeneration197. I/R and OS injuries can also take place in different causes of ALF such as toxic, post hepatectomy or post transplantation injury. The prognosis of ALF is directly linked to liver regeneration, which in 40% of the cases can overcome the hepatocyte destruction.
1. Pre-clinical Acute Liver Failure Studies
To circumvent organ donnor shortage, replacing injured hepatocytes by stem cells initially appeared as the main aim of liver-oriented cell-based therapy. Although several studies showed that MSC can transdifferenciate towards a hepatocyte phenotype in vitro and in vivo202, the beneficial effects of MSC are more complex, encompassing regenerative203–207, immunoregulatory206–208 and anti-OS injury117 pathways.
Most preclinical studies using MSC used mice and rats with carbon tetrachloride30,76,209–212, thioacetamide118, D-galactosamine29,74,75,213,214 or I/R-induced liver injury116,215,216. However, two studies used D-galactosamine induced fulminant hepatic failure in pigs29,213. Therapeutic dose ranged from 2 to 10 × 106 cells/kg217. Most of the studies used intravenous MSC administration, but others chose the intra-portal route29,215 aiming at circumventing trapping in the pulmonary circulation215. Overall, MSC decreased the severity of histological liver injury74,75,210,213–215, improved liver function75,210–212,215 and finally enhanced survival29,74,75,210,213–215. In contrast, Boeykens et al. did not find any benficial effects of intraportally administrated MSC in terms of improved liver recovery218. However, the authors used MSC in a complex liver injury model, combining a partial hepatectomy in a previously steatotic liver which may not be applicable to ALF. Regardless all these promising findings, no clinical trial has been carried out in this field.
MESENCHYMAL STEM CELLS IN ACUTE BRAIN INJURY STROKE
Stroke causes 15 million death worldwide every year219. In United States of America, it remains the leading cause of disability and the third leading cause of mortality behind cardiovascular disease and cancer220. Currently, tissue plasminogen activator administration within 4.5 hours of the onset of ischemia is the only validated treatment for ischemic stroke. Alternate or complementary therapeutics are urgently needed.
In acute stroke, reduction in the oxygen and glucose supplies lead to neuronal cell death through several mechanisms including intracellular calcium movement and energetic metabolism impairment221–224. Secondary, restoration of the cerebral blood flow leads to I/R. As in the other organs, I/R injury in the brain triggers ROS production as well as pro-inflammatory pathways225,226. Microglia cells secrete pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β227,228. Taken together, all these mechanisms increase neuronal cell damage.
1. Pre-clinical Stroke Studies
Most preclinical animal studies using MSC have involved rodent, preferentially rats, in models of middle cerebral artery occlusion. Although some teams carried out a permanent occlusion model77,134,229–232, most of the studies used a transient middle cerebral artery occlusion model ranging from 90 to 120 minutes of ischemic time. Three routes of MSC administration have been investigated: intracerebral77,132,229,231–236, intracarotid237,238 and intravenous133–135,230,239–242. Time of treatment delivery after stroke varied from 2 hours236 to 1 month32. Both routes of MSC administration, intracerebral132,233–236 or intravenous243, decreased infarct size and improved neurological outcomes in rats. Either intravenous or intracarotid administration of MSC also improved behavioral outcomes244. However, it remains unclear which route, intracerebral243 or intravenous route245, is more efficatious. The MSC doses range from 4 × 105 77 to 1.2 × 108 240 cells/kg depending on the model. A relation between cell dose and efficacy have been demonstrated with both neurological outcomes240 and neurotrophic factors secretion77.
2. Clinical Trials
Based on the accumulation of these preclinical studies, clinical trials using MSC in stroke have increased dramatically. The number of clinical trials involving MSC in stroke (ischemic, hemorrhagic, acute, subacute or chronic) rose from one completed phase I study in 2009246,247 to 22 phase I/II clinical trials248. Bang et al. carried out the first phase I study for assessing feasibility and safety of intravenous administration of 108 autologous MSC in patients with severe neurological deficits due to subacute ischemic stroke246. Five patients were included in the treatment group versus 25 in the control group. Although intravenous cell infusion appeared safe and feasible, the small sample size in the treated group and the non-blinded design of this study prevented any conclusions concerning the potential therapeutic benefits of MSC on neurological outcomes. Five years later, the same authors published a randomized placebo-controlled long-term follow-up study carried out on 52 subacute ischemic stroke patients249. In this study, 16 patients were included in the intravenous MSC group. No difference was observed between groups concerning adverse events. More importantly, some of the neurological recovery scores were improved in the MSC group compared to the placebo group. Currently 9 studies are underway to investigate the effect of intravenous or intra-arterial administration of MSC in acute ischemic stroke patients (See table, Supplemental Digital Content 1, which lists the ongoing clinical trials). All are phase I/II studies except one phase III. Four of the trials use autologous whereas 5 use allogeneic MSC. Time of MSC administration ranges from 1 day to 6 weeks after the onset of clinical signs of stroke. The therapeutic dose ranges from 1 to 2.5 × 106 cells/kg. Primary outcomes are safety, feasability, tolerance, improvement of functional recovery assessed by neurological scores, and size of infarct. The maximum follow-up ranges from 1 month to 24 months after MSC administration. Despite the number of clinical trials, little data is yet available to demonstrate the potential therapeutic use of MSC in stroke management. Results of on-going trials are expected soon, especially long-term safety data and the potential impact of MSC on neurological outcomes.
TRAUMATIC BRAIN INJURY
TBI remains a significant cause of morbidity, mortality and disability among patients250. After the initial trauma, multiple pathological pathways converge, generating secondary lesions and leading to increased neuronal cell death and brain damage. These different pathways include increased neurotransmitter release, ROS generation with OS injury, calcium-mediated signaling and increased apoptosis, mitochondrial dysfunction and pro-inflammatory response.
1. Pre-clinical Traumatic Brain Injury Studies
MSC can both suppress these different injury mechanisms and also express neuronal and glial markers251, although regeneration may not be a significant therapeutic mechanism. Most preclinical studies in TBI have used BM-MSC39,136,137,139,252–255, except two studies which used peripheral blood-derived141 or umbilical cord-derived MSC91. Rats were the most frequent small animal used39,137,139,141,252–256, although, a few studies with TBI have been performed in mice91. In these studies, MSC were typically given from 24 hours to 7 days following TBI and, doses varied from 6 × 106 91 to 3.2 × 108 254 cells/kg depending on the administration route, which included intravenous39,136,137,139,140,252–255,257 or intracerebral91,140,141. MSC route of administration in TBI remains controversial. Multiple studies demonstrated that in rat models of TBI, most of the MSC are initially trapped in the lungs, liver and spleen, leaving a small portion of cells, ranging from 0.0005%256 to 1.4%33, to cross the blood brain barrier to reach the cerebral parenchyma. Harting et al. showed that intravenous MSC treatment failed to improve any motor or cognitive outcomes in a rat TBI model256. Although some studies highlighted the beneficial effects of intravenous MSC in TBI39,136,137,140,253–255, most of studies were from the same experimental team. Interestingly, Mahmood et al. compared the intravenous with the intracerebral route of administration of MSC at doses of 3 × 106 and 7 × 106 cells/kg in a rat TBI model140. They found differences in terms of localization of the induced neuronal cells proliferation but none regarding neurological functional recovery. Overall, the beneficial effects of MSC have been demonstrated in terms of functional neurologic improvements from 15 to 90 days after TBI39,136,137,140,141,253–255,257. MSC are believed to migrate into the injured brain parenchyma91,141,255 with a high affinity for the periphery of the lesions253, leading to a decrease in the contusion volume measured one month after the TBI91. Possibly due to the small number of published preclinical animal studies and to the unresolved issue of optiminal route of delivery, no clinical trial using MSC in TBI have been yet carried out.
MESENCHYMAL STEM CELLS IN SEPSIS AND MULTIPLE ORGAN DYSFUNCTION SYNDROME
Despite decades of clinical trials and improvement in antibiotic and supportive care, sepsis remains a challenging life-threatening disease in critically ill patients and the leading cause of morbidity and mortality in ICU patients258. In the United States, sepsis is responsible for more than 200,000 patient deaths and utilizes US$17 billion per year259,260. Sepsis results from a complex host-pathogen interaction leading to a dysregulation of the host response in terms of inflammation and coagulation. Pro-apoptotic pathways, metabolomic disorders, OS and I/R injuries are also involved in patients treated for sepsis. Eventually, sepsis can evolve toward septic shock, MODS, and death. Currently, all clinical trials using therapeutics targetting a single specific pathway have failed to demonstrate any clinical benefits261–264 such as high dose corticoids265,266 or activated protein C267. Consequently, immunomodulatory approach using a mutli-faceted therapy is required to overcome the inflammatory imbalance. MSC is an attractive approach due to its ability to home to injured sites, mitigate the pro-inflammatory cascade, modulate multiple immune cell types, promote cell survival, protect against OS injuries and exhibit some anti-bacterial properties. In addition, another advantage of cell-based therapy in sepsis is that stem cells can potentially interact with their environment, so that they can adopt some dynamic phenotypes and secrete a variety array of soluble factors depending on the pathological context268,269.
1. Pre-clinical Sepsis Studies
In this review, we have excluded studies using endotoxin-induced injury models of sepsis and focused only on pre-clinical studies usings live bacteria. Although lipopolysaccharide represents one part of the multiple bacterial factors involved in the septic process, these models have obvious limitations270. Thus, we considered the live bacteria models more clinically relevant. The therapeutic use of MSC has been used in three different sepsis models: cecal ligation and puncture70,78,79, P. aeruginosa peritonitis110, and E.coli pneumonia86,88. The cecal ligation and puncture model is the only one that generates a polymicrobial sepsis, since the procedure exposes directly the peritoneum to the gut microbiome. Intravenous70,79,110, intraperitoneal78 and intratracheal87,88 route of MSC administration have been used. Dose of MSC ranged from 1 × 107 79 to 4 × 108 88 cells/kg. The main findings were that MSC were able to enhance bacteria clearance and attenuate septic organ injury in lungs, liver and kidneys70,78,79.
Although MSC have been extensievly studied in heart I/R injury and used in clinical trials in patients with acute myocardial infarction271, no data have been published concerning their potential therapeutic effects in sepsis-induced cardiac injury272,273. And yet, half of patients with severe sepsis and septic shock present with reversible left ventricular systolic or diastolic dysfunction274 which is associated with increased mortality. Since the main pathways involved in this sepsis-related heart injury are those encountered in inflammation and I/R injuries, it seems to be important to study MSC in this context.
Beyond their ability of organ functional improvement in sepsis-induced injury, several studies showed a significant survival advantage in mice treated with MSC in peritonitis70,78,79 or pneumonia models87. In addition, Lee et al. demonstrated a similar beneficial effect of MSC on macrophage phagocytosis and bacteria clearance in an E.coli bacterial-induced lung injury in an ex vivo human lung preparation88. Eventhough most of these studies highlighted promising therapeutic properties of MSC within the early inflammatory phase of sepsis, it is still unknown whether they could be beneficial or harmful during the later anti-inflammatory phases while immunity is impaired275. However, what makes the therpaeutic use of MSC unusual is that their phenotype can be skewed either towards a pro or anti-inflammatory side depending on the surrounding milieu268,269,276. This importance of this property of MSC needs to be studied, such as their use in the later phase of sepsis.
Possibly due to the heterogeneity of the animal septic models and the lack of data comparing MSC to the multiple therapeutics commonly used in sepsis, no clinical trial has been carried out yet.
REMAINING QUESTIONS AND LIMITATIONS IN CLINICAL USE OF MESENCHYMAL STEM CELLS
As we described previously, the dose of MSC used in the pre-clinical small animal studies are extremely large and varies substantially (from 4 × 105 to 4 × 108 cells/kg). The optimal dose remains unknown in clinical trials although the typical dose in human is 5 – 10 × 106 cell/kg per dose. Additionally, the optimal route of delivery to generate the best therapeutic effect is still largely unknown between systemic and local administration. For example, the two clinical trials (NCT02097641, NCT01902082) in ARDS use intravenous administration whereas, in bronchopulmonary dysplasia in neonates, the only clinical trial (NCT01297205) uses intra-bronchial administration.
Most injury models have shown benefits of MSC administration shortly after injury. Given that organ injury is a dynamic process over time, it is still unknown whether any beneficial effects might be found if MSC was given at a later phase such as during the resolution of injury; thus, it is unclear whether a second dose of MSC is needed for the resolution phase. Overall, the optimal dose, route and time-sequence remain to be determined.
Even though organ failure is associated with poor outcome, it remains unclear whether organ failure or the initial underlying cause of injury or both is responsible for death. Organ failure has been even seen by others as an adaptive process of the organism in response to injury. Consequently, MSC should be considered as an adjuvant therapy; treating the initial cause of injury still remain the priority. For example, MSC should be considered an adjuvant therapy to ARDS caused by bacterial pneumonia, not supplanting antibiotics or other supportive therapies.
In addition, although MSC have anti-microbial properties in pre-clinical animal models, it is still worth questioning whether an immunosuppressive therapy such as MSC is appropriate during injury from an infectious etiology. For example, recent studies suggest that MSC fail to improve outcomes in acute phase of severe influenza277. Whether this is a limitation to the murine model used needs to be studied further.
And finally, although a recent meta-anaylsis demonstrated no severe adverse outcome associated with MSC therapy278, the potential of malignant transformation of MSC or the ability of MSC to enhance pre-existing tumors still remains a serious clinical question, especially in light of the limitations of the tests available to detect cancer (i.e. computerized tomography scan).
CONCLUSION
The beneficial effects of cell-based therapy with MSC are apparent in multiple preclinical injury models involving all the organs in MODS. Attracted by signals from the injured and inflamed tissues, MSC appear to migrate to the site of damage and secrete an array of soluble factors and/or exosomes/microvesicles which suppress the injury. This review highlights the pre-clinical evidence which provided the underlying rationale for several phase I/II clinical trials in ARDS, AKI and stroke. Based on promising preliminary results, further phase II and III trials are underway, the results of which are pending. However, no clinical studies are underway for ALF, TBI, sepsis and MODS.
Some concerns still remain with MSC cell-based therapy which will need to be addressed in on-going Phase I/II clinical trials such as the long term adverse effects of systemic immune suppression, potential for ectopic tissue formation and MSC immunogenicity. Although very promising, the evidence is still unclear whether MSC cell-based therapy is superior to current therapies. We still await the results from the clinical trials.
Supplementary Material
Summary Statement.
There are currently >350 clinical trials utilizing the adult stem cell, mesenchymal stem or stromal cells. The review summarizes the underlying rationale and pre-clinical studies using mesenchymal stem cells for acute organ injury.
Acknowledgments
Dr. Monsel was funded by the International Research Grant from the Société Française d’Anesthésie-Réanimation (Paris, France). Dr. Lee was supported by the National Heart, Lung, and Blood Institute Grant HL-113022 (USA) & Hamilton Endowment Funds (UCSF Department of Anesthesiology, San Francisco, CA).
Footnotes
The authors declare no competing interests.
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Awad SS. State-of-the-art therapy for severe sepsis and multisystem organ dysfunction. Am J Surg. 2003;186:S23–30. doi: 10.1016/j.amjsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Mongardon N, Dyson A, Singer M. Is MOF an outcome parameter or a transient, adaptive state in critical illness? Curr Opin Crit Care. 2009;15:431–6. doi: 10.1097/MCC.0b013e3283307a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall J-R, Payen D Investigators SOiAIP. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 5.Uchino S, Kellum MJA, Bellomo MR, Doig MGS, Morimatsu PH, Morgera MS, Schetz MM, Tan M, Bouman M, Macedo ME, Gibney MN, Tolwani MA, Ronco MC. Acute Renal Failure in Critically Ill Patients: A Multinational, Multicenter Study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y-G, Hao Q, Monsel A, Feng X-M, Lee JW. Adult Stem Cells for Acute Lung Injury: Remaining Questions and Concerns. Respirology. 2013;18:744–56. doi: 10.1111/resp.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee WM. Recent developments in acute liver failure. Best Pract Res Clin Gastroenterol. 2012;26:3–16. doi: 10.1016/j.bpg.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 12.Tögel FE, Westenfelder C. Mesenchymal stem cells: A new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6:179–83. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- 13.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 14.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–7. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 15.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 16.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037–42. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 17.Bruno S, Bussolati B. Therapeutic effects of mesenchymal stem cells on renal ischemia-reperfusion injury: A matter of genetic transfer? Stem Cell Res Ther. 2013;4:55. doi: 10.1186/scrt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–11. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y-G, Feng X-M, Abbott J, Fang X-H, Hao Q, Monsel A, Qu J-M, Matthay MA, Lee JW. Human Mesenchymal Stem Cell Microvesicles for Treatment of E. coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells. 2014;32:116–25. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–92. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–9. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J am Soc Nephrol. 2004;15:1794–804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 26.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–35. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 27.Tögel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2009;13:2109–14. doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezzat T. Dynamic tracking of stem cells in an acute liver failure model. World J Gastroenterol. 2012;18:507–16. doi: 10.3748/wjg.v18.i6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T, Jin L, Li J, Zhou P, Hao S, Cao H, Li L. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 2012;56:1044–52. doi: 10.1002/hep.25722. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Chen L, Liu T, Zhang B, Xiang D, Wang Z, Wang Y. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012;18:1352–64. doi: 10.1089/ten.tea.2011.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park B-N, Shim W, Lee G, Bang OY, An Y-S, Yoon J-K, Ahn YH. Early distribution of intravenously injected mesenchymal stem cells in rats with acute brain trauma evaluated by 99mTc-HMPAO labeling. Nucl Med Biol. 2011;38:1175–82. doi: 10.1016/j.nucmedbio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Shen LH, Li Y, Chen J, Zacharek A, Gao Q, Kapke A, Lu M, Raginski K, Vanguri P, Smith A, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- 33.Yoon J-K, Park B-N, Shim W-Y, Shin JY, Lee G, Ahn YH. In vivo tracking of 111In-labeled bone marrow mesenchymal stem cells in acute brain trauma model. Nucl Med Biol. 2010;37:381–8. doi: 10.1016/j.nucmedbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 35.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, Sher D, Weissman S, Ferrer K, Mosca J, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–55. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 36.Wynn RF, Hart CA, Corradi-Perini C, O’Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–5. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 37.Chapel A, Bertho JM, Bensidhoum M, Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier Fdr, Mathieu E, Trompier Fo, Dudoignon N, Germain C, Mazurier C, Aigueperse J, Borneman J, Gorin NC, Gourmelon P, Thierry D. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gen Med. 2003;5:1028–38. doi: 10.1002/jgm.452. [DOI] [PubMed] [Google Scholar]
- 38.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–41. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 39.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- 40.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–41. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 43.Hung S-C, Pochampally RR, Hsu S-C, Sanchez C, Chen S-C, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji JF, He BP, Dheen ST, Tay SSW. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415–27. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- 45.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: Comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–45. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 46.Tögel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–84. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 47.Goransson V, Johnsson C, Jacobson A, Heldin P, Hallgren R, Hansell P. Renal hyaluronan accumulation and hyaluronan synthase expression after ischaemia-reperfusion injury in the rat. Nephrol Dial Transplant. 2004;19:823–30. doi: 10.1093/ndt/gfh003. [DOI] [PubMed] [Google Scholar]
- 48.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–35. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 49.Nace G, Evankovich J, Eid R, Tsung A. Dendritic cells and damage-associated molecular patterns: Endogenous danger signals linking innate and adaptive immunity. J Innate Immun. 2012;4:6–15. doi: 10.1159/000334245. [DOI] [PubMed] [Google Scholar]
- 50.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 51.Cain SM, Curtis SE. Experimental models of pathologic oxygen supply dependency. Crit Care Med. 1991;19:603–12. doi: 10.1097/00003246-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Deitch EA. Organ Failure. Ann Surg. 2005;216:117–34. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosano GMC, Fini M, Caminiti G, Barbaro G. Cardiac metabolism in myocardial ischemia. Curr Pharm Des. 2008;14:2551–62. doi: 10.2174/138161208786071317. [DOI] [PubMed] [Google Scholar]
- 54.Townsend MC, Hampton WW, Haybron DM, Schirmer WJ, Fry DE. Effective organ blood flow and bioenergy status in murine peritonitis. Surgery. 1986;100:205–13. [PubMed] [Google Scholar]
- 55.Gurusamy N, Goswami S, Malik G, Das DK. Oxidative injury induces selective rather than global inhibition of proteasomal activity. J Mol Cell Cardiol. 2008;44:419–28. doi: 10.1016/j.yjmcc.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Mohammadzadeh M, Halabian R, Gharehbaghian A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM, Roudkenar MH. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones. 2012;17:553–65. doi: 10.1007/s12192-012-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan B, Singla DK. Transplanted Induced Pluripotent Stem Cells Mitigate Oxidative Stress and Improve Cardiac Function through the Akt Cell Survival Pathway in Diabetic Cardiomyopathy. Mol Pharm. 2013;10:3425–32. doi: 10.1021/mp400258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–75. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 59.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia-reperfusion injury. Stem Cell Res. 2013;10:301–12. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Lai RC, Yeo RWY, Tan KH, Lim SK. Mesenchymal stem cell exosome ameliorates reperfusion injury through proteomic complementation. Regen Med. 2013;8:197–209. doi: 10.2217/rme.13.4. [DOI] [PubMed] [Google Scholar]
- 61.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–70. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 62.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–41. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 63.Reilly PM, Schiller HJ, Bulkley GB. Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am J Surg. 1991;161:488–503. doi: 10.1016/0002-9610(91)91120-8. [DOI] [PubMed] [Google Scholar]
- 64.Berthier S, Nguyen MV, Baillet A, Hograindleur MA, Paclet MH, Polack B, Morel F. Molecular interface of S100A8 with cytochrome b558 and NADPH oxidase activation. PLoS One. 2012;7:e40277. doi: 10.1371/journal.pone.0040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X, Arslan F, Ren Y, Adav SS, Poh KK, Sorokin V, Lee CN, de Kleijn D, Lim SK, Sze SK. Metabolic adaptation to a disruption in oxygen supply during myocardial ischemia and reperfusion is underpinned by temporal and quantitative changes in the cardiac proteome. J Proteome Res. 2012;11:2331–46. doi: 10.1021/pr201025m. [DOI] [PubMed] [Google Scholar]
- 66.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 67.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 68.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 69.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, Sullivan DE. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2:27–42. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–9. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manning E, Pham S, Li S, Vazquez-Padron RI, Mathew J, Ruiz P, Salgar SK. Interleukin-10 delivery via mesenchymal stem cells: A novel gene therapy approach to prevent lung ischemia-reperfusion injury. Hum Gene Ther. 2010;21:713–27. doi: 10.1089/hum.2009.147. [DOI] [PubMed] [Google Scholar]
- 72.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 74.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–43. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 75.Zheng YB, Zhang XH, Huang ZL, Lin CS, Lai J, Gu YR, Lin BL, Xie DY, Xie SB, Peng L, Gao ZL. Amniotic-Fluid–Derived Mesenchymal Stem Cells Overexpressing Interleukin-1 Receptor Antagonist Improve Fulminant Hepatic Failure. PLoS One. 2012;7:e41392. doi: 10.1371/journal.pone.0041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burra P, Arcidiacono D, Bizzaro D, Chioato T, Di Liddo R, Banerjee A, Cappon A, Bo P, Conconi MT, Parnigotto PP, Mirandola S, Gringeri E, Carraro A, Cillo U, Russo FP. Systemic administration of a novel human umbilical cord mesenchymal stem cells population accelerates the resolution of acute liver injury. BMC Gastroenterol. 2012;12:88. doi: 10.1186/1471-230X-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, Carroll J, Hess DC. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010:108–16. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 78.Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–39. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]
- 79.Mei SHJ, Haitsma JJ, Dos Santos CC, Deng Y, Lai PFH, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am J Respir Crit Care Med. 2010;182:1047–57. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–53. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thébaud B. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–77. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim ES, Chang YS, Choi SJ, Kim JK, Yoo HS, Ahn SY, Sung DK, Kim SY, Park YR, Park WS. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respir Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liang ZX, Sun JP, Wang P, Tian Q, Yang Z, Chen LA. Bone marrow-derived mesenchymal stem cells protect rats from endotoxin-induced acute lung injury. Chin Med J. 2011;124:2715–22. [PubMed] [Google Scholar]
- 86.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–63. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 87.Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533–9. doi: 10.1136/thoraxjnl-2011-201176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751–60. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–43. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zanier ER, Montinaro M, Vigano M, Villa P, Fumagalli S, Pischiutta F, Longhi L, Leoni ML, Rebulla P, Stocchetti N, Lazzari L, De Simoni MG. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. 2011;39:2501–10. doi: 10.1097/CCM.0b013e31822629ba. [DOI] [PubMed] [Google Scholar]
- 92.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 93.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 94.Poggi A, Prevosto C, Massaro AM, Negrini S, Urbani S, Pierri I, Saccardi R, Gobbi M, Zocchi MR. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: Role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–60. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 95.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 96.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: Evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–90. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 97.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 98.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: Functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–34. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 99.Duffy MM, Pindjakova J, Hanley SA, McCarthy C, Weidhofer GA, Sweeney EM, English K, Shaw G, Murphy JM, Barry FP, Mahon BP, Belton O, Ceredig R, Griffin MD. Mesenchymal stem cell inhibition of T-helper 17 cell- differentiation is triggered by cell-cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol. 2011;41:2840–51. doi: 10.1002/eji.201141499. [DOI] [PubMed] [Google Scholar]
- 100.Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–12. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 101.Tatara R, Ozaki K, Kikuchi Y, Hatanaka K, Oh I, Meguro A, Matsu H, Sato K, Ozawa K. Mesenchymal stromal cells inhibit Th17 but not regulatory T-cell differentiation. Cytotherapy. 2011;13:686–94. doi: 10.3109/14653249.2010.542456. [DOI] [PubMed] [Google Scholar]
- 102.Sun J, Han ZB, Liao W, Yang SG, Yang Z, Yu J, Meng L, Wu R, Han ZC. Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cell Physiol Biochem. 2011;27:587–96. doi: 10.1159/000329980. [DOI] [PubMed] [Google Scholar]
- 103.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 104.Chien MH, Bien MY, Ku CC, Chang YC, Pao HY, Yang YL, Hsiao M, Chen CL, Ho JH. Systemic human orbital fat-derived stem/stromal cell transplantation ameliorates acute inflammation in lipopolysaccharide-induced acute lung injury. Crit Care Med. 2012;40:1245–53. doi: 10.1097/CCM.0b013e31823bc89a. [DOI] [PubMed] [Google Scholar]
- 105.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells. 2010;28:2229–38. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meisel R, Brockers S, Heseler K, Degistirici O, Bülle H, Woite C, Stuhlsatz S, Schwippert W, Jäger M, Sorg R, Henschler R, Seissler J, Dilloo D, Däubener W. Human but not murine multipotent mesenchymal stromal cells exhibit broad-spectrum antimicrobial effector function mediated by indoleamine 2,3-dioxygenase. Leukemia. 2011;25:648–54. doi: 10.1038/leu.2010.310. [DOI] [PubMed] [Google Scholar]
- 107.Brandau S, Jakob M, Hemeda H, Bruderek K, Janeschik S, Bootz F, Lang S. Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J Leukoc Biol. 2010;88:1005–15. doi: 10.1189/jlb.0410207. [DOI] [PubMed] [Google Scholar]
- 108.Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, Calzetti F, Pelletier M, Pizzolo G, Krampera M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29:1001–11. doi: 10.1002/stem.651. [DOI] [PubMed] [Google Scholar]
- 109.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–62. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 110.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–13. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiu J, Li W, Feng S, Wang M, He Z. Transplantation of bone marrow-derived endothelial progenitor cells attenuates cerebral ischemia and reperfusion injury by inhibiting neuronal apoptosis, oxidative stress and nuclear factor-κB expression. Int J Mol Med. 2013;31:91–8. doi: 10.3892/ijmm.2012.1180. [DOI] [PubMed] [Google Scholar]
- 112.Whone AL, Kemp K, Sun M, Wilkins A, Scolding NJ. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res. 2012;1431:86–96. doi: 10.1016/j.brainres.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 113.Chen YT, Sun CK, Lin YC, Chang LT, Chen YL, Tsai TH, Chung SY, Chua S, Kao YH, Yen CH, Shao PL, Chang KC, Leu S, Yip HK. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu H, McTaggart SJ, Johnson DW, Gobe GC. Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy. 2012;14:162–72. doi: 10.3109/14653249.2011.613927. [DOI] [PubMed] [Google Scholar]
- 115.Zhuo W, Liao L, Xu T, Wu W, Yang S, Tan J. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol Int. 2011;86:191–6. doi: 10.1159/000319366. [DOI] [PubMed] [Google Scholar]
- 116.Jin G, Qiu G, Wu D, Hu Y, Qiao P, Fan C, Gao F. Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int J Mol Med. 2013;31:1395–401. doi: 10.3892/ijmm.2013.1340. [DOI] [PubMed] [Google Scholar]
- 117.Nyamandi VZ, Johnsen VL, Hughey CC, Hittel DS, Khan A, Newell C, Shearer J. Enhanced stem cell engraftment and modulation of hepatic reactive oxygen species production in diet-induced obesity. Obesity (Silver Spring, Md) 2014;22:721–9. doi: 10.1002/oby.20580. [DOI] [PubMed] [Google Scholar]
- 118.Quintanilha LF, Takami T, Hirose Y, Fujisawa K, Murata Y, Yamamoto N, Dos Santos Goldenberg RC, Terai S, Sakaida I. Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res. 2013 doi: 10.1111/hepr12204. [DOI] [PubMed] [Google Scholar]
- 119.Sun CK, Chang CL, Lin YC, Kao YH, Chang LT, Yen CH, Shao PL, Chen CH, Leu S, Yip HK. Systemic administration of autologous adipose-derived mesenchymal stem cells alleviates hepatic ischemia-reperfusion injury in rats. Crit Care Med. 2012;40:1279–90. doi: 10.1097/CCM.0b013e31823dae23. [DOI] [PubMed] [Google Scholar]
- 120.Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. doi: 10.3389/fphys.2012.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thebaud B, Stewart DJ. Exosomes: Cell Garbage Can, Therapeutic Carrier, or Trojan Horse? Circulation. 2012;126:2553–5. doi: 10.1161/CIRCULATIONAHA.112.146738. [DOI] [PubMed] [Google Scholar]
- 123.Lai RC, Yeo RW, Tan SS, Zhang B, Yin Y, Sze NSK, Choo A, Lim SK. In: Mesenchymal Stem Cell Exosomes: The Future MSC-Based Therapy? Mesenchymal Stem Cell Therapy. Chase LG, Vemuri MC, editors. New York: Humana Press; 2013. pp. 39–62. [Google Scholar]
- 124.Beiral HJV, Rodrigues-Ferreira C, Fernandes AM, Gonsalez SR, Mortari NC, Takiya CM, Sorenson MM, Figueiredo-Freitas C, Galina A, Vieyra A. The Impact of Stem Cells on Electron Fluxes, Proton Translocation and ATP Synthesis in Kidney Mitochondria After Ischemia/Reperfusion. Cell Transplant. 2014;23:207–20. doi: 10.3727/096368912X659862. [DOI] [PubMed] [Google Scholar]
- 125.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 126.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers E-M, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte’t Hoen ENM, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TSK, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Théry C, Valadi H, van Balkom BWM, Vázquez J, Vidal M, Wauben MHM, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–65. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J, Abbate M, Zoja C, Remuzzi G. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J Am Soc Nephrol. 2007;18:2921–8. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 129.Chen Y, Qian H, Zhu W, Zhang X, Yan Y, Ye S, Peng X, Li W, Xu W. Hepatocyte growth factor modification promotes the amelioration effects of human umbilical cord mesenchymal stem cells on rat acute kidney injury. Stem Cells Dev. 2011;20:103–13. doi: 10.1089/scd.2009.0495. [DOI] [PubMed] [Google Scholar]
- 130.Yuan L, Wu MJ, Sun HY, Xiong J, Zhang Y, Liu CY, Fu LL, Liu DM, Liu HQ, Mei CL. VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;300:F207–18. doi: 10.1152/ajprenal.00073.2010. [DOI] [PubMed] [Google Scholar]
- 131.Tomasoni S, Longaretti L, Rota C, Morigi M, Conti S, Gotti E, Capelli C, Introna M, Remuzzi G, Benigni A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013;22:772–80. doi: 10.1089/scd.2012.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther. 2004;9:189–97. doi: 10.1016/j.ymthe.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 133.Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 134.Zhang J, Li Y, Chen J, Yang M, Katakowski M, Lu M, Chopp M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004;1030:19–27. doi: 10.1016/j.brainres.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 135.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 136.Kim H-J, Lee J-H, Kim S-H. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: Secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27:131–8. doi: 10.1089/neu.2008.0818. [DOI] [PubMed] [Google Scholar]
- 137.Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33–9. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- 138.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci. 2012;124:165–76. doi: 10.1042/CS20120226. [DOI] [PubMed] [Google Scholar]
- 140.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–93. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 141.Nichols JE, Niles JA, DeWitt D, Prough D, Parsley M, Vega S, Cantu A, Lee E, Cortiella J. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+CXCR4+ mesenchymal stem cells: Development of autologous cell-based therapeutics for traumatic brain injury. Stem Cell Res Ther. 2013;4:3. doi: 10.1186/scrt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang M, Tsai BM, Crisostomo PR, Meldrum DR. Pretreatment with adult progenitor cells improves recovery and decreases native myocardial proinflammatory signaling after ischemia. Shock. 2006;25:454–9. doi: 10.1097/01.shk.0000209536.68682.90. [DOI] [PubMed] [Google Scholar]
- 143.Chen S, Chen L, Wu X, Lin J, Fang J, Chen X, Wei S, Xu J, Gao Q, Kang M. Ischemia postconditioning and mesenchymal stem cells engraftment synergistically attenuate ischemia reperfusion-induced lung injury in rats. J Surg Res. 2012;178:81–91. doi: 10.1016/j.jss.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 144.Pati S, Gerber MH, Menge TD, Wataha KA, Zhao Y, Baumgartner JA, Zhao J, Letourneau PA, Huby MP, Baer LA, Salsbury JR, Kozar RA, Wade CE, Walker PA, Dash PK, Cox CS, Doursout MF, Holcomb JB. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6:e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun CK, Yen CH, Lin YC, Tsai TH, Chang LT, Kao YH, Chua S, Fu M, Ko SF, Leu S, Yip HK. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med. 2011;9:118. doi: 10.1186/1479-5876-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112:42–9. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Gonçalves GM, Cenedeze MA, Wang PMH, Teixeira VPA, Reis MA, Pacheco-Silva A, Câmara NOS. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–82. doi: 10.1016/j.intimp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 149.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127–34. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 150.Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-α-regulated mechanism. World J Gastroenterol. 2013;19:3583–95. doi: 10.3748/wjg.v19.i23.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhuo W, Liao L, Fu Y, Xu T, Wu W, Yang S, Tan J. Efficiency of Endovenous Versus Arterial Administration of Mesenchymal Stem Cells for Ischemia-Reperfusion–Induced Renal Dysfunction in Rats. Transplant Proc. 2013;45:503–10. doi: 10.1016/j.transproceed.2012.07.162. [DOI] [PubMed] [Google Scholar]
- 152.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 153.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 154.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 155.National Heart L, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Hite RD, Harabin AL Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 156.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, Group PS. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 157.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: Recent progress. Am J Respir Cell Mol Biol. 2006;35:10–9. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guery BP, Mason CM, Dobard EP, Beaucaire G, Summer WR, Nelson S. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am J Respir Crit Care Med. 1997;155:1777–84. doi: 10.1164/ajrccm.155.5.9154891. [DOI] [PubMed] [Google Scholar]
- 159.Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G, Bonnet D, Janes SM. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One. 2009;4:e8013. doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Curley GF, Hayes M, Ansari B, Shaw G, Ryan A, Barry F, O’Brien T, O’Toole D, Laffey JG. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax. 2012;67:496–501. doi: 10.1136/thoraxjnl-2011-201059. [DOI] [PubMed] [Google Scholar]
- 161.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–62. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–7. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 163.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–22. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mei SHJ, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu J, Qu J, Cao L, Sai Y, Chen C, He L, Yu L. Mesenchymal stem cell-based angiopoietin-1 gene therapy for acute lung injury induced by lipopolysaccharide in mice. J Pathol. 2008;214:472–81. doi: 10.1002/path.2302. [DOI] [PubMed] [Google Scholar]
- 166.Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflam (Lond) 2012;9:33. doi: 10.1186/1476-9255-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Prota LFM, Lassance RM, Maron-Gutierrez T, Castiglione RC, Garcia CSB, Santana MCE, Souza-Menezes J, Abreu SC, Samoto V, Santiago MF, Capelozzi VL, Takiya CM, Rocco PRM, Morales MM. Bone marrow mononuclear cell therapy led to alveolar-capillary membrane repair, improving lung mechanics in endotoxin-induced acute lung injury. Cell Transplant. 2010;19:965–71. doi: 10.3727/096368910X506845. [DOI] [PubMed] [Google Scholar]
- 168.Hsu C-Y, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–12. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bagshaw SM, George C, Bellomo R, Committee ADM. Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hoste EAJ, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–66. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 172.Heyman SN, Lieberthal W, Rogiers P, Bonventre JV. Animal models of acute tubular necrosis. Curr Opin Crit Care. 2002;8:526–34. doi: 10.1097/00075198-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 173.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: Biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77:9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 174.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, Dhawan R. Animal models of acute renal failure. Pharmacol Rep. 2012;64:31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 175.Thuillier R, Favreau F, Celhay O, Macchi L, Milin S, Hauet T. Thrombin inhibition during kidney ischemia-reperfusion reduces chronic graft inflammation and tubular atrophy. Transplantation. 2010;90:612–21. doi: 10.1097/tp.0b013e3181d72117. [DOI] [PubMed] [Google Scholar]
- 176.Versteilen AMG, Blaauw N, Di Maggio F, Groeneveld ABJ, Sipkema P, Musters RJP, Tangelder GJ. Rho-Kinase inhibition reduces early microvascular leukocyte accumulation in the rat kidney following ischemia-reperfusion injury: Roles of nitric oxide and blood flow. Nephron Nephron Exp Nephrol. 2011;118:e79–86. doi: 10.1159/000322605. [DOI] [PubMed] [Google Scholar]
- 177.Kwon O, Hong SM, Ramesh G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am J Physiol Renal Physiol. 2009;296:F25–33. doi: 10.1152/ajprenal.90531.2008. [DOI] [PubMed] [Google Scholar]
- 178.Kato N, Yuzawa Y, Kosugi T, Hobo A, Sato W, Miwa Y, Sakamoto K, Matsuo S, Kadomatsu K. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol. 2009;20:1565–76. doi: 10.1681/ASN.2008090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol. 2007;123:7–13. doi: 10.1016/j.clim.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol. 2003;23:511–21. doi: 10.1053/s0270-9295(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 182.Moreau R, Lebrec D. Acute kidney injury: New concepts. Hepatorenal syndrome: The role of vasopressors. Nephron Physiol. 2008;109:73–9. doi: 10.1159/000142939. [DOI] [PubMed] [Google Scholar]
- 183.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–69. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 184.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: New perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1153–67. doi: 10.1152/ajpregu.00402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–96. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 186.Eliopoulos N, Zhao J, Bouchentouf M, Forner K, Birman E, Yuan S, Boivin MN, Martineau D. Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. Am J Physiol Renal Physiol. 2010;299:F1288–98. doi: 10.1152/ajprenal.00671.2009. [DOI] [PubMed] [Google Scholar]
- 187.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–41. [PubMed] [Google Scholar]
- 188.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–82. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 189.Morigi M, Rota C, Montemurro T, Montelatici E, Lo Cicero V, Imberti B, Abbate M, Zoja C, Cassis P, Longaretti L, Rebulla P, Introna M, Capelli C, Benigni A, Remuzzi G, Lazzari L. Life-sparing effect of human cord blood-mesenchymal stem cells in experimental acute kidney injury. Stem Cells. 2010;28:513–22. doi: 10.1002/stem.293. [DOI] [PubMed] [Google Scholar]
- 190.Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, Gagliardini E, Xinaris C, Benedetti V, Fabricio ASC, Squarcina E, Abbate M, Benigni A, Remuzzi G, Morigi M. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012;21:1911–23. doi: 10.1089/scd.2011.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cao H, Qian H, Xu W, Zhu W, Zhang X, Chen Y, Wang M, Yan Y, Xie Y. Mesenchymal stem cells derived from human umbilical cord ameliorate ischemia/reperfusion-induced acute renal failure in rats. Biotechnol Lett. 2010;32:725–32. doi: 10.1007/s10529-010-0207-y. [DOI] [PubMed] [Google Scholar]
- 192.Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther. 2008;19:807–19. doi: 10.1089/hum.2008.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tögel F, Cohen A, Zhang P, Yang Y, Hu Z, Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475–85. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28:788–93. doi: 10.1093/ndt/gfs556. [DOI] [PubMed] [Google Scholar]
- 195.Gaspari F, Cravedi P, Mandala M, Perico N, de Leon FR, Stucchi N, Ferrari S, Labianca R, Remuzzi G, Ruggenenti P. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: A pilot prospective case-control study. Nephron Clin Pract. 2010;115:c154–60. doi: 10.1159/000312879. [DOI] [PubMed] [Google Scholar]
- 196.Gooch A, Doty J, Flores J, Swenson L, Toegel FE, Reiss GR, Lange C, Zander AR, Hu Z, Poole S, Zhang P, Westenfelder C. Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery. Cell Ther Transplant. 2008;1:31–5. [Google Scholar]
- 197.Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, Lee WM. Pathogenesis of Liver Injury in Acute Liver Failure. Gastroenterology. 2012;143:e1–7. doi: 10.1053/j.gastro.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leifeld L, Dumoulin F-L, Purr I, Janberg K, Trautwein C, Wolff M, Manns MP, Sauerbruch T, Spengler U. Early up-regulation of chemokine expression in fulminant hepatic failure. J Pathol. 2003;199:335–44. doi: 10.1002/path.1298. [DOI] [PubMed] [Google Scholar]
- 199.Sgroi A, Gonelle-Gispert C, Morel P, Baertschiger RM, Niclauss N, Mentha G, Majno P, Serre-Beinier V, Buhler L. Interleukin-1 receptor antagonist modulates the early phase of liver regeneration after partial hepatectomy in mice. PLoS One. 2011;6:e25442. doi: 10.1371/journal.pone.0025442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yumoto E, Higashi T, Nouso K, Nakatsukasa H, Fujiwara K, Hanafusa T, Yumoto Y, Tanimoto T, Kurimoto M, Tanaka N, Tsuji T. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J Gastroenterol Hepatol. 2002;17:285–94. doi: 10.1046/j.1440-1746.2002.02690.x. [DOI] [PubMed] [Google Scholar]
- 201.Tron K, Novosyadlyy R, Dudas J, Samoylenko A, Kietzmann T, Ramadori G. Upregulation of heme oxygenase-1 gene by turpentine oil-induced localized inflammation: Involvement of interleukin-6. Lab Invest. 2005;85:376–87. doi: 10.1038/labinvest.3700228. [DOI] [PubMed] [Google Scholar]
- 202.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–63. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 203.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–4. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 204.Muraca M, Ferraresso C, Vilei MT, Granato A, Quarta M, Cozzi E, Rugge M, Pauwelyn KA, Caruso M, Avital I, Inderbitzin D, Demetriou AA, Forbes SJ, Realdi G. Liver repopulation with bone marrow derived cells improves the metabolic disorder in the Gunn rat. Gut. 2007;56:1725–35. doi: 10.1136/gut.2007.127969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679–88. doi: 10.1242/jcs.115.13.2679. [DOI] [PubMed] [Google Scholar]
- 206.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 207.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–92. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 208.Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy. 2006;8:559–61. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 209.Cho KA, Woo SY, Seoh JY, Han HS, Ryu KH. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell Biol Int. 2012;36:1267–74. doi: 10.1042/CBI20110634. [DOI] [PubMed] [Google Scholar]
- 210.Gruttadauria S, Grosso G, Pagano D, Biondi A, Echeverri GJ, Seria E, Pietrosi G, Liotta R, Basile F, Gridelli B. Marrow-Derived Mesenchymal Stem Cells Restore Biochemical Markers of Acute Liver Injury in Experimental Model. Transplant Proc. 2013;45:480–6. doi: 10.1016/j.transproceed.2012.06.087. [DOI] [PubMed] [Google Scholar]
- 211.Jin SZ, Liu BR, Xu J, Gao FL, Hu ZJ, Wang XH, Pei FH, Hong Y, Hu HY, Han MZ. Ex vivo-expanded bone marrow stem cells home to the liver and ameliorate functional recovery in a mouse model of acute hepatic injury. Hepatobiliary Pancrat Dis Int. 2012;11:66–73. doi: 10.1016/s1499-3872(11)60127-6. [DOI] [PubMed] [Google Scholar]
- 212.Yukawa H, Noguchi H, Oishi K, Takagi S, Hamaguchi M, Hamajima N, Hayashi S. Cell transplantation of adipose tissue-derived stem cells in combination with heparin attenuated acute liver failure in mice. Cell Transplant. 2009;18:611–8. doi: 10.1177/096368970901805-617. [DOI] [PubMed] [Google Scholar]
- 213.Cao H, Yang J, Yu J, Pan Q, Li J, Zhou P, Li Y, Pan X, Li J, Wang Y, Li L. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012;10:56. doi: 10.1186/1741-7015-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal Stem Cell-Derived Molecules Reverse Fulminant Hepatic Failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kanazawa H, Fujimoto Y, Teratani T, Iwasaki J, Kasahara N, Negishi K, Tsuruyama T, Uemoto S, Kobayashi E. Bone marrow-derived mesenchymal stem cells ameliorate hepatic ischemia reperfusion injury in a rat model. PLoS One. 2011;6:e19195. doi: 10.1371/journal.pone.0019195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pan GZ, Yang Y, Zhang J, Liu W, Wang GY, Zhang YC, Yang Q, Zhai FX, Tai Y, Liu JR, Zhang Q, Chen GH. Bone marrow mesenchymal stem cells ameliorate hepatic ischemia/reperfusion injuries via inactivation of the MEK/ERK signaling pathway in rats. J Surg Res. 2012;178:935–48. doi: 10.1016/j.jss.2012.04.070. [DOI] [PubMed] [Google Scholar]
- 217.Dahlke MH, Hoogduijn M, Eggenhofer E, Popp FC, Renner P, Slowik P, Rosenauer A, Piso P, Geissler EK, Lange C, Chabannes D, Mazzanti B, Bigenzahn S, Bertolino P, Kunter U, Introna M, Rambaldi A, Capelli C, Perico N, Casiraghi F, Noris M, Gotti E, Seifert M, Saccardi R, Verspaget HW, van Hoek B, Bartholomew A, Wekerle T, Volk HD, Remuzzi G, Deans R, Lazarus H, Schlitt HJ, Baan CC. Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation. 2009;88:614–9. doi: 10.1097/TP.0b013e3181b4425a. [DOI] [PubMed] [Google Scholar]
- 218.Boeykens N, Ponsaerts P, Van der Linden A, Berneman Z, Ysebaert D, De Greef K. Injury-Dependent Retention of Intraportally Administered Mesenchymal Stromal Cells Following Partial Hepatectomy of Steatotic Liver Does Not Lead to Improved Liver Recovery. PLoS One. 2013;8:e69092. doi: 10.1371/journal.pone.0069092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. The Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 220.Kissela B, Broderick J, Woo D, Kothari R, Miller R, Khoury J, Brott T, Pancioli A, Jauch E, Gebel J, Shukla R, Alwell K, Tomsick T. Greater Cincinnati/Northern Kentucky Stroke Study: Volume of first-ever ischemic stroke among blacks in a population-based study. Stroke. 2001;32:1285–90. doi: 10.1161/01.str.32.6.1285. [DOI] [PubMed] [Google Scholar]
- 221.Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, Endres M. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med. 2001;7:255–60. [PubMed] [Google Scholar]
- 222.Li X, Klaus JA, Zhang J, Xu Z, Kibler KK, Andrabi SA, Rao K, Yang ZJ, Dawson TM, Dawson VL, Koehler RC. Contributions of poly(ADP-ribose) polymerase-1 and -2 to nuclear translocation of apoptosis-inducing factor and injury from focal cerebral ischemia. J Neurochem. 2010;113:1012–22. doi: 10.1111/j.1471-4159.2010.06667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mattson MP, Culmsee C, Yu ZF. Apoptotic and antiapoptotic mechanisms in stroke. Cell Tissue Res. 2000;301:173–87. doi: 10.1007/s004419900154. [DOI] [PubMed] [Google Scholar]
- 224.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 225.Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, Tamatani T, Miyasaka M, Kogure K. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res. 1994;656:344–52. doi: 10.1016/0006-8993(94)91478-8. [DOI] [PubMed] [Google Scholar]
- 226.Zhang RL, Chopp M, Zhang ZG, Phillips ML, Rosenbloom CL, Cruz R, Manning A. E-selectin in focal cerebral ischemia and reperfusion in the rat. J Cereb Bood Flow Metab. 1996;16:1126–36. doi: 10.1097/00004647-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 227.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–6. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14:3574–89. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 229.Lee J, Kuroda S, Shichinohe H, Ikeda J, Seki T, Hida K, Tada M, Sawada K-i, Iwasaki Y. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology. 2003;23:169–80. doi: 10.1046/j.1440-1789.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 230.Liu H, Honmou O, Harada K, Nakamura K, Houkin K, Hamada H, Kocsis JD. Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia. Brain. 2006;129:2734–45. doi: 10.1093/brain/awl207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Shichinohe H, Kuroda S, Yano S, Ohnishi T, Tamagami H, Hida K, Iwasaki Y. Improved expression of gamma-aminobutyric acid receptor in mice with cerebral infarct and transplanted bone marrow stromal cells: An autoradiographic and histologic analysis. J Nucl Med. 2006;47:486–91. [PubMed] [Google Scholar]
- 232.Yano S, Kuroda S, Shichinohe H, Hida K, Iwasaki Y. Do bone marrow stromal cells proliferate after transplantation into mice cerebral infarct?--a double labeling study. Brain Res. 2005;1065:60–7. doi: 10.1016/j.brainres.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 233.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 234.Ikeda N, Nonoguchi N, Zhao MZ, Watanabe T, Kajimoto Y, Furutama D, Kimura F, Dezawa M, Coffin RS, Otsuki Y, Kuroiwa T, Miyatake SI. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36:2725–30. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- 235.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Bood Flow Metab. 2000;20:1311–9. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 236.Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, Funakoshi H, Kajimoto Y, Nakamura T, Dezawa M, Shibata M-A, Otsuki Y, Coffin RS, Liu W-D, Kuroiwa T, Miyatake SI. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Bood Flow Metab. 2006;26:1176–88. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- 237.Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–72. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 238.Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–9. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- 239.Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003;73:778–86. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- 240.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 241.Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–85. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–17. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 243.Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017–25. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 244.Gutierrez-Fernandez M, Rodriguez-Frutos B, Alvarez-Grech J, Vallejo-Cremades MT, Exposito-Alcaide M, Merino J, Roda JM, Diez-Tejedor E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. doi: 10.1016/j.neuroscience.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 245.Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 246.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 247.De Keyser J. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;58:653–4. doi: 10.1002/ana.20612. [DOI] [PubMed] [Google Scholar]
- 248.Smith HK, Gavins FNE. The potential of stem cell therapy for stroke: Is PISCES the sign? FASEB J. 2012;26:2239–52. doi: 10.1096/fj.11-195719. [DOI] [PubMed] [Google Scholar]
- 249.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 250.Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AIR. Early prognosis in traumatic brain injury: From prophecies to predictions. Lancet Neurol. 2010;9:543–54. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 251.Munoz-Elias G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: Stem cell and precursor functions. Stem Cells. 2003;21:437–48. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- 252.Chopp M, Mahmood A, Lu D, Li Y. Editorial. Mesenchymal stem cell treatment of traumatic brain injury. J Neurosurg. 2009;110:1186–8. doi: 10.3171/2008.10.JNS081254. [DOI] [PubMed] [Google Scholar]
- 253.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Human marrow stromal cell treatment provides long-lasting benefit after traumatic brain injury in rats. Neurosurgery. 2005;57:1026–31. doi: 10.1227/01.neu.0000181369.76323.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 2006;104:272–7. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- 255.Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–203. [PubMed] [Google Scholar]
- 256.Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, Cox CS. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110:1189–97. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lu D, Mahmood A, Wang L, Li Y, Lu M, Chopp M. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport. 2001;12:559–63. doi: 10.1097/00001756-200103050-00025. [DOI] [PubMed] [Google Scholar]
- 258.Wannemuehler TJ, Manukyan MC, Brewster BD, Rouch J, Poynter JA, Wang Y, Meldrum DR. Advances in mesenchymal stem cell research in sepsis. J Surg Res. 2012;173:113–26. doi: 10.1016/j.jss.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 259.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 260.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 261.Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934–41. [PubMed] [Google Scholar]
- 262.Cohen J, Carlet J. INTERSEPT: An international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-alpha in patients with sepsis. International Sepsis Trial Study Group. Crit Care Med. 1996;24:1431–40. doi: 10.1097/00003246-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 263.Fisher CJJ, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271:1836–43. [PubMed] [Google Scholar]
- 264.Fisher CJJ, Slotman GJ, Opal SM, Pribble JP, Bone RC, Emmanuel G, Ng D, Bloedow DC, Catalano MA. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: A randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22:12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 265.Effect of high-dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. The Veterans Administration Systemic Sepsis Cooperative Study Group. N Engl J Med. 1987;317:659–65. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- 266.Bone RC, Fisher CJJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–8. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 267.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 268.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES, Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rittirsch D, Hoesel LM, Ward PA. The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol. 2007;81:137–43. doi: 10.1189/jlb.0806542. [DOI] [PubMed] [Google Scholar]
- 271.de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: A meta-analysis and update on clinical trials. Circ Cardiovasc Interv. 2014;7:156–67. doi: 10.1161/CIRCINTERVENTIONS.113.001009. [DOI] [PubMed] [Google Scholar]
- 272.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res. 2004;95:1140–53. doi: 10.1161/01.RES.0000150734.79804.92. [DOI] [PubMed] [Google Scholar]
- 273.Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7:163–83. doi: 10.2174/157340311798220494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Feger F, Rouby JJ. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit Care Med. 2008;36:766–74. doi: 10.1097/CCM.0B013E31816596BC. [DOI] [PubMed] [Google Scholar]
- 275.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–8. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.van den Akker F, de Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: Immunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm. 2013;2013:181020. doi: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Darwish I, Banner D, Mubareka S, Kim H, Besla R, Kelvin DJ, Kain KC, Liles WC. Mesenchymal stromal (stem) cell therapy fails to improve outcomes in experimental severe influenza. PLoS One. 2013;8:e71761. doi: 10.1371/journal.pone.0071761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ Canadian Critical Care Trials G. Safety of cell therapy with mesenchymal stromal cells (SafeCell): A systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.