Abstract
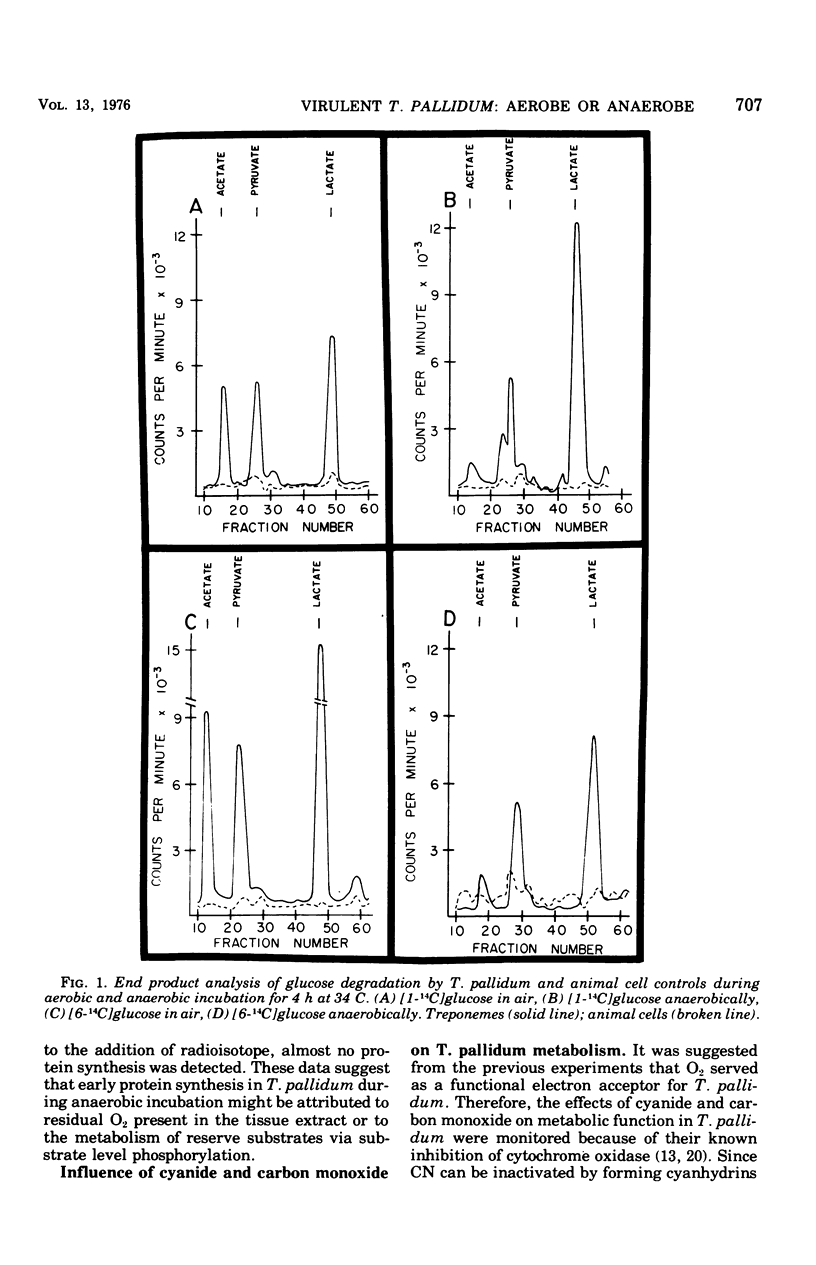
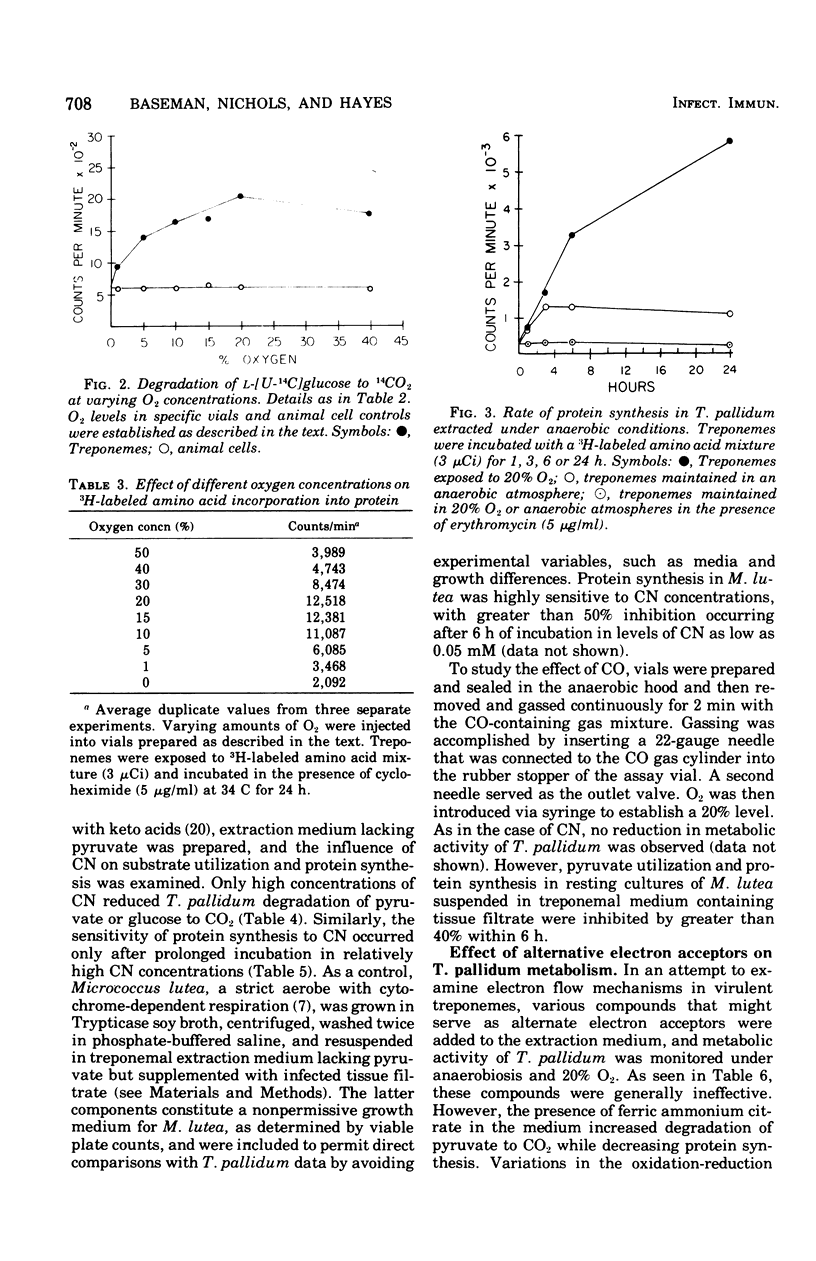
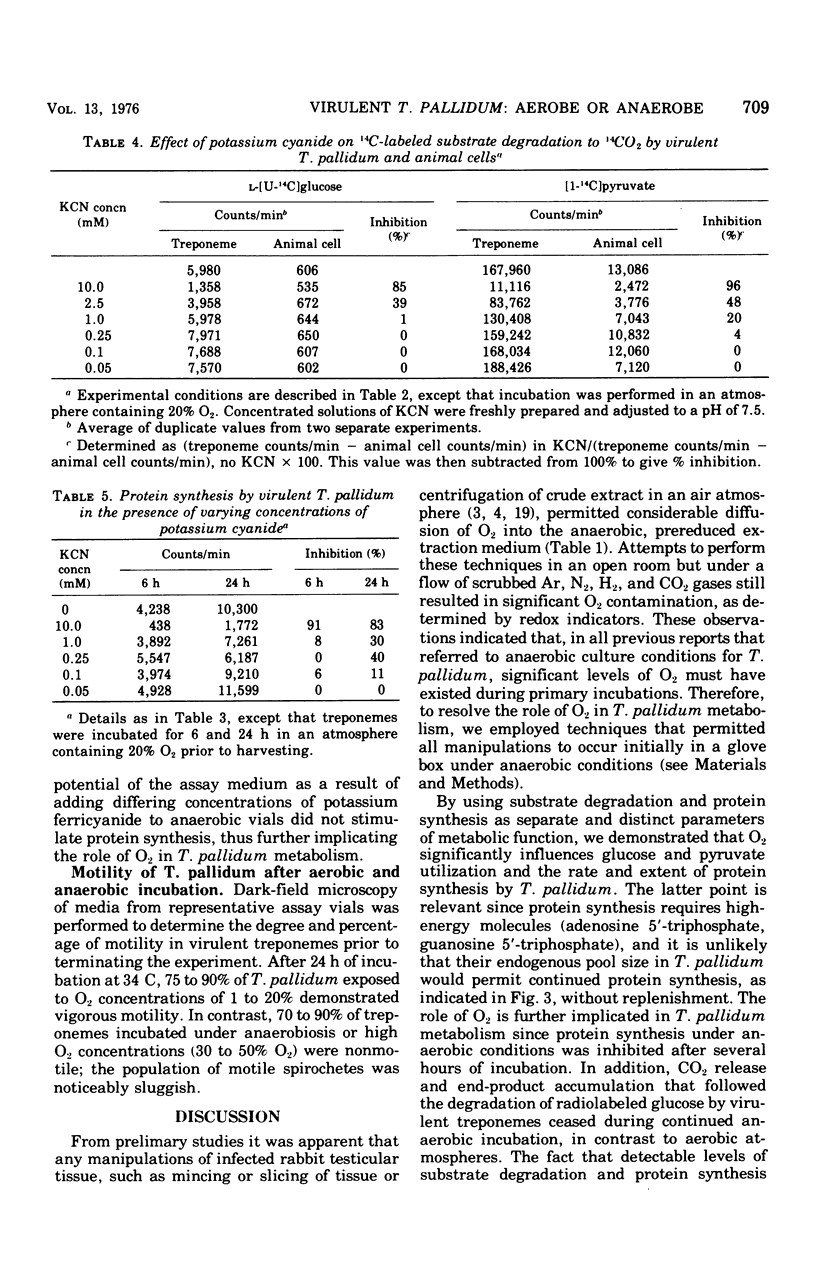
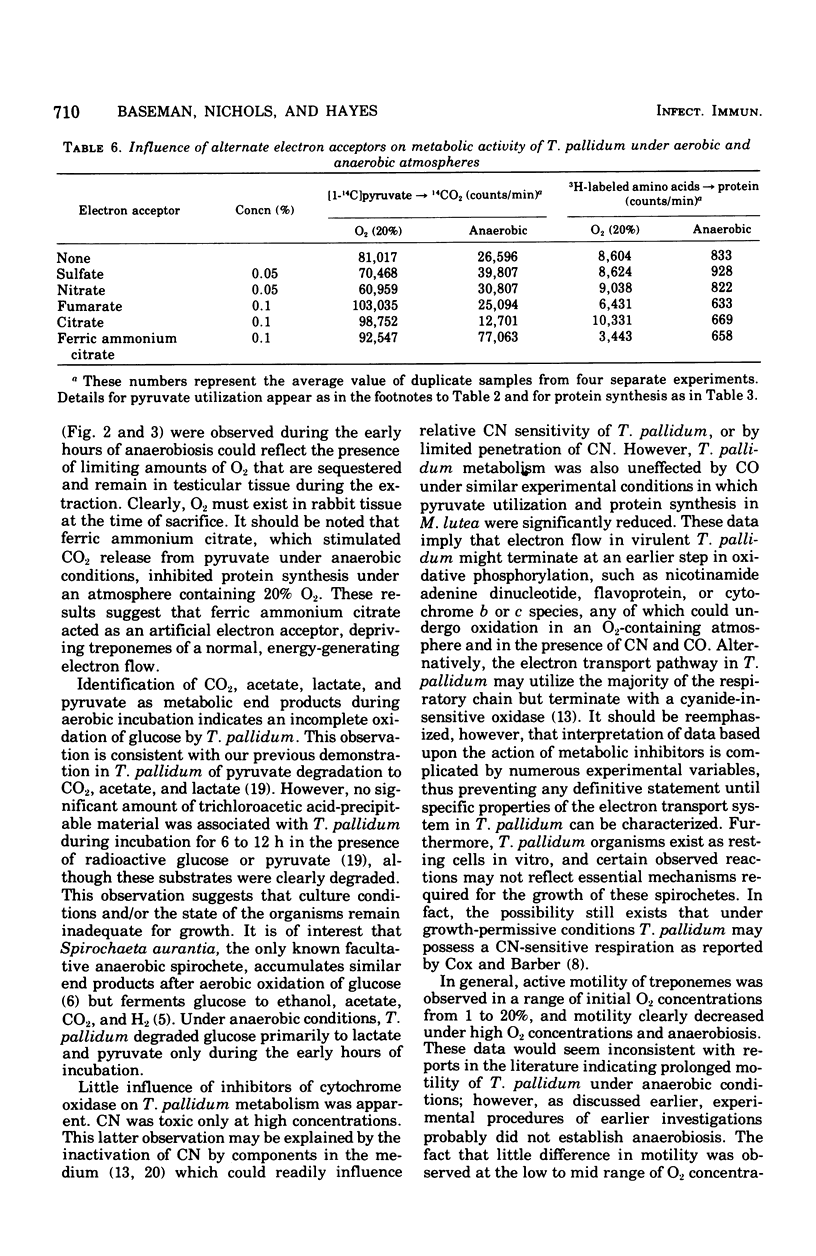
Substrate degradation and protein synthesis served as indicators of metabolism in virulent Treponema pallidum. Opitmal metabolic activity in these spirochetes was observed at 10 to 20% O2 concentrations, with markedly reduced activity at higher or lower O2 levels or under anaerobiosis; alternate functioning electron acceptors that might substitute for O2 were not found. Carbon monoxide and cyanide at concentrations that inactivate cytochrome oxidase were not effective metabolic poisons for T. pallidum, although Micrococcus lutea, a strict aerobe with cytochrome-dependent respiration, was inhibited under similar experimental conditions. Motility of virulent T. pallidum was vigorous in the presence of O2 and sluggish or inhibited in its absence, reinforcing the role of O2 in T. pallidum metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Cox C. D. Intermediate energy metabolism of Leptospira. J Bacteriol. 1969 Mar;97(3):992–1000. doi: 10.1128/jb.97.3.992-1000.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cox C. D. Terminal electron transport in Leptospira. J Bacteriol. 1969 Mar;97(3):1001–1004. doi: 10.1128/jb.97.3.1001-1004.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Protein synthesis by Treponema pallidum extracted from infected rabbit tissue. Infect Immun. 1974 Dec;10(6):1350–1355. doi: 10.1128/iai.10.6.1350-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Nichols J. C., Rumpp J. W., Hayes N. S. Purification of Treponema pallidum from Infected Rabbit Tissue: Resolution into Two Treponemal Populations. Infect Immun. 1974 Nov;10(5):1062–1067. doi: 10.1128/iai.10.5.1062-1067.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Metabolism of Spirochaeta aurantia. I. Anaerobic energy-yielding pathways. Arch Mikrobiol. 1972;83(4):261–277. doi: 10.1007/BF00425239. [DOI] [PubMed] [Google Scholar]
- Breznak J. A., Canale-Parola E. Metabolism of Spirochaeta aurantia. II. Aerobic oxidation oxidation of carbohydrates. Arch Mikrobiol. 1972;83(4):278–292. doi: 10.1007/BF00425240. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Barber M. K. Oxygen uptake by Treponema pallidum. Infect Immun. 1974 Jul;10(1):123–127. doi: 10.1128/iai.10.1.123-127.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves S. R., Sandok P. L., Jenkin H. M., Johnson R. C. Retention of motility and virulence of Treponema pallidum (Nichols strain) in vitro. Infect Immun. 1975 Nov;12(5):1116–1120. doi: 10.1128/iai.12.5.1116-1120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. L., Kinyon J. M., Mullin M. T., Glock R. D. Isolation and propagation of spirochetes from the colon of swine dysentery affected pigs. Can J Comp Med. 1972 Jan;36(1):74–76. [PMC free article] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Kelly R. Cultivation of Borrelia hermsi. Science. 1971 Jul 30;173(3995):443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- MERKEL J. R., NICKERSON W. J. Riboflavin as a photocatalyst and hydrogen carrier in photochemical reduction. Biochim Biophys Acta. 1954 Jul;14(3):303–311. doi: 10.1016/0006-3002(54)90188-2. [DOI] [PubMed] [Google Scholar]
- Metzger M., Smogór W. Study of the effect of pH and Eh values of the Nelson-Diesendruck medium on the survival of virulent Treponema pallidum. Arch Immunol Ther Exp (Warsz) 1966;14(4):445–453. [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H., Jr The use of freeze-preserved treponemes in the Treponema pallidum immobilization test. Cryobiology. 1972 Oct;9(5):404–410. doi: 10.1016/0011-2240(72)90157-5. [DOI] [PubMed] [Google Scholar]
- Nichols J. C., Baseman J. B. Carbon sources utilized by virulent Treponema pallidum. Infect Immun. 1975 Nov;12(5):1044–1050. doi: 10.1128/iai.12.5.1044-1050.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Growth properties of Rhodospirillum rubrum mutants and fermentation of pyruvate in anaerobic, dart conditions. J Bacteriol. 1973 Nov;116(2):874–884. doi: 10.1128/jb.116.2.874-884.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER M. M. Factors influencing the in vitro survival of Treponema pallidum. Am J Hyg. 1960 May;71:401–417. doi: 10.1093/oxfordjournals.aje.a120123. [DOI] [PubMed] [Google Scholar]
- Willcox R. R., Guthe T. Treponema pallidum. A bibliographical review of the morphology, culture and survival of T. pallidum and associated organisms. Bull World Health Organ. 1966;35:1–169. [PMC free article] [PubMed] [Google Scholar]
- Willis M. V., Baseman J. B., Amos H. Noncoordinate control of RNA synthesis in eucaryotic cells. Cell. 1974 Oct;3(2):179–184. doi: 10.1016/0092-8674(74)90123-8. [DOI] [PubMed] [Google Scholar]


