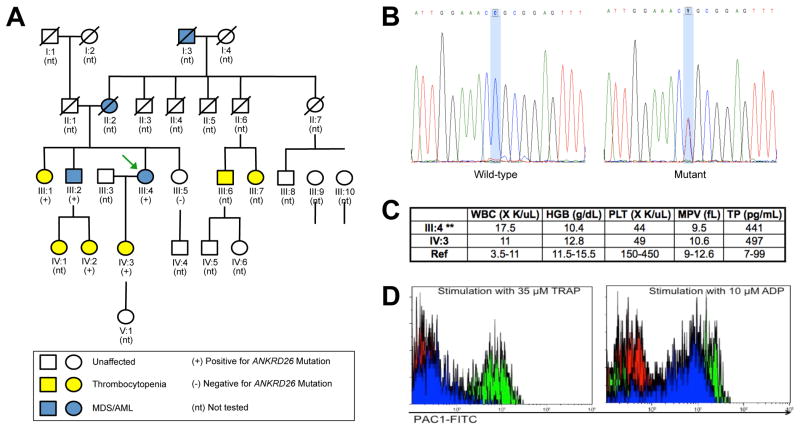
Recently a mother-daughter pair with life-long thrombocytopenia presented for consultation regarding their presumed diagnoses of autoimmune-based idiopathic thrombocytopenic purpura (ITP) and their extended family history of bleeding and myeloid malignancies (Figure 1A). The proband/mother (III:4) had been diagnosed with myelodysplastic syndrome (MDS), and the daughter (IV:3) was 32 weeks pregnant. A family history revealed 10 of 28 family members with thrombocytopenia, 4 of whom also had MDS/acute myeloid leukemia (AML). Consideration was given to known alleles associated with congenital thrombocytopenia with predisposition to MDS/AML. Among RUNX1, GATA2, and CEPBA, three genes in which germline mutations predispose to myeloid malignancies, only familial platelet disorder (FPD)/germline RUNX1 mutation is associated with thrombocytopenia and platelet dysfunction.1 Recently, however, mutations within the 5′ untranslated region (UTR) of ANKRD26 on chromosome 10p12 have been associated with Thrombocytopenia 2 (THC2), an autosomal-dominant congenital thrombocytopenia, and in one series a 30-fold increase in the frequency of MDS/AML. 2–5
Figure 1.
Characterization of the family and its germline ANKRD26 mutation. (A) Five-generation family pedigree, showing the mother (III:4; green arrow) and daughter (IV:3) pair. Family members with thrombocytopenia are shown in yellow, those with MDS/AML are in blue, and those tested for the presence of the ANKRD26 mutation are indicated by + (mutation present), or − (mutation absent). (B) Electropherograms of the sequenced wild-type control versus the proband with an ANKRD26 c.-118 C>T mutation. (C) Pertinent laboratory results of the mother and daughter are shown: WBC, white blood count; HGB, hemoglobin in g/dL; PLT, platelet count; MPV, mean platelet volume; TP, thrombopoietin in pg/mL; Ref, reference range. **Note that the blood counts obtained from the mother were analyzed when she had MDS. (D) Platelet flow cytometry. Red: control plus PBS; Green: control plus agonist; blue: patient (III:4) plus agonist. Left panel: Assay run with Thrombin Receptor Activation Peptide (TRAP) as the agonist; Right panel: Assay run with ADP as the agonist. Platelet activation was quantified by the binding of FITC-labeled PAC-1 IgM antibody (BD-Biosciences, New Jersey, USA), which selectively recognizes the activated conformation of the platelet fibrinogen receptor, GPIIb/IIIa. This approach interrogates platelets at the receptor activation step, which occurs just prior to actual aggregation in the presence of fibrinogen. Results are expressed as the percentage of events in the platelet gate that exceed the defined positivity threshold for PAC-1 binding.
Initially, we carried out gene sequencing on the mother-daughter pair for RUNX1, GATA2, and CEBPA, all of which were wild-type. We then sequenced the 5′ UTR of ANKRD26, and identified a c.-118 C>T mutation in both individuals (Figure 1B), confirmed by Clinical Laboratory Improvement Amendments (CLIA)-based testing. Subsequent sequencing of the mother’s thrombocytopenic brother with MDS (III:2), additional thrombocytopenic relatives (III:1, IV:2, IV:3), and non-thrombocytopenic sister (III:5), showed a perfect correlation between the presence of the mutant allele and thrombocytopenia (Figure 1A). ACBD5 and MASTL, earlier candidates for the causative mutation of THC2, were wild-type. 6
Platelet dysfunction in THC2 has been characterized in a series of families with ANKRD26 mutations and moderate thrombocytopenia, demonstrating normal mean platelet volumes, elevated thrombopoietin levels, variable aggregation defects in response to collagen, ADP, or ristocetin, and bone marrow examinations revealing dysmegakaryopoiesis with small megakaryoctyes and hypolobulated nuclei.3,5 Complete blood counts from the mother and daughter revealed moderate thrombocytopenia, normal mean platelet volumes, and elevated thrombopoietin levels (Figure 1C). Bone marrow examination from the mother (III:4) revealed a normal karyotype MDS with uni-lineage dysplasia, with prominent dysmegakaryopoiesis, including small megakaryocytes and hypolobated nuclei. Molecular testing showed no mutation in NPM1. Bone marrow examination of the brother with MDS (III:2) also showed MDS with uni-lineage dysplasia, like his sister, the proband. Review of the AML diagnosed in the proband’s mother (II:2) was not possible, given the length of time that transpired from this patient’s diagnosis and our analysis. Flow cytometry-based platelet studies of the mother’s platelets exhibited 2% activation to Thrombin Receptor Activation Peptide (TRAP) stimulation (controls, 40–46%; Figure 1D) as well as minimal responses to arachidonic acid, the thromboxane analogue U46619, and the calcium ionophore A23187 (data not shown). ADP was the only agonist tested that elicited a normal platelet response, indicating abnormal platelet function.
The identification of this family supports the association of ANKRD26 mutations with THC2 and a predisposition to myeloid malignancies, as observed for individuals with germline RUNX1 mutations. When patients present with suspected autoimmune-based ITP, any family history of bleeding and/or MDS/AML should prompt consideration of either RUNX1 or ANKRD26 mutations. If either is confirmed, the patient and at-risk family members should receive genetic counseling, appropriate screening, and a discussion of management options and the risks of developing myeloid malignancy. 7 In this case, because of the observed platelet defects, we advised the daughter’s obstetrician to use peripartum platelet transfusions to ≥130 × 109/L to reduce the likelihood of postpartum hemorrhage. This recommendation was followed, and the daughter gave birth without complications. We urge hematologists to test for germline predisposition to thrombocytopenia and myeloid malignancies when evaluating individuals with a similar personal or family history to our patients.
Acknowledgments
We gratefully acknowledge the members of this family who were willing to participate in research. This work was supported by the National Institutes of Health K12CA139160 (JEC).
Footnotes
Contributions: NAM and IS first identified this family clinically; LAG supervised the clinical evaluation of members of this family and the associated research; RL provided genetic counseling; BN and RL established the detailed pedigree; SG and JM performed platelet aggregation studies; RM performed laboratory experiments; JEC and LAG analyzed the data; and all authors wrote and edited the manuscript.
Conflict of Interest disclosure: The authors declare no competing financial interests.
Contributor Information
Rafael Marquez, The University of Chicago Department of Medicine, Chicago, IL.
Andrew Hantel, The University of Chicago Department of Medicine, Chicago, IL.
Rachelle Lorenz, The University of Chicago Department of Medicine & Center for Clinical Cancer Genetics, Chicago, IL.
Barbara Neistadt, The University of Chicago Department of Medicine, Chicago, IL.
Jerry Wong, The University of Chicago Department of Pathology, Chicago, IL.
Jane E. Churpek, The University of Chicago Department of Medicine, Comprehensive Cancer Center, & Center for Clinical Cancer Genetics, Chicago, IL
Nameer Al Mardini, Swedish American Health System, Rockford, IL.
Ismael Shaukat, OSF HealthCare, Rockford, IL.
Sandeep Gurbuxani, The University of Chicago Department of Pathology, Chicago, IL.
Jonathan L. Miller, The University of Chicago Department of Pathology, Chicago, IL
Lucy A. Godley, The University of Chicago Department of Medicine, Comprehensive Cancer Center, & Center for Clinical Cancer Genetics, Chicago, IL
References
- 1.Nickels EM, Soodalter J, Churpek JE, Godley LA. Recognizing familial myeloid leukemia in adults. Ther Adv Hematol. 2013;4(4):254–269. doi: 10.1177/2040620713487399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pippucci T, Savoia A, Perrotta S, et al. Mutations in the 5′ UTR of ANKRD26, the ankirin repeat domain 26 gene, cause an autosomal-dominant form of inherited thrombocytopenia, THC2. Am J Hum Genet. 2011;88(1):115–120. doi: 10.1016/j.ajhg.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noris P, Perrotta S, Seri M, et al. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: analysis of 78 patients from 21 families. Blood. 2011;117(24):6673–6680. doi: 10.1182/blood-2011-02-336537. [DOI] [PubMed] [Google Scholar]
- 4.Al Daama SA, Housawi YH, Dridi W, et al. A missense mutation in ANKRD26 segregates with thrombocytopenia. Blood. 2013;122(3):461–462. doi: 10.1182/blood-2013-03-489344. [DOI] [PubMed] [Google Scholar]
- 5.Noris P, Favier R, Alessi MC, et al. ANKRD26-related thrombocytopenia and myeloid malignancies. Blood. 2013;122(11):1987–1989. doi: 10.1182/blood-2013-04-499319. [DOI] [PubMed] [Google Scholar]
- 6.Punzo F, Mientjes EJ, Rohe CF, et al. A mutation in the acyl-coenzyme A binding domain-containing protein 5 gene (ACBD5) identified in autosomal dominant thrombocytopenia. J Thromb Haemost. 2010;8(9):2085–2087. doi: 10.1111/j.1538-7836.2010.03979.x. [DOI] [PubMed] [Google Scholar]
- 7.Churpek JE, Lorenz R, Nedumgottil S, et al. Proposal for the clinical detection and management of patients and their family members with familial myelodysplastic syndrome/acute leukemia predisposition syndromes. Leuk Lymphoma. 2013;54(1):28–35. doi: 10.3109/10428194.2012.701738. [DOI] [PubMed] [Google Scholar]