Abstract
Little is known about the etiology of intracranial germ cell tumors (iGCTs), although international incidence data suggest that the highest incidence rates occur in Asian countries. In this analysis, we used 1992–2010 data from the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program to determine whether rates of iGCT were also high in Asian/Pacific Islanders living in the United States. Frequencies, incidence rates and survival rates were evaluated for the entire cohort and for demographic subgroups based on sex, age category (0–9 and 10–29 years), race (white, black, and Asian/Pacific Islander), and tumor location (pineal gland vs. other) as sample size permitted. Analyses were conducted using SEER*Stat 8.1.2. We observed a significantly higher incidence rate of iGCT in Asian/Pacific Islanders compared with whites (RR = 2.05, 95 % CI 1.57–2.64, RR = 3.04, 95 % CI 1.75–5.12 for males and females, respectively) in the 10–29 year age group. This difference was observed for tumors located both in the pineal gland and for tumors in other locations. Five-year relative survival differed by demographic and tumor characteristics, although these differences were not observed in comparisons limited to cases treated with radiation. Increased incidence rates of iGCT in individuals of Asian descent in the SEER registry are in agreement with data from the International Agency for Research on Cancer, where Japan and Singapore were among the countries with highest incidence. The increased incidence in individuals of Asian ancestry in the United States suggests that underlying genetic susceptibility may play a role in the etiology of iGCT.
Keywords: Pediatric cancer, Germ cell tumors, Intracranial, SEER, Incidence
Introduction
Pediatric germ cell tumors (GCTs) are rare and heterogeneous tumors that are grouped together due to their presumed common cell of origin, the primordial germ cell (PGC) [1]. GCTs typically occur in the testes or ovaries; however, abnormal prenatal PGC migration along with lack of apopotosis can result in tumors in extragonadal locations [2]. Of these extragonadal tumors, the central nervous system is the most common location [3]. Little is known about the etiology of intracranial and intraspinal GCTs (iGCTs).
Similar to gonadal GCTs, iGCTs are grouped into two broad classes including germinomas and nongerminomas, with teratomas comprising the majority of the latter group [4]. Germinomas are the most common histologic subtype, accounting for 70–80 % of iGCTs, and the pineal gland is the most common tumor location [5, 6]. Incidence peaks in the second decade of life [3, 7, 8].
Incidence rates of iGCTs vary greatly by country. Based on data from the International Incidence of Childhood Cancer, countries with the highest incidence of pediatric iGCTs include Japan, Denmark, Singapore, and the United Kingdom for males, and Japan, Italy, Denmark and Singapore for females [9]. The high rates of iGCT in males in Japan and Singapore are in direct contrast to trends in testicular GCT, where Japan and Singapore have lower incidence rates in international comparisons [10]. Inherited genetic variation could lead to this variation in rates of iGCT.
Potential explanations for variation in incidence rates internationally include differing exposure to some as yet undiscovered environmental risk factor, differing frequency of a genetic risk allele, or some combination of these. One potential way to determine if there is underlying genetic susceptibility is to evaluate incidence rates in Asians who have migrated to a different country with lower incidence rates. In this analysis, we evaluated incidence rates (1992–2010) of iGCTs in the United States using data from the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) Program with a specific focus on differences in incidence rates across racial and ethnic groups. We included males and females up to age 29 years in our analysis in order to capture the age range with peak incidence of intracranial GCT.
Materials and methods
Using data from the NCI's SEER Program [11], we analyzed incidence and survival of pediatric and young adult intracranial GCTs in males and females [12, 13]. We used data from the SEER 13 registries, which actively collects information on demographics, tumor site and morphology, stage at diagnosis, treatment, and vital status from thirteen cancer registries in five states (Connecticut, Hawaii, Iowa, New Mexico, and Utah), six metropolitan areas (Atlanta, Detroit, San Francisco-Oakland, Seattle-Puget Sound, Los Angeles, San Jose-Monterey) and two other regions (Rural Georgia and the Alaskan Native Tumor Registry). The SEER 13 registries represent ~14 % of the U.S. population [14] with an estimated case ascertainment rate of 98 % [15]. We included first malignancies diagnosed from 1992–2010 among individuals ≤29 years of age.
We identified iGCTs using International Classification of Disease for Oncology, 3rd edition (ICD-O-3) [16] histology and topography codes included in the International Classification of Childhood Cancer (ICCC), 3rd Edition [17] category Xa. (malignant intracranial germ cell tumors). All histology and topography codes were combined in the analysis of GCTs overall. To evaluate differences by histology, we stratified the tumors into germinoma (ICCC 9060–9065), teratoma (9080–9084), and other (9085, 9070–9072, 9100). Differences by tumor location were also evaluated by classifying tumors as pineal gland (topography code 75.3) and other (topography codes 70.0–72.9, 75.1, 75.2).
This analysis used existing data with no personal identifiers; therefore, the study was exempt from review by the University of Minnesota Institutional Review Board.
Statistical analysis
Frequencies and age-adjusted incidence rates during the period 1992–2010 were calculated using SEER*STAT software [18]; incidence rates were reported as the number of cases per 1,000,000 person-years of follow-up. The U.S. 2000 standard population (age<1, ages 1–4, ages 5–9, ages 10–14, ages 15–19, ages 20–24 and ages 25–29 years) was used in direct age standardization. Rate ratios (RR) were used to compare incidence rates in demographic subgroups.
The life tables method in SEER*Stat [18, 19] was used to calculate five-year relative survival rates and corresponding standard errors. Observed rates included all individuals diagnosed with a first malignancy between 1992 and 2005 who were followed actively through 2010. SEER follow-up rates into 2010 were high for both males and females aged 0–29 years (93 and 91 %, respectively) [14]. Relative survival rates are ratios of observed-to-expected survival and are reported as percentages. The expected rates were based on data from the National Center for Health Statistics and take into account differences in distributions of age, sex, race, and year of diagnosis. Relative rates were adjusted if they exceeded 100 %, increased over time, or involved heterogeneity in withdrawal (exact method).
All analyses were stratified by sex. Incidence and survival were evaluated for the entire cohort and for demographic subgroups based on age category (0–9 and 10–29 years), race (white, black, Asian/Pacific Islander and American Indian/Alaskan Native), and ethnicity (non-Hispanic and Hispanic) as sample size permitted. Because reporting of brain tumors to cancer registries in the United States was revised to include non-malignant tumors in 2004 [20], we also conducted stratified analyses of cases diagnosed before and after 2004 to ensure that our results were not influenced by this reporting change.
Results
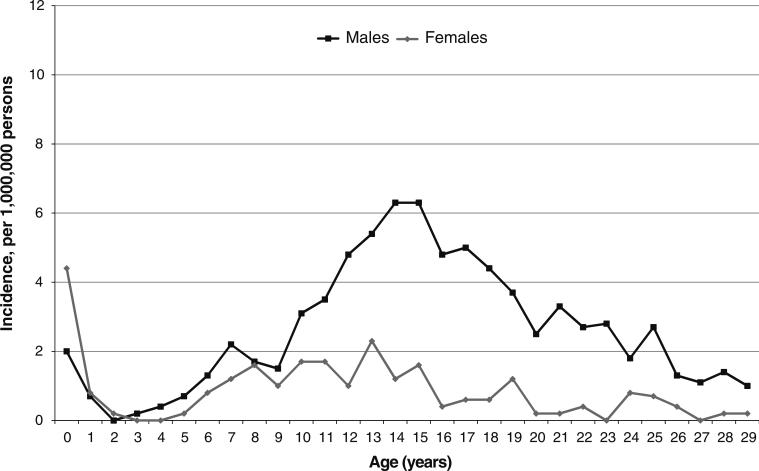
From 1992 to 2010, 554 intracranial GCTs were reported in the SEER registry, including 423 in males and 131 in females. Intracranial GCTs accounted for 5.3 % of all GCTs in males and 8.9 % of all GCTs in females ages 0–29 years. Similar to the incidence of GCTs overall, the age-specific incidence of intracranial GCT followed a bimodal distribution, with an initial peak after birth and a second peak during adolescence (Fig. 1). There was no significant difference in incidence rates by sex in children ages 0–9 years (RR 0.92, 95 % CI 0.62, 1.37). In contrast, incidence was significantly lower in females compared to males in the 10–29 year age group (0.8 vs. 3.4 per 1,000,000 persons, respectively; RR 0.23, 95 % CI 0.18, 0.29). When we examined incidence in males and females by year of diagnosis, there were no significant changes in incidence rates from 1992 to 2010 in males or females in either age group (data not shown).
Fig. 1.
Incidence of intracranial germ cell tumors in males and females ages 0–29 years in the SEER registry, 1992–2010
We observed significant differences in incidence rates by race and ethnicity in both males and females, although these differences varied by age at diagnosis (Table 1). Incidence rates were higher in both males and females of Asian/Pacific Islander descent, and these differences were statistically significant in all groups except males ages 0–9 years. Similar to rates for GCTs overall, incidence of iGCTs was significantly lower among black males compared with white males in both age groups, although these analyses were based on small numbers of black males. No difference was observed between black and white females. When we evaluated rates by ethnicity, we observed a significantly lower rate of iGCT among Hispanic compared with white males in the 10–29 year age group. No other differences in rates by ethnicity were observed.
Table 1.
Frequency and incidence rates of pediatric and young adult intracranial germ cell tumors by race, ethnicity, and age group in males and females in the NCI Surveillance, Epidemiology and End Results Registry, 1992–2010
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| Na | Incidence rate (95 % CI) | RR (95 % CI) | Na | Incidence rate (95 % CI) | RR (95 % CI) | |
| Total | 423 | 2.6 (2.4, 2.9) | 1.0 (Ref) | 131 | 0.9 (0.7, 1.0) | 0.33 (0.27, 0.40) |
| 0–9 years | ||||||
| Total | 58 | 1.1 (0.8, 1.4) | 1.0 (Ref) | 52 | 1.0 (0.7, 1.3) | 0.92 (0.62, 1.37) |
| Race | ||||||
| White | 46 | 1.2 (0.9, 1.6) | 1.0 (Ref) | 33 | 0.9 (0.6, 1.2) | 1.0 (Ref) |
| Black | 1 | 0.1b (0, 0.8) | 0.12 (0.003, 0.70) | 8 | 1.2 (0.5, 2.3) | 1.33 (0.53, 2.94) |
| Asian/Pacific Islander | 11 | 2.0 (1.0, 3.5) | 1.68 (0.78, 3.28) | 11 | 2.0b (1.0, 3.6) | 2.29 (1.04, 4.66) |
| Ethnicity | ||||||
| Non-hispanic | 44 | 1.1 (0.8, 1.5) | 1.0 (Ref) | 40 | 1.1 (0.8, 1.5) | 1.0 (Ref) |
| Hispanic | 14 | 0.9 (0.5, 1.6) | 0.82 (0.41, 1.53) | 12 | 0.8 (0.4, 1.4) | 0.75 (0.36, 1.46) |
| 10–29 years | ||||||
| Total | 365 | 3.4 (3.1, 3.8) | 1.0 (Ref) | 79 | 0.8 (0.6, 1.0) | 0.23 (0.18, 0.29) |
| Raced | ||||||
| White | 257 | 3.3 (2.9, 3.7) | 1.0 (Ref) | 49 | 0.7 (0.5, 0.9) | 1.0 (Ref) |
| Black | 24 | 1.8b (1.2, 2.7) | 0.56 (0.35, 0.85) | 8 | 0.6 (0.3, 1.2) | 0.93 (0.38, 1.97) |
| Asian/Pacific Islander | 80 | 6.7b (5.3, 8.4) | 2.05 (1.57, 2.64) | 22 | 2.0b (1.3, 3.1) | 3.04 (1.75, 5.12) |
| Ethnicity | ||||||
| Non-hispanic | 288 | 3.7 (3.3, 4.1) | 1.0 (Ref) | 62 | 0.8 (0.6, 1.1) | 1.0 (Ref) |
| Hispanic | 77 | 2.8c (2.2, 3.5) | 0.77 (0.59, 0.99) | 17 | 0.7 (0.4, 1.1) | 0.80 (0.44, 1.38) |
Number of cases in SEER registry, 1992–2010
Rate is significantly different than rate in white
Rate is significantly different than rate in non-Hispanic
N does not sum to total because incidence rates were not presented for American Indian/Native Alaskan (N = 3) and other (N = 1) categories
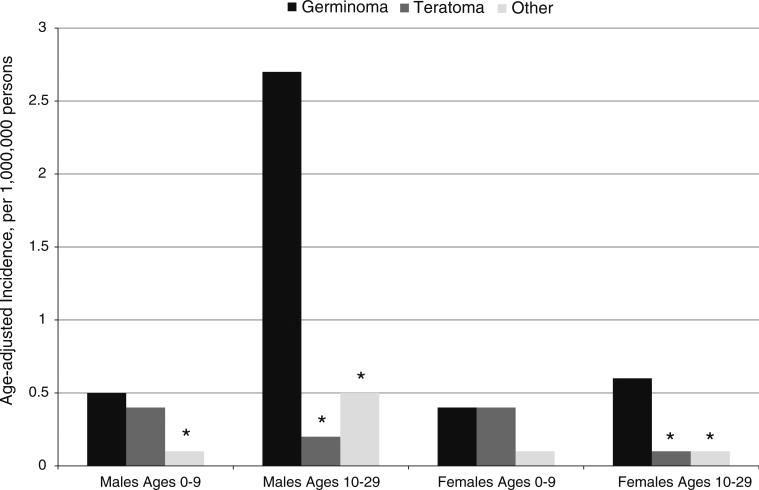
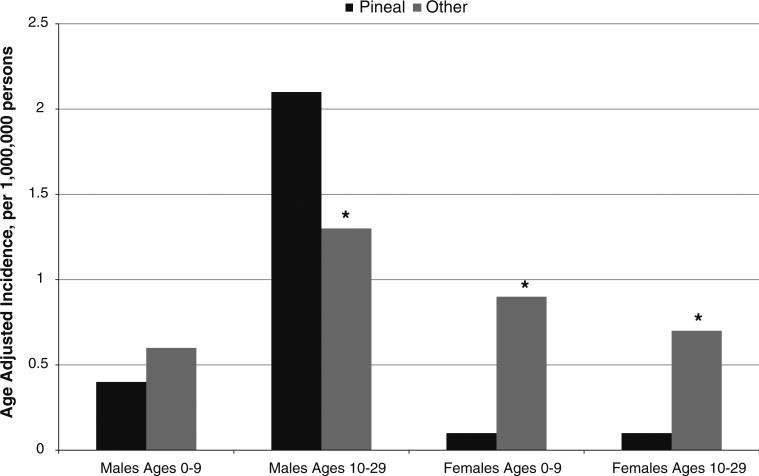
Germinomas were the most common histologic subtype of tumors in males overall and in adolescent females, while germinomas and teratomas were equally common in females ages 0–9 years (Fig. 2). When we evaluated incidence of iGCTs by tumor location (pineal gland vs. other), we observed different patterns in males and females (Fig. 3). For males, the pineal gland was the most common location (Fig. 3). In contrast, iGCTs in the pineal gland were very rare in females. Tumors located in other areas of the CNS were equally frequent in males and females. The higher incidence of iGCT in Asians overall was also observed in the analyses by tumor location and histology (data not shown).
Fig. 2.
Incidence of intracranial germ cell tumors by histology in males and females. Asterisk indicates that rate is significantly different from rate for germinoma
Fig. 3.
Incidence of intracranial germ cell tumors by tumor location in males and females. Asterisk indicates that rate is significantly different from rate for tumors in the pineal gland
Overall, the majority of iGCTs in the SEER registry were of malignant histology (522/553; 94 %). In the analyses stratified by year of diagnosis, there was no evidence that incidence increased due to the inclusion of benign brain tumors in the years following 2004. The incidence was significantly increased following the 2004 reporting change only in the Asian males ages 0–9 years. Interestingly, this was the one group where we did not see an increased incidence in Asians compared with other groups, although these analyses were based on very small numbers. Incidence rates remained stable in all of the categories before and after the 2004 reporting change. Exclusion of the non-malignant iGCTs did not substantially change the results of any analysis.
Overall, 5-year relative survival is favorable for iGCTs (85.9 %, 95 % CI 81.8, 89.1). Males had higher 5-year relative survival rates than females overall, although this difference was entirely explained by differences in survival in cases not treated with radiation therapy (Table 2). Survival was higher for germinoma than for either teratoma or other histology and tumors located in the pineal gland also had a more favorable outcome, although not all differences reached statistical significance. When the comparisons were restricted to patients who received radiation, there were no significant differences in 5-year relative survival by race, sex, or tumor location (data not shown).
Table 2.
5-year relative survival by race, tumor histology, and tumor location in males and females in the NCI Surveillance, Epidemiology and End Results Registry, 1992–2005
| Males |
Females |
|||
|---|---|---|---|---|
| N (%) | 5-year relative survival (95 % CI) | N | 5-year relative survival (95 % CI) | |
| All | 292 (100) | 88.9 % (84.6, 92.1) | 79 (100) | 74.7 % (63.5, 82.9) |
| Racea | ||||
| White | 212 (73) | 90.8 % (85.8, 94.1) | 49 (62) | 73.5 % (58.7, 83.7) |
| Black | 19 (7) | 84.7 % (58.5, 95.0) | 7 (9) | 71.5 % (25.8, 92.1) |
| Asian/Pacific Islander | 58 (20) | 82.9 % (70.4, 90.5) | 23 (29) | 77.9 % (54.6, 90.2) |
| Tumor histology | ||||
| Germinoma | 228 (78) | 91.9 % (87.3, 94.9) | 53 (67) | 84.9 % (71.9, 92.2) |
| Teratoma | 23 (8) | 82.8 % (60.1, 93.3) | 13 (16) | 38.5 % (14.1, 62.9) |
| Other | 41 (14) | 75.7 % (59.5, 86.2) | 13 (16) | 69.3 % (37.3, 87.2) |
| Tumor location | ||||
| Pineal gland | 172 (59) | 91.0 % (85.4, 94.5) | 9 (11) | 88.9 % (43.2, 98.4) |
| Other | 120 (41) | 85.9 % (78.1, 91.1) | 70 (89) | 72.9 % (60.7, 81.8) |
| Radiation therapy | ||||
| Yes | 233 (80) | 90.4 % (85.7, 93.6) | 50 (63) | 90.1 % (77.6, 95.8) |
| No | 56 (19) | 82.0 % (68.9, 90.0) | 26 (33) | 46.3 % (26.7, 63.8) |
| Unknown | 3 (1) | 100% | 3 (4) | 66.7 % (5.4, 94.5) |
Does not sum to total because rates were not present for American Indian/Alaskan Natives due to small numbers (N = 3)
Discussion
In this analysis of the SEER Program data from 1992 to 2010, we have shown that incidence and survival rates for iGCTs differ by demographic and tumor characteristics. Of particular interest is the finding that Asians and Pacific Islanders living in the United States have the highest incidence rates, in support of international data indicating that incidence rates are high in Asian countries [9] and an earlier study using the SEER data [21]. We also found differences in tumor location in adolescent and young adult males and females with iGCTs, with tumors of the pineal gland comprising the majority of tumors in males overall and non-pineal gland tumors comprising the majority of tumors in females. Differences in 5-year relative survival were also observed, and may be explained by differences in the percentage of patients who are treated with radiation therapy.
While Japan and Singapore had some of the highest incidence rates of intracranial GCTs based on the international data, few countries had sufficient data available to evaluate incidence rates given the rarity of the tumor and the lack of data for individuals over age 14 years [9]. Few registry based studies have reported incidence rates of iGCTs [3, 6, 22], making international comparisons difficult. One registry based study including data from Japan and the United States did not find a higher rate of iGCT in Japan (0.096 per 100,000 for the Japan Cancer Surveillance Research Group in 2004–2006 vs. 0.075 per 100,000 for SEER in 2004–2008) [6]; however, it is likely that there is considerable variation in incidence within Asian countries. In our analysis, we were unable to stratify the Asian/Pacific Islander group to evaluate country specific heterogeneity in incidence rates. Additional studies have reported only the percentage of CNS tumors that are iGCT. Unsurprisingly, there is also considerable variation, with percentages ranging from 3 to 5 % in the United States [8] and France [23] to ~14 % in Japan [22] and Taiwan [24]. Of additional interest is the fact that previous registry based reports suggest that incidence of gonadal GCTs is also higher in Asians in the pediatric age group, both living in the United States [25, 26] and in Asia [9]. The biological mechanisms that would explain this increased incidence only for GCTs in the pediatric age group is intriguing and warrants further investigation.
The higher incidence of iGCT in males overall is largely explained by the higher incidence of tumors in the pineal gland, also reported in a previous study [8], but the explanation in not clear. The higher incidence of gonadal GCTs in males is thought to be due to the more limited number of germ cells in females in the mature ovaries [27, 28]; however, this is unlikely to explain the higher incidence of iGCTs in males. The primary function of the pineal gland is the production of melatonin, although several other hormones are also produced. Of note, disruption of the circadian rhythm through shift work has been classified as a probable carcinogen [29], with a recent meta-analysis suggesting that higher 6-sulfatoxymelatonin levels were associated with a modest decrease in risk of breast cancer [30]. The pineal gland plays a well-established role in puberty [31, 32], with evidence that melatonin levels are involved in the timing of puberty [33, 34]. Given the peak incidence of iGCTs during puberty, it is possible that changing hormone levels in the pineal gland during puberty are triggering the growth of germ cells that aberrantly migrated to the pineal gland during early development. One pineal hormone, 5-methyoxytryptophol (ML), increases significantly in males but decreases in females during puberty [35], suggesting that this may be a good candidate for further investigation [29, 30].
Clinical presentation of iGCT varies depending on tumor location and common symptoms include headache, nausea and vomiting, and hydrocephalus [4, 36, 37]. Other more rare symptoms have also been reported, including seizures and other psychiatric symptoms [36, 38]. Auto-immune encephalitis (AE) is a paraneoplastic event characterized by abnormal behavior, seizures, and movement disorders [39–43]; a number of case reports find AE in conjunction with ovarian teratoma [44–48]. We note with interest that 30 % of AE patients in a recent registry based study in California were Asian/Pacific Islander [49]. This high frequency of AE in Asians and the co-occurrence with GCT in case reports is intriguing. While published studies have reported only ovarian teratomas, it is possible that AE could be triggered by PGCs abnormally present in the cranio-spatial axis prior to GCT diagnosis. It will be of interest to determine whether a diagnosis of AE precedes iGCT. The role of the immune system is likely to differ in iGCT compared with TGCT given that the testis is an immune privileged site [50, 51] while the CNS has tightly regulated interactions with the immune system [52, 53].
Five year relative survival rates for patients with iGCTs are lower than rates for GCTs in other locations [25] but are higher than rates for pediatric CNS overall [13, 54]. Previous reports have documented the improved survival rates associated with radiation therapy in iGCT [8, 55]. Delayed diagnosis has been reported in iGCT, although outcomes are typically favorable even in children with disseminated disease [4, 36, 38]. We observed lower survival for females with iGCTs compared with males, which may be explained by the lower percentage of female cases treated with radiation therapy (80 % in males vs. 63 % in females). Reasons for not undergoing therapy are not included in the SEER database so we were unable to evaluate the underlying reasons for treatment differences. A previous study of data from the United States National Cancer Database also reported lower survival in female iGCT patients compared to males [6], although the frequency of radiation therapy was not reported. We also observed lower survival rates in Asian males compared with white males. Potential explanations for this finding include the higher incidence of tumors of non-germinoma histology, the higher incidence of tumors located outside the pineal gland, or differences in treatment patterns. The number of tumors in the SEER database is not sufficient to distinguish between these potential hypotheses.
The SEER dataset has many strengths, including a high rate of case ascertainment and high quality data. However; limitations must also be considered. The SEER 13 registries provide population-based ascertainment of cancer cases for approximately 14 % of the US population. Differences in demographic characteristics [56] and cancer incidence rates [57] may exist in the 86 % of the population not covered by the SEER 13 registries. The small numbers in some analyses, particularly for analyses in females, is also a limitation as unstable estimates caused by small cells could lead to spurious findings.
In summary, we report higher rates of iGCT in Asians/Pacific Islanders living in the United States using population-based incidence data from the SEER program. These data are in agreement with data from the International Incidence of Childhood Cancer Vol II [9], and persisted across demographic and tumor characteristics. The explanation for these rate differences has not yet been investigated; however, the increased incidence in individuals of Asian ancestry in the United States suggests that underlying genetic susceptibility may play an important role in the etiology of iGCT.
Acknowledgments
National Institutes of Health Grants (R01 CA151284 to J.N.P., K05 CA157439 to J.A.R.) and the Children's Cancer Research Fund, Minneapolis, MN, USA.
Footnotes
Conflict of interest The authors have no financial disclosures.
Contributor Information
Jenny N. Poynter, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, 420 Delaware St SE MMC 715, Minneapolis, MN 55455, USA Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Rachel Fonstad, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, 420 Delaware St SE MMC 715, Minneapolis, MN 55455, USA.
Jakub Tolar, Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA; Division of Blood and Marrow Transplantation, Department of Pediatrics, University of Minnesota, Minneapolis, MN 55455, USA; Stem Cell Institute, University of Minnesota, Minneapolis, MN 55455, USA.
Logan G. Spector, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, 420 Delaware St SE MMC 715, Minneapolis, MN 55455, USA Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
Julie A. Ross, Division of Epidemiology and Clinical Research, Department of Pediatrics, University of Minnesota, 420 Delaware St SE MMC 715, Minneapolis, MN 55455, USA Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA.
References
- 1.Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. doi:10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- 2.Oosterhuis JW, Stoop H, Honecker F, Looijenga LH. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl. 2007;30:256–263. doi: 10.1111/j.1365-2605.2007.00793.x. discussion 263–254 doi: 10.1111/j.1365-2605.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- 3.Arora RS, Alston RD, Eden TO, Geraci M, Birch JM. Comparative incidence patterns and trends of gonadal and extragonadal germ cell tumors in England, 1979 to 2003. Cancer. 2012;118:4290–4297. doi: 10.1002/cncr.27403. doi:10.1002/cncr.27403. [DOI] [PubMed] [Google Scholar]
- 4.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. doi:10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 5.Carlos Chung KH, Owler BK, Dexter M, Chaseling R. Paediatric germ cell tumours of the central nervous system: results and experience from a tertiary-referral paediatric institution in Australia. J Clin Neurosci. 2013;20:514–519. doi: 10.1016/j.jocn.2012.04.017. doi:10.1016/j.jocn.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy BJ, Shibui S, Kayama T, Miyaoka E, Narita Y, Murakami M, Matsuda A, Matsuda T, Sobue T, Palis BE, Dolecek TA, Kruchko C, Engelhard HH, Villano JL. Primary CNS germ cell tumors in Japan and the United States: an analysis of 4 tumor registries. Neuro Oncol. 2012;14:1194–1200. doi: 10.1093/neuonc/nos155. doi:10.1093/neuonc/nos155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jellinger K. Primary intracranial germ cell tumours. Acta Neuropathol. 1973;25:291–306. doi: 10.1007/BF00691757. [DOI] [PubMed] [Google Scholar]
- 8.Villano JL, Propp JM, Porter KR, Stewart AK, Valyi-Nagy T, Li X, Engelhard HH, McCarthy BJ. Malignant pineal germ-cell tumors: an analysis of cases from three tumor registries. Neuro Oncol. 2008;10:121–130. doi: 10.1215/15228517-2007-054. doi:10.1215/15228517-2007-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkin DM, Kramarova E, Draper GJ, Masuyer E, Michaelis J, Neglia J, Qureshi S, Stiller CA, editors. International Agency for Research on Cancer. World Health Organization; Lyon: 1998. International incidence of childhood cancer. [Google Scholar]
- 10.Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, McGlynn KA. International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiol Biomarkers Prev. 2010;19:1151–1159. doi: 10.1158/1055-9965.EPI-10-0031. doi:10.1158/1055-9965.EPI-10-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance Research Program, Cancer Statistics Branch. National Cancer Institute, DCCPS; Bethesda, Md: Surveillance, epidemiology, and end results (SEER) program. SEER*Stat Database: incidence—SEER 14 Regs Research Data, Nov 2011 Sub (1992–2010). released April 2013, based on the November 2012 submission. http://www.seer.cancer.gov. [Google Scholar]
- 12.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. doi:10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 13.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–2596. doi: 10.1002/cncr.23866. doi:10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howlader N, Noone A, Krapcho M, Garshell J, Neyman N, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER cancer statistics review, 1975–2010. National cancer institute; 2013. http://seer.cancer.gov/csr/1975_2010/</csr/1975_2009_pops09/>, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. [Google Scholar]
- 15.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . International classification of diseases for oncology. 3rd edn. WHO; Geneva: 2000. [Google Scholar]
- 17.Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International classification of childhood cancer. Cancer. (third edition.) 2005;103:1457–1467. doi: 10.1002/cncr.20910. doi:10.1002/cncr.20910. [DOI] [PubMed] [Google Scholar]
- 18.SEER*Stat software Version 8.1.2. national cancer institute; Surveillance research program. www.seer.cancer.gov/seerstat. [Google Scholar]
- 19.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 20.McCarthy BJ, Kruchko C, Central Brain Tumor Registry of the United States Consensus conference on cancer registration of brain and central nervous system tumors. Neuro Oncol. 2005;7:196–201. doi: 10.1215/S115285170400050X. doi:10.1215/S115285170400050X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin TL, Sainani K, Fisher PG. Incidence patterns of central nervous system germ cell tumors: a SEER Study. J Pediatr Hematol Oncol. 2009;31:541–544. doi: 10.1097/MPH.0b013e3181983af5. doi:10.1097/MPH.0b013e3181983af5. [DOI] [PubMed] [Google Scholar]
- 22.Makino K, Nakamura H, Yano S, Kuratsu J. Population-based epidemiological study of primary intracranial tumors in childhood. Childs Nerv Syst. 2010;26:1029–1034. doi: 10.1007/s00381-010-1126-x. doi:10.1007/s00381-010-1126-x. [DOI] [PubMed] [Google Scholar]
- 23.Bauchet L, Rigau V, Mathieu-Daude H, Fabbro-Peray P, Palenzuela G, Figarella-Branger D, Moritz J, Puget S, Bauchet F, Pallusseau L, Duffau H, Coubes P, Tretarre B, Labrousse F, Dhellemmes P. Clinical epidemiology for childhood primary central nervous system tumors. J Neurooncol. 2009;92:87–98. doi: 10.1007/s11060-008-9740-0. doi:10.1007/s11060-008-9740-0. [DOI] [PubMed] [Google Scholar]
- 24.Wong TT, Ho DM, Chang KP, Yen SH, Guo WY, Chang FC, Liang ML, Pan HC, Chung WY. Primary pediatric brain tumors: statistics of Taipei VGH, Taiwan (1975-2004). Cancer. 2005;104:2156–2167. doi: 10.1002/cncr.21430. doi:10.1002/cncr.21430. [DOI] [PubMed] [Google Scholar]
- 25.Poynter JN, Amatruda JF, Ross JA. Trends in incidence and survival of pediatric and adolescent patients with germ cell tumors in the United States, 1975 to 2006. Cancer. 2010;116:4882–4891. doi: 10.1002/cncr.25454. doi:10.1002/cncr.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ducore JM, Parikh-Patel A, Gold EB. Cancer occurrence in Southeast Asian children in California. J Pediatr Hematol Oncol. 2004;26:613–618. [PubMed] [Google Scholar]
- 27.Motta PM, Nottola SA, Makabe S. Natural history of the female germ cell from its origin to full maturation through prenatal ovarian development. Eur J Obstet Gynecol Reprod Biol. 1997;75:5–10. doi: 10.1016/s0301-2115(97)00216-9. [DOI] [PubMed] [Google Scholar]
- 28.Moller H, Evans H. Epidemiology of gonadal germ cell cancer in males and females. Apmis. 2003;111:43–46. doi: 10.1034/j.1600-0463.2003.11101071.x. discussion 46–48. [DOI] [PubMed] [Google Scholar]
- 29.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Benbrahim-Tallaa L, Cogliano V. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 30.Wang XS, Tipper S, Appleby PN, Allen NE, Key TJ, Travis RC. First-morning urinary melatonin and breast cancer risk in the Guernsey Study. Am J Epidemiol. 2014;179:584–593. doi: 10.1093/aje/kwt302. doi:10.1093/aje/kwt302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. doi:10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasan V, Spence WD, Pandi-Perumal SR, Zakharia R, Bhatnagar KP, Brzezinski A. Melatonin and human reproduction: shedding light on the darkness hormone. Gynecol Endocrinol. 2009;25:779–785. doi: 10.3109/09513590903159649. doi:10.3109/09513590903159649. [DOI] [PubMed] [Google Scholar]
- 33.Cavallo A. Melatonin and human puberty: current perspectives. J Pineal Res. 1993;15:115–121. doi: 10.1111/j.1600-079x.1993.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 34.Cohen HN, Hay ID, Annesley TM, Beastall GH, Wallace AM, Spooner R, Thomson JA, Eastwold P, Klee GG. Serum immunoreactive melatonin in boys with delayed puberty. Clin Endocrinol. 1982;17:517–521. doi: 10.1111/j.1365-2265.1982.tb01623.x. [DOI] [PubMed] [Google Scholar]
- 35.Molina-Carballo A, Munoz-Hoyos A, Martin-Garcia JA, Uberos-Fernandez J, Rodriguez-Cabezas T, Acuna-Castroviejo D. 5-Methoxytryptophol and melatonin in children: differences due to age and sex. J Pineal Res. 1996;21:73–79. doi: 10.1111/j.1600-079x.1996.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 36.Sethi RV, Marino R, Niemierko A, Tarbell NJ, Yock TI, Macdonald SM. Delayed diagnosis in children with intracranial germ cell tumors. J Pediatr. 2013 doi: 10.1016/j.jpeds.2013.06.024. doi:10.1016/j.jpeds.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Jorsal T, Rorth M. Intracranial germ cell tumours. A review with special reference to endocrine manifestations. Acta Oncol. 2012;51:3–9. doi: 10.3109/0284186X.2011.586000. doi:10.3109/0284186X.2011.586000. [DOI] [PubMed] [Google Scholar]
- 38.Crawford JR, Santi MR, Vezina G, Myseros JS, Keating RF, LaFond DA, Rood BR, MacDonald TJ, Packer RJ. CNS germ cell tumor (CNSGCT) of childhood: presentation and delayed diagnosis. Neurology. 2007;68:1668–1673. doi: 10.1212/01.wnl.0000261908.36803.ac. doi:10.1212/01.wnl.0000261908.36803.ac. [DOI] [PubMed] [Google Scholar]
- 39.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Florance NR, Davis RL, Lam C, Szperka C, Zhou L, Ahmad S, Campen CJ, Moss H, Peter N, Gleichman AJ, Glaser CA, Lynch DR, Rosenfeld MR, Dalmau J. Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. doi:10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. doi:10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luca N, Daengsuwan T, Dalmau J, Jones K, deVeber G, Kobayashi J, Laxer RM, Benseler SM. Anti-N-methyl-D-aspartate receptor encephalitis: a newly recognized inflammatory brain disease in children. Arthritis Rheum. 2011;63:2516–2522. doi: 10.1002/art.30437. doi:10.1002/art.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune encephalitis in children. J Child Neurol. 2012;27:1460–1469. doi: 10.1177/0883073812448838. doi:10.1177/0883073812448838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneo-plastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. doi:10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo JW, Leung EY, Ng BL, Fu MH, Yip KK, Chan RT, Chang CM. Anti-N-methyl-D-aspartate receptor encephalitis in a young woman with an ovarian tumour. Hong Kong Med J. 2010;16:313–316. [PubMed] [Google Scholar]
- 46.Roberts R, MacDougall NJ, O'Brien P, Abdelaziz K, Christie J, Swingler R. Not hysteria: ovarian teratoma-associated anti-N-methyl-D-aspartate receptor encephalitis. Scott Med J. 2012;57:182. doi: 10.1258/smj.2012.012026. doi:10.1258/smj.2012.012026. [DOI] [PubMed] [Google Scholar]
- 47.Dabner M, McCluggage WG, Bundell C, Carr A, Leung Y, Sharma R, Stewart CJ. Ovarian teratoma associated with anti-N-methyl D-aspartate receptor encephalitis: a report of 5 cases documenting prominent intratumoral lymphoid infiltrates. Int J Gynecol Pathol. 2012;31:429–437. doi: 10.1097/PGP.0b013e31824a1de2. doi:10.1097/PGP.0b013e31824a1de2. [DOI] [PubMed] [Google Scholar]
- 48.Dulcey I, Cespedes MU, Ballesteros JL, Preda O, Aneiros-Fernandez J, Clavero PA, Nogales FF. Necrotic mature ovarian teratoma associated with anti-N-methyl-D-aspartate receptor encephalitis. Pathol Res Pract. 2012;208:497–500. doi: 10.1016/j.prp.2012.05.018. doi:10.1016/j.prp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 49.Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA. The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California Encephalitis Project. Clin Infect Dis. 2012;54:899–904. doi: 10.1093/cid/cir1038. doi:10.1093/cid/cir1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fijak M, Meinhardt A. The testis in immune privilege. Immunol Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. doi:10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 51.Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–68. doi: 10.1016/j.mce.2010.03.022. doi:10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. doi:10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 53.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. doi:10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. doi:10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bamberg M, Kortmann RD, Calaminus G, Becker G, Meisner C, Harms D, Gobel U. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585–2592. doi: 10.1200/JCO.1999.17.8.2585. [DOI] [PubMed] [Google Scholar]
- 56.SEER Characteristics of the SEER population compared with the total United States population. National Cancer Institute; http://seer.cancer.gov/registries/characteristics.html. [Google Scholar]
- 57.Merrill RM, Dearden KA. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States? Cancer Causes Control. 2004;15:1027–1034. doi: 10.1007/s10552-004-1324-5. [DOI] [PubMed] [Google Scholar]