Abstract
Purpose
To evaluate the effect of storage temperature on the morphology, viability, cell number and metabolism of cultured human conjunctival epithelial cells (HCjE).
Materials & methods
Three-day cultured HCjE were stored at nine different temperatures between 4°C and 37°C for four and seven days. Phenotype was assessed by immunofluorescence microscopy, morphology by scanning electron microscopy, viability and cell number by a microplate fluorometer and glucose metabolism by a blood gas analyzer.
Results
Cultured cells not subjected to storage expressed the conjunctival cytokeratins 7 and 19, and the proliferation marker PCNA. Cell morphology was best maintained following four-day storage between 12°C and 28°C, and following 12°C storage after seven days. Assessed by propidium iodide uptake, the percentage of viable cells after four-day storage was maintained only between 12°C and 28°C, while it had decreased in all other groups (P<0.05; n=4). After seven days this percentage was maintained in the 12°C group, but it had decreased in all other groups, compared to the control (P<0.05; n=4). The total number of cells remaining in the cultures after four-day storage, compared to the control, had declined in all groups (P<0.05; n=4), except 12°C and 20°C. Following seven days this number had decreased in all groups (P<0.01; n=4), except 12°C. Four-day storage at 12°C demonstrated superior preservation of the number of calcein-stained viable cells (P<0.05) and the least accumulation of ethidium homodimer 1-stained dead cells (P<0.001), compared to storage at 4°C and 24°C (n=6). The total metabolism of glucose to lactate after four-day storage were higher in the 24°C group compared to 4°C and 12°C, as well as the control (P<0.001; n=3).
Conclusions
Storage at 12°C appears optimal for preserving morphology, viability and total cell number in stored HCjE cultures. The superior cell preservation at 12°C may be related to temperature-associated effects on cell metabolism.
Keywords: Conjunctival epithelium, cell culture, storage temperature, transplantation, limbal stem cell deficiency
INTRODUCTION
The conjunctival epithelium consists of stratified squamous cells and goblet cells. It covers the inside of the eyelids, in addition to the anterior surface of the eye, excluding the cornea. Goblet cells are necessary for a normal tear film, secreting gel-forming mucins that contribute to tear film stability and thereby to the optical clarity of the cornea. Loss of a healthy conjunctiva leads to chronic ocular surface inflammation, which can ultimately result in corneal scarring and blindness with concurrent loss of stem cells of the cornea, the limbal stem cells (LSC).1, 2 In addition to repairing conjunctival defects,2–4 transplantation of cultured human conjunctival epithelial cells (HCjE) represents a promising new treatment strategy for blindness caused by bilateral limbal stem cell deficiency (LSCD).2, 5–7 Moreover, the long-term stability of LSC transplantation to the cornea often relies on conjunctival epithelial restoration.2
The possibility of storing cultured cells for at least a few days could ease logistical difficulties8 regarding transplantation of tissue and potentially improve outcomes by providing time for quality control of the transplant.9 Furthermore, a reliable storage technology is mandatory to enable transportation of tissue,10 which will become increasingly important in regenerative medicine as various technologies leave the experimental stage to be used in mainstream medicine. Studies on the preservation of cultured ocular epithelia are still limited, however. Whereas cryopreservation is the standard method for storing cells in suspension, cryopreservation of adherent stratified ocular epithelia has been less successful.11 In addition, when only short-term storage is required, above-zero degree storage methods are more convenient than cryopreservation, which necessitates expensive cryopreservation facilities and expertise.
Storing cells at temperatures below 37°C reduces their metabolism by slowing down the rate of enzymatic reactions.12 Although this may preserve the stored tissue, by decreasing the rate of injurious cell processes, temperatures that are too cold (e.g., 0°C to 10°C) can conversely have a detrimental effect on cell viability12 owing to electrolyte disturbances and oxidative stress.12 Hence, a delicate balance appears to exist. To our knowledge, however, no studies have investigated the whole 4°C to 37°C range with respect to defining the best storage temperature for cultured HCjE.
In the current study, we hypothesized that cultured HCjE should be stored at a low enough temperature to decrease metabolism but at a high enough temperature to avoid cold-induced cell damage. In this study, we used commercially available HCjE and cultured the cells on conventional multiwell plates to reduce the biological variation to a minimum, thereby isolating the effect of storage temperature. The effect of storage at nine different temperatures between 4°C and 37°C on the morphology, viability, cell number and metabolism of cultured HCjE was investigated. Storage at 12°C appeared optimal for preserving morphology, viability and total cell number of cultured HCjE for up to seven days.
MATERIALS AND METHODS
Normal HCjE and serum-free culture medium (CEpiCM) were obtained from ScienCell Research Laboratories (San Diego, CA). Trypsin-EDTA, HEPES, sodium bicarbonate, gentamycin, propidium iodide (PI) and 4',6-diamidino-2-phenylindole (DAPI) were obtained from Sigma Aldrich (St Louis, MO). Minimum essential medium (MEM) and the calcein-acetoxymethyl ester (CAM)/ethidium homodimer 1 (EH-1) Live/Dead viability kit were obtained from Invitrogen (Carlsbad, CA). Nunclon Δ surface 24-well plates, glass coverslips, pipettes and other routine plastics were obtained from VWR International (West Chester, PA). Mouse monoclonal anti-cytokeratin (CK) 7 (clone OV-TL 12/30) and mouse monoclonal anti-proliferating cell nuclear antigen (PCNA; clone PC10) were from DAKO (Glostrup, Denmark). Rabbit polyclonal anti-CK19 (ab53119) and secondary FITC-conjugated anti-mouse and anti-rabbit antibodies were obtained from Abcam (Cambridge, UK).
Cell Culture
HCjE were seeded (5000 cells/cm2) in serum-free CEpiCM culture medium on Nunclon Δ surface 24-well plates or glass coverslips. Cultures were refed with CEpiCM after two days and cultured under routine culture conditions of 95% air and 5% CO2 at 37°C for three days, to obtain a confluent monolayer.
Cell Storage
Following culture, each multiwell plate was sealed and randomly selected for storage in custom-built temperature-regulating cabinets13 at one of nine different temperatures (4°C, 8°C, 12°C, 16°C, 20°C, 24°C, 28°C, 32°C and 37°C) for four or seven days. The standard deviation of the temperature in each temperature cabinet was ±0.4°C, as reported elsewhere.13 The storage medium consisted of 1 mL MEM with 12.5 mM Hepes, 22.3mM sodium bicarbonate and 50µg/ml gentamycin. Cultured HCjE that were not subjected to storage served as controls.
Immunofluorescence Microscopy
Cells cultured for three days in Nunclon multiwell plates were rinsed in phosphate-buffered saline (PBS) and fixed in 100% methanol for 15 minutes at room temperature before being returned to fresh PBS. Fixed cells were incubated in a blocking buffer that consisted of 1% bovine serum albumin (BSA) and 0.1% Tween-20 in PBS for one hour at room temperature. For phenotypic characterization the three-day cultured cells were incubated overnight at 4°C with the following antibodies diluted in PBS with 1% BSA: anti-CK19 (1:50), a conjunctival epithelial cell marker;14 the OV-TL 12/30 clone of anti-CK7 (1:100), a marker which is found in conjunctival epithelial cells;14 and anti-PCNA (1:1000), indicative for proliferating cells. The FITC-conjugated secondary antibody was diluted 1:250 in PBS with 1% BSA and incubated for one hour at room temperature. Negative control consisted of replacing the primary antibody with PBS. The cultures were washed three times in PBS containing 1µg/mL DAPI to stain the cell nuclei.
Scanning Electron Microscopy
HCjE cultured on glass coverslips were used for scanning electron microscopy (SEM) as described elsewhere.15 Glutaraldehyde-fixed samples (n=3) were dehydrated in increasing ethanol concentrations, then dried according to the critical point method (Polaron E3100 Critical Point Drier; Polaron Equipment Ltd., Watford, UK) with CO2 as the transitional fluid. The specimens were attached to carbon stubs and coated with a 30 nm thick layer of platinum in a Polaron E5100 sputter coater before being photographed with an XL30 ESEM electron microscope (Philips, Amsterdam, the Netherlands).
Quantification of Viability and Total Cell Number after Storage
After storage at the nine temperatures mentioned above, the HCjE (n=4) were incubated with PI (5µg/mL in PBS) at room temperature for three minutes, in order to stain the nuclei of the dead cells, which were mostly detached from the culture well. The PI fluorescence of the dead cell nuclei was then measured with a microplate fluorometer (Fluoroskan Ascent, Thermo Scientific, Waltham, MA) with the excitation/emission filter pair 530/620. The cells were thereafter fixed with 100% methanol for 15 minutes and rinsed thoroughly three times with PBS to remove the non-adherent dead cells, before being incubated a second time with PI (5µg/mL in PBS) for three minutes to stain all the nuclei of the remaining adherent cells in the culture. Finally, the PI fluorescence of the fixed adherent cells was measured with the microplate fluorometer. Background fluorescence, measured in wells containing PI but not cells, was subtracted from all values. From pilot studies it became apparent that relatively few dead cells remained adherent in the fixed cultures compared to the number of dead cells that detached during processing for the viability analysis. Therefore, the percentage of viable cells was calculated as follows: 100-(PI fluorescence of dead cells/(PI fluorescence of dead cells + PI fluorescence of fixed cells))%. The PI fluorescence of fixed cells denotes the total number of cells remaining in the cultures.
In addition to the PI viability analysis, the percentage of remaining viable and dead cells following four-day storage at 4°C, 12°C and 24°C was measured using a CAM/EH-1 Live/Dead viability kit as described previously,16 with some modifications. Calcein-acetoxymethyl enters the cytosol where it is enzymatically cleaved to the green fluorescent calcein by esterases that are present in living cells.17 Viable cells thereafter retain calcein, as it cannot traverse healthy cell membranes. EH-1 stains the nuclei of dead cells red, as it can only transverse cell membranes of dead cells. After storage the HCjE were incubated in PBS containing 1µM CAM and 1µM EH-1 at room temperature for one hour. Thereafter, calcein and EH-1 fluorescence was measured by the microplate fluorometer (Fluoroskan Ascent) with the excitation/emission filter pairs 485/538 and 530/620, respectively. Background fluorescence, measured in wells containing CAM/EH-1-reagents, but not cells, was subtracted from all values before calculating mean fluorescence for the groups. The validity of the CAM and EH-1 stainings were confirmed by epifluorescence microscopy using HCjE cultured for three days as positive control and HCjE exposed to methanol for 15 minutes as negative control (data not shown).
Metabolic Analysis
For measurement of the amount of glucose remaining and lactate accumulated in the storage medium, 200µL of storage medium was withdrawn from each culture well following four-day storage with a 2mL syringe. Each sample was run immediately in a Radiometer ABL 700 blood gas analyzer (Radiometer Copenhagen, Denmark).
Statistical Analysis
One-way ANOVA with Tukey’s post hoc pair-wise comparisons (SPSS ver. 19.0) was used to compare the groups. Data were expressed as mean ± standard deviation, and values were considered significant if P<0.05.
RESULTS
Phenotypic Characterization of Cultured HCjE
To characterize the HCjE, three-day cultured cells without subsequent storage were evaluated by immunofluorescence microscopy. Cultured HCjE expressed the conjunctival epithelial markers CK7 (n=6) and CK19 (n=3), in addition to the proliferation marker PCNA (n=7) (data not shown).
Morphology of Cultured HCjE Following Storage
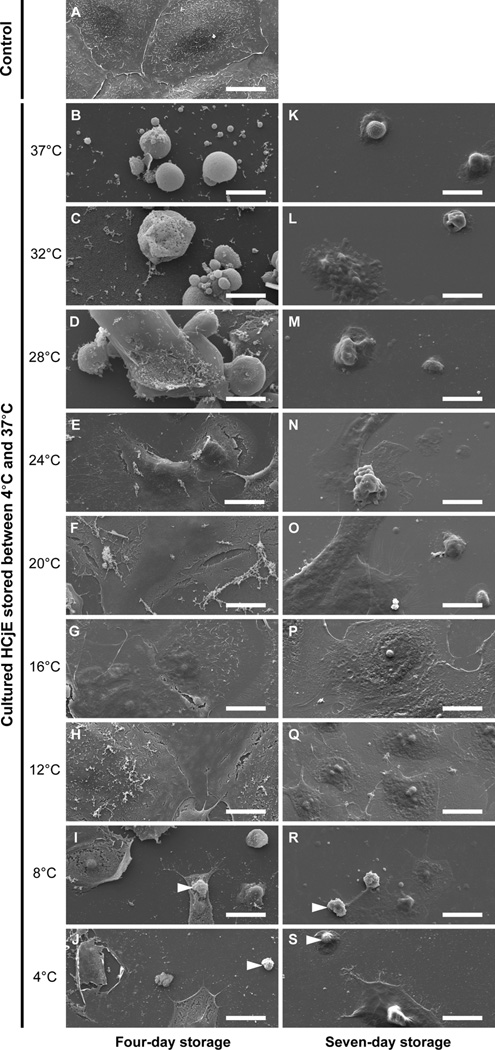
HCjE cultured for three days, but not stored (n=3), were tightly adjoined (Figure 1A). Following four-day storage (n=3), cell-cell attachment was optimally conserved in the 12°C, 16°C, 20°C and 24°C groups (Figure 1E–H). Cold temperature storage (4°C and 8°C), as well as relatively high temperature storage (32°C and 37°C), yielded poor results with large cell loss after both four and seven days, with the 4°C and 8°C groups displaying cytosol and nuclear shrinkage (Figure 1I–J and R–S), and the 32°C and 37°C groups showing extensive cell membrane blebbing (Figure 1B–C and K–L). Seven-day storage (n=3), as opposed to four-day storage, resulted in large loss of cell-cell contact and cell-substrate attachment in the 20°C, 24°C and 28°C groups. In contrast, only the 12°C group, and to a certain degree the 16°C group, preserved a confluent layer of cells without apparent signs of cell damage. Collectively, these results indicate that the morphology of cultured HCjE can be preserved at a relatively wide temperature range (e.g., 12°C to 24°C) when stored for short periods (i.e., four days), whereas for longer storage periods (i.e., seven days), the storage temperature becomes more critical and should be lowered to about 12°C.
Figure 1.
Scanning electron photomicrographs of cultured human conjunctival epithelial cells (HCjE) stored at nine different temperatures between 4°C and 37°C for four and seven days. Images are representative of three experiments. Arrowheads indicate cytoplasmic and nuclear shrinkage. Scale bars are 20µm in length.
Viability and Total Cell Number Following Storage
Cell viability after storage for four and seven days was quantified by measuring PI uptake (n=4). Cultured HCjE not subjected to storage were for the most part PI-negative (data not shown). In contrast, exposure to 100% methanol killed most cells making them essentially all PI-positive (data not shown). Thus PI staining can be used to determine the percentage of viable HCjE cells after storage and methanol can be used to kill the adherent cells.
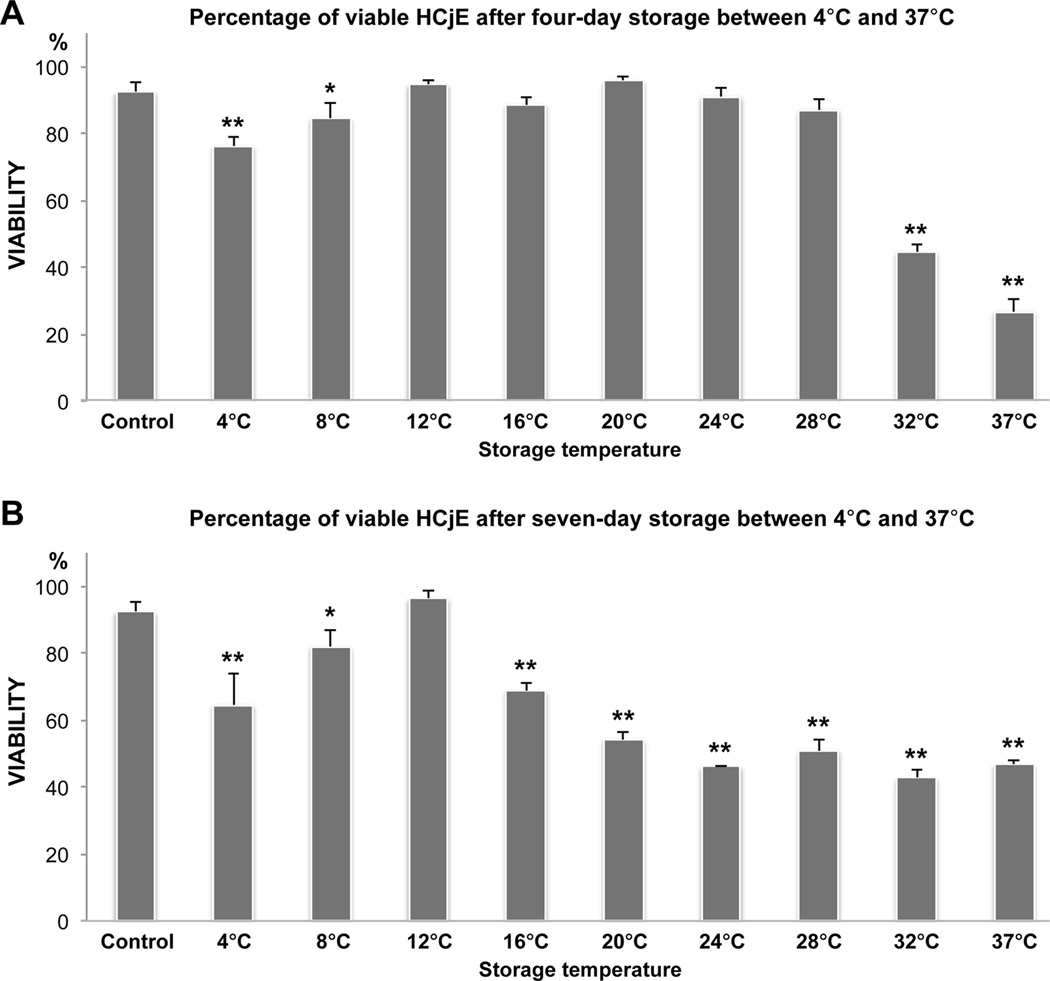
Although SEM analysis of the stored cultures demonstrated morphological changes suggestive of cell death, following storage most dead cells appeared to already have detached from the culture well and were seen floating in the storage medium. After four days of storage, compared with the control, the percentage of viable cells was maintained in the 12°C, 16°C, 20°C, 24°C and 28°C groups, but had decreased in the low (4°C and 8°C; P<0.01 and <0.05, respectively) and to a greater extent in the high (32°C and 37°C; P<0.01) temperature groups (Figure 2A). In contrast, subsequent to seven days of storage, 12°C storage was the only group that maintained viability (Figure 2B). Thus, the PI viability results were in line with the morphology results, confirming that the optimal storage temperature range becomes narrower with extended storage time and that 12°C is better for preserving cells up to one week.
Figure 2.
Bar charts illustrating the percentage of viable human conjunctival epithelial cells after four (A) and seven (B) days of storage at nine different temperatures between 4°C and 37°C. Propidium iodide (PI) fluorescence was measured by a microplate fluorometer to quantify the percentage of viable cells. Cultured HCjE not subjected to storage served as a control. N=4. Error bars indicate standard deviation. * P<0.05 and ** P<0.01, compared with the control.
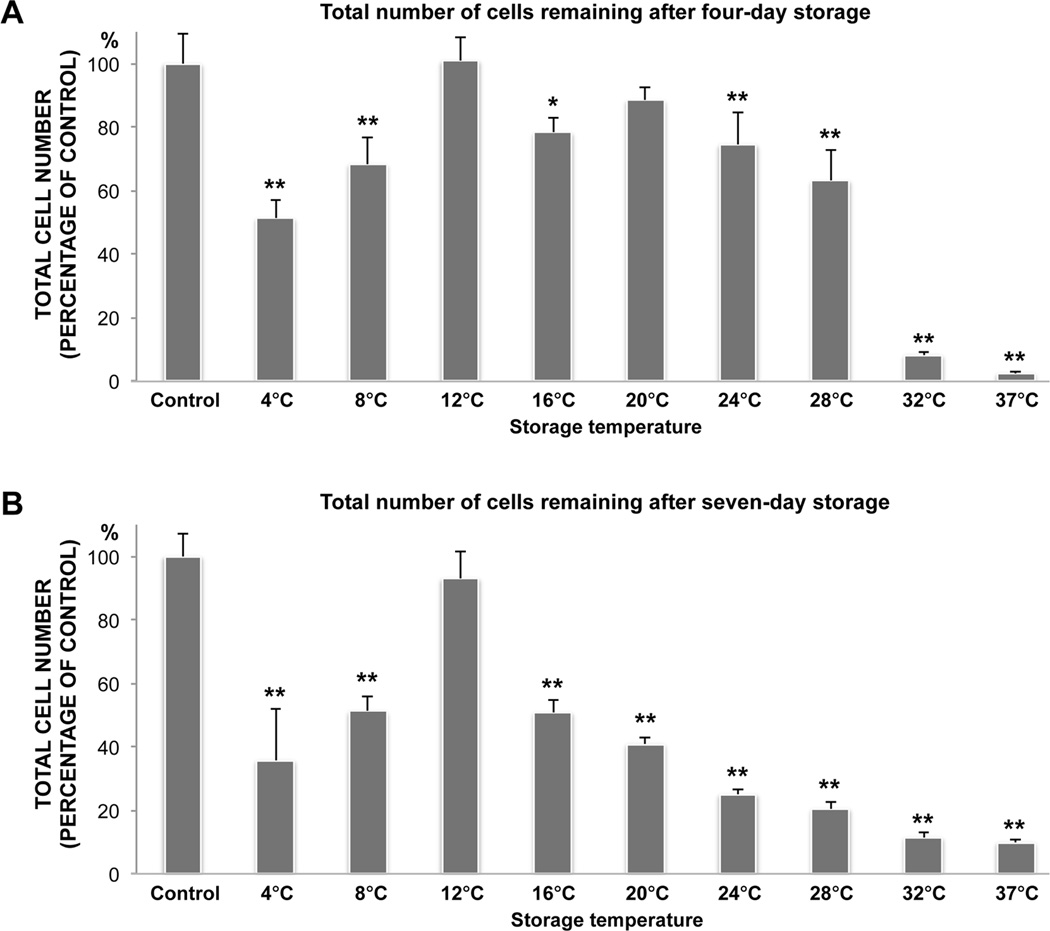
The number of cells remaining in the cultures after storage compared to the unstored control cultures was quantified by measuring the PI uptake of methanol-fixed cultures (n=4). After four-day storage the number of adherent cells remaining had decreased in all storage groups (P<0.05), except in the 12°C and 20°C groups (Figure 3A). Following seven days of storage, this number was solely maintained in the 12°C storage group (Figure 3B). Hence, these data were in agreement with those from the PI viability analysis, collectively demonstrating that viability and total number of adherent cells are generally best preserved between 12°C and 24°C after four-days, and at 12°C following seven days of storage.
Figure 3.
Bar charts illustrating the total number of cells (dead and viable) remaining after four (A) and seven (B) days of storage at nine different temperatures between 4°C and 37°C. After storage the samples were methanol-fixed to kill all the remaining adherent cells. Propidium iodide (PI) uptake in fixed cells was then measured by a microplate fluorometer to determine the total number of remaining cells. Cultured HCjE not subjected to storage served as a control. N=4. Error bars indicate standard deviation. * P<0.01 and ** P<0.001, compared with the control.
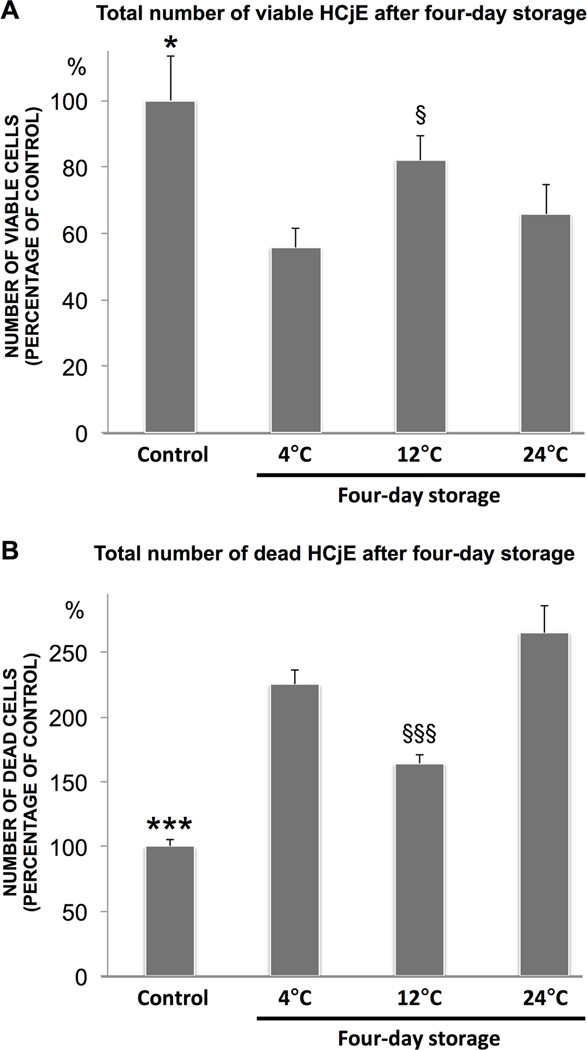
To validate the main finding that cell survival is optimal subsequent to storage at 12°C, cultured HCjE stored at 4°C, 12°C and 24°C for four days were assayed with a CAM/EH-1 Live/Dead viability kit (n=6). HCjE not subjected to storage served as control. The number of viable cells, expressed as a percentage of the control, decreased following storage at all three temperatures (P<0.05) (Figure 4A). In line with the PI viability analysis, however, the percentage of remaining viable cells was superior after 12°C storage (82% ± 7%) compared to 4°C (56% ± 6%; P<0.001) and 24°C (66% ± 9%; P<0.05) storage. The number of dead cells, expressed as a percentage of the control, increased after storage at all three temperatures (P<0.001) (Figure 4B). In support of the viability results, the percentage of dead cells relative to the control was lowest following storage at 12°C (164% ± 7%) compared to 4°C (225% ± 11%) and 24°C (265% ± 21%) storage (P<0.001). Collectively, the results from the CAM/EH-1 Live/Dead viability kit demonstrated optimal cell survival following 12°C storage, when compared to storage at 4°C and 24°C, thereby supporting the PI viability results.
Figure 4.
Viability of cultured HCjE stored for four days at 4°C, 12 and 24°C was quantified with a microplate fluorometer using a calcein-acetoxymethyl ester/ethidium homodimer 1 (EH-1) Live/Dead kit. Calcein-acetoxymethyl ester is converted to the green fluorescent calcein by living cells, while EH-1 stains the nuclei of dead cells red. The bar charts illustrate the total number of calcein-stained live cells (A) and EH-1-stained dead cells (B). Cultured HCjE not subjected to storage served as control. N=6. Error bars indicate standard deviation. * P<0.05 and *** P<0.001, compared to all other groups; § P<0.05, compared to 4°C and 24°C; §§§ P<0.001 compared to 4°C and 24°C.
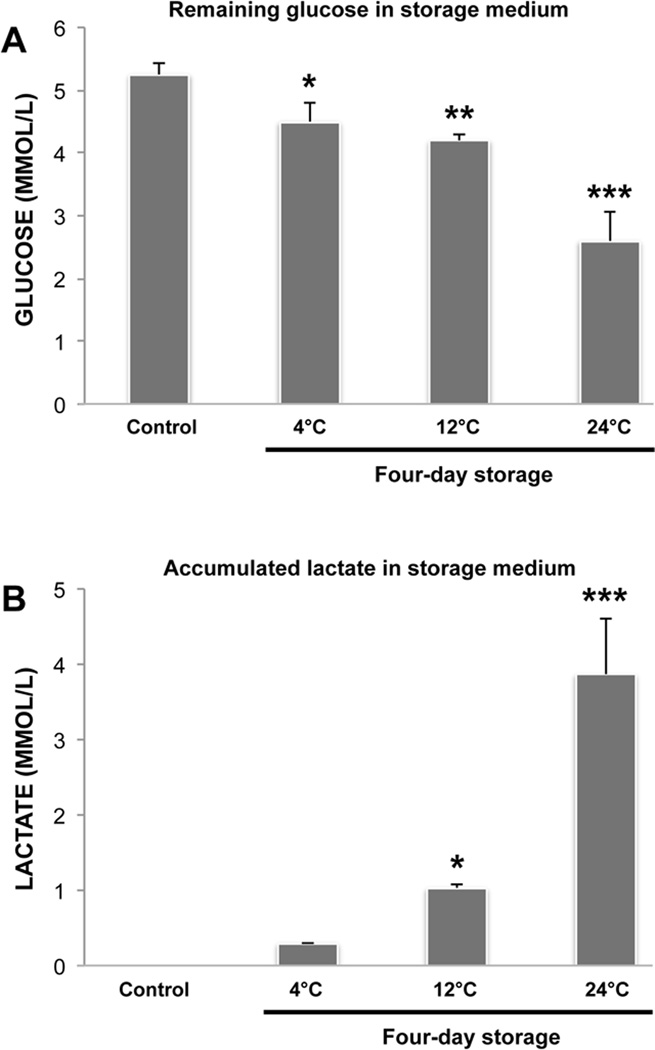
Measurements of Glucose and Lactate Concentrations in the Storage Medium
Compared to the non-stored control, cell morphology and viability after four-day storage was generally preserved at temperatures between 12°C and 24°C. After seven days, however, cells were only well preserved in the 12°C group. To explore if this was associated with differences in storage medium glucose and lactate concentrations, we measured the concentrations of remaining glucose and accumulated lactate after four-day storage at 4°C, 12°C and 24°C (n=3). Compared to fresh storage medium without cells (5.3mmol/L ± 0.2), the glucose concentrations had decreased significantly in the 4°C (4.5mmol/L ± 0.3; P<0.05), 12°C (4.2mmol/L ± 0.1; P<0.01) and 24°C (2.6mmol/L ± 0.5; P<0.001) groups (Figure 5A). The lactate concentration demonstrated a corresponding increase with storage temperature and was significantly higher in the 12°C (1.0mmol/L ± 0.1; P<0.05) and 24°C (3.9mmol/L ± 0.7; P<0.001) groups, compared to the control (0mmol/L ± 0) (Figure 5B). Moreover, lactate and glucose concentrations were significantly (P<0.001) higher and lower, respectively, in the 24°C group, compared to in the 4°C and 12°C groups. Thus, these data show that the metabolism of glucose to lactate is associated with storage temperature and is more pronounced when storing HCjE at 24°C than at 4°C or 12°C.
Figure 5.
Bar charts showing the remaining storage medium glucose and accumulated lactate after four-day storage at 4°C, 12°C and 24°C. Fresh storage medium without cells served as control. N=3. Error bars indicate standard deviation. * P<0.05, ** P<0.01 and *** P<0.001, compared with the control.
DISCUSSION
Ambient storage temperature (i.e., around 20°C) has proven superior to conventional cold-storage for corneal epithelial cells,18, 19 but to find the best storage temperature a wide temperature range must be explored. The current study demonstrated that when cells were assessed for morphology, viability, cell number and glucose conversion to lactate by metabolically active cells only 12°C, out of all nine storage temperatures tested, preserved the HCjE for up to seven days. This stresses the necessity of investigating multiple temperatures spanning the whole range between 4°C and 37°C when attempting to pinpoint the optimal storage temperature. In addition to showing that successful HCjE preservation is highly temperature-dependent, our study indicates that choosing the optimal temperature becomes even more critical when extending the storage time from four to seven days.
The normal HCjE used in the current study were commercially available cells that expressed the conjunctival epithelial cytokeratins CK19 and CK7, hence supporting a conjunctival origin. The cells did not stratify in culture when following the supplier’s culture protocol, but formed a confluent cobblestone monolayer after three days. Although the native conjunctival and corneal epithelium form a multilayered, organized structure in vivo, the use of monolayer cultures is still clinically relevant, as successful ocular surface restoration by transplanting monolayer limbal and conjunctival cultures has been demonstrated in multiple studies.20–23 The use of monolayer cultures was also necessary to avoid biases related to different degrees of culture stratification when performing microplate fluorometer measurements.
Morphology was optimally conserved between 12°C and 24°C after four days and at 12°C after seven days of storage. Morphological signs of apoptosis were observed at both low (4°C and 8°C) and high (32°C and 37°C) storage temperatures, in the form of cytosol and nuclear shrinkage, as well as membrane blebs.24 Cold storage is known to induce both apoptosis and necrosis, whereas loss of cell contacts has been shown to trigger apoptosis.25 Poor cell-cell contact, observed especially subsequent to storage at the lowest (4°C and 8°C) and highest (32°C and 37°C) temperatures, might therefore explain some of the reduction in viability at these temperatures. Alongside loss of cell-cell contact, cell detachment was especially evident at the lowest and highest temperatures. This agrees with our previous study on the storage of cultured human limbal epithelial cells, which demonstrated pronounced cell detachment following storage at 5°C and 31°C.19 Studies on the preservation of whole corneas further support these findings.26, 27
Viability was preserved after four-day storage between 12°C and 28°C, whereas only the 12°C group maintained cell viability after seven days of storage, assessed by PI uptake. Similar results were obtained when quantifying the total number of cells remaining in the cultures in the experimental groups, compared to the unstored control cells, thereby corroborating the PI viability analysis. Although the CAM/EH-1 viability analysis demonstrated a decreased number of viable cells following storage at all three tested temperatures, the results obtained with this analysis confirmed the superior preservation of live cells and minimal accumulation of dead cells following storage at 12°C, compared to storage at lower (4°C) and higher (24°C) temperatures. We previously investigated the effect of 5°C, 23°C and 31°C storage on the viability of cultured human limbal epithelial cells, concluding that 5°C and 31°C storage yielded inferior viability.19 Storage at 23°C, or ambient temperature, has proven suitable in several studies,9, 18, 19, 28, 29 including a recent study on the storage of cultured HCjE.30 Corneal epithelial cells encapsulated in alginate reportedly maintained a higher percentage of viable cells after storage at ambient temperature (18°C–22°C) than when stored at 4°C or 31°C.18 Thus, the results of the present study concur with previous work on ocular cell storage and with a study examining ovarian cells by Hunt and colleagues,31 which demonstrated that 17°C storage resulted in the highest cell viability and the lowest viability followed storage at 4°C and 37°C. It should be noted, however, that Hunt and colleagues stored their cells as a suspension, and did not investigate temperatures between 6°C and 17°C or between 22°C and 37°C.
The viability analysis following storage was performed at ambient temperature without rewarming the cultures to 37°C. This may have inhibited potential latent cold-induced cell damage from developing, which has been demonstrated upon returning cells to 37°C after hypothermic storage.32 This type of damage causes apoptosis through caspase-3 activation due to release of reactive oxygen species during rewarming.12 The likelihood of developing cold-induced apoptosis differs between cell types and depends on the duration of cold-exposure. This type of apoptosis has been described in several cell types upon rewarming following storage at 4°C.12 As the viability analyses in the current study involved a maximum incubation time of one hour at room temperature after storage, this may not have been sufficiently warm or long enough for any significant rewarming-injury to develop. Therefore, the loss of viability in this study may mostly stem from injury inflicted upon the cells during storage.
We sampled storage medium for glucose and lactate measurements to investigate whether the superior cell preservation following 12°C storage, compared to lower (4°C) or higher (24°C) temperatures, was associated with differences in cell metabolism. Our results showed increasing breakdown of glucose to lactate with higher storage temperature. This is in line with the fact that the enzymatic reaction rate is dependent on temperature.33 Furthermore, a previous study also demonstrated progressively larger concentrations of lactate in the medium following corneal storage at 4°C, 21°C and 31°C.34 The glucose and lactate concentrations following storage at 4°C was maintained in our study, compared to the unstored control. Thus, lack of glucose or lactate accumulation does not appear to contribute to the poor cell preservation at 4°C.
We have demonstrated that the storage temperature is critical for preservation of HCjE. Storage at 12°C appears optimal for preserving morphology, viability and number of cells in the HCjE cultures for up to seven days. The superior cell preservation at 12°C may be related to temperature-associated effects on cell metabolism. Maintaining the optimal temperature range during storage and transportation of the cultured cells to the transplantation clinics may improve future ocular surface therapy with cultured HCjE. In addition, the surprising finding that “sub-ambient” storage at 12°C is optimal for preservation of HCjE may have implications for storage of cell types and tissues beyond that of conjunctival epithelial cells.
ACKNOWLEDGMENTS
The authors thank Steinar Stølen (Department of Oral Biology, Faculty of Dentistry, University of Oslo, Oslo, Norway); Lara Pasovic, Peder Aabel and Catherine Jackson (Department of Medical Biochemistry, Oslo University Hospital, Oslo, Norway); Xiangjun Chen (SynsLaser Kirurgi AS, Oslo/Tromsø, Norway) and Astrid Østerud (Department of Ophthalmology, Oslo University Hospital, Oslo, Norway) for their excellent assistance and support.
Declaration of funding: This work was supported by the Norwegian Research Council and South-Eastern Norway Regional Health Authority.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCES
- 1.Schrader S, Notara M, Beaconsfield M, Tuft SJ, Daniels JT, Geerling G. Tissue engineering for conjunctival reconstruction: established methods and future outlooks. Current eye research. 2009;34:913–924. doi: 10.3109/02713680903198045. [DOI] [PubMed] [Google Scholar]
- 2.Mason SL, Stewart RM, Kearns VR, Williams RL, Sheridan CM. Ocular epithelial transplantation: current uses and future potential. Regenerative medicine. 2011;6:767–782. doi: 10.2217/rme.11.94. [DOI] [PubMed] [Google Scholar]
- 3.Ang LP, Tan DT. Autologous cultivated conjunctival transplantation for recurrent viral papillomata. American journal of ophthalmology. 2005;140:136–138. doi: 10.1016/j.ajo.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Ang LP, Tan DT, Cajucom-Uy H, Beuerman RW. Autologous cultivated conjunctival transplantation for pterygium surgery. American journal of ophthalmology. 2005;139:611–619. doi: 10.1016/j.ajo.2004.10.056. [DOI] [PubMed] [Google Scholar]
- 5.Ang LP, Tanioka H, Kawasaki S, et al. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Investigative ophthalmology & visual science. 2010;51:758–764. doi: 10.1167/iovs.09-3379. [DOI] [PubMed] [Google Scholar]
- 6.Ono K, Yokoo S, Mimura T, et al. Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Molecular vision. 2007;13:1138–1143. [PMC free article] [PubMed] [Google Scholar]
- 7.Tanioka H, Kawasaki S, Yamasaki K, et al. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Investigative ophthalmology & visual science. 2006;47:3820–3827. doi: 10.1167/iovs.06-0293. [DOI] [PubMed] [Google Scholar]
- 8.O'Callaghan AR, Daniels JT. Concise review: limbal epithelial stem cell therapy: controversies and challenges. Stem Cells. 2011;29:1923–1932. doi: 10.1002/stem.756. [DOI] [PubMed] [Google Scholar]
- 9.Utheim TP, Raeder S, Utheim OA, de la Paz M, Roald B, Lyberg T. Sterility control and long-term eye-bank storage of cultured human limbal epithelial cells for transplantation. The British journal of ophthalmology. 2009;93:980–983. doi: 10.1136/bjo.2008.149591. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad S, Osei-Bempong C, Dana R, Jurkunas U. The culture and transplantation of human limbal stem cells. Journal of cellular physiology. 2010;225:15–19. doi: 10.1002/jcp.22251. [DOI] [PubMed] [Google Scholar]
- 11.Yeh HJ, Yao CL, Chen HI, Cheng HC, Hwang SM. Cryopreservation of human limbal stem cells ex vivo expanded on amniotic membrane. Cornea. 2008;27:327–333. doi: 10.1097/ICO.0b013e31815dcfaf. [DOI] [PubMed] [Google Scholar]
- 12.Rauen U, de Groot H. Mammalian cell injury induced by hypothermia- the emerging role for reactive oxygen species. Biological chemistry. 2002;383:477–488. doi: 10.1515/BC.2002.050. [DOI] [PubMed] [Google Scholar]
- 13.Pasovic L, Utheim TP, Maria R, et al. Optimization of Storage Temperature for Cultured ARPE-19 Cells. J Ophthalmol. 2013;2013:11. doi: 10.1155/2013/216359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jirsova K, Dudakova L, Kalasova S, Vesela V, Merjava S. The OV-TL 12/30 clone of anti-cytokeratin 7 antibody as a new marker of corneal conjunctivalization in patients with limbal stem cell deficiency. Investigative ophthalmology & visual science. 2011;52:5892–5898. doi: 10.1167/iovs.10-6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raeder S, Utheim TP, Utheim OA, et al. Effect of limbal explant orientation on the histology, phenotype, ultrastructure and barrier function of cultured limbal epithelial cells. Acta Ophthalmol Scand. 2007;85:377–386. doi: 10.1111/j.1600-0420.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 16.Utheim TP, Raeder S, Utheim OA, et al. Sterility control and long-term eye bank storage of cultured human limbal epithelial cells for transplantation. Br J Ophthalmol. 2009 doi: 10.1136/bjo.2008.149591. [DOI] [PubMed] [Google Scholar]
- 17.Poole CA, Brookes NH, Clover GM. Keratocyte networks visualised in the living cornea using vital dyes. Journal of cell science. 1993;106(Pt 2):685–691. doi: 10.1242/jcs.106.2.685. [DOI] [PubMed] [Google Scholar]
- 18.Wright B, Cave RA, Cook JP, et al. Enhanced viability of corneal epithelial cells for efficient transport/storage using a structurally modified calcium alginate hydrogel. Regenerative medicine. 2012;7:295–307. doi: 10.2217/rme.12.7. [DOI] [PubMed] [Google Scholar]
- 19.Raeder S, Utheim TP, Utheim OA, et al. Effects of organ culture and Optisol-GS storage on structural integrity, phenotypes, and apoptosis in cultured corneal epithelium. Investigative ophthalmology & visual science. 2007;48:5484–5493. doi: 10.1167/iovs.07-0494. [DOI] [PubMed] [Google Scholar]
- 20.Sangwan VS, Vemuganti GK, Iftekhar G, Bansal AK, Rao GN. Use of autologous cultured limbal and conjunctival epithelium in a patient with severe bilateral ocular surface disease induced by acid injury: a case report of unique application. Cornea. 2003;22:478–481. doi: 10.1097/00003226-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Sangwan VS, Matalia HP, Vemuganti GK, et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian journal of ophthalmology. 2006;54:29–34. doi: 10.4103/0301-4738.21611. [DOI] [PubMed] [Google Scholar]
- 22.Sangwan VS, Matalia HP, Vemuganti GK, et al. Early results of penetrating keratoplasty after cultivated limbal epithelium transplantation. Archives of ophthalmology. 2005;123:334–340. doi: 10.1001/archopht.123.3.334. [DOI] [PubMed] [Google Scholar]
- 23.Basu S, Mohamed A, Chaurasia S, Sejpal K, Vemuganti GK, Sangwan VS. Clinical outcomes of penetrating keratoplasty after autologous cultivated limbal epithelial transplantation for ocular surface burns. American journal of ophthalmology. 2011;152:917–924. doi: 10.1016/j.ajo.2011.05.019. e911. [DOI] [PubMed] [Google Scholar]
- 24.Metcalfe A, Streuli C. Epithelial apoptosis. BioEssays : news and reviews in molecular, cellular and developmental biology. 1997;19:711–720. doi: 10.1002/bies.950190812. [DOI] [PubMed] [Google Scholar]
- 25.Bates RC, Buret A, van Helden DF, Horton MA, Burns GF. Apoptosis induced by inhibition of intercellular contact. The Journal of cell biology. 1994;125:403–415. doi: 10.1083/jcb.125.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borderie VM, Kantelip BM, Delbosc BY, Oppermann MT, Laroche L. Morphology, histology, and ultrastructure of human C31 organ-cultured corneas. Cornea. 1995;14:300–310. doi: 10.1097/00003226-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Crewe JM, Armitage WJ. Integrity of epithelium and endothelium in organ-cultured human corneas. Investigative ophthalmology & visual science. 2001;42:1757–1761. [PubMed] [Google Scholar]
- 28.Raeder S, Utheim TP, Messelt E, Lyberg T. The impact of de-epithelialization of the amniotic membrane matrix on morphology of cultured human limbal epithelial cells subject to eye bank storage. Cornea. 2010;29:439–445. doi: 10.1097/ICO.0b013e3181ba0c94. [DOI] [PubMed] [Google Scholar]
- 29.Utheim TP, Raeder S, Utheim OA, et al. A novel method for preserving cultured limbal epithelial cells. The British journal of ophthalmology. 2007;91:797–800. doi: 10.1136/bjo.2006.103218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eidet JR, Utheim OA, Raeder S, et al. Effects of serum-free storage on morphology, phenotype, and viability of ex vivo cultured human conjunctival epithelium. Experimental eye research. 2012;94:109–116. doi: 10.1016/j.exer.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Hunt L, Hacker DL, Grosjean F, et al. Low-temperature pausing of cultivated mammalian cells. Biotechnology and bioengineering. 2005;89:157–163. doi: 10.1002/bit.20320. [DOI] [PubMed] [Google Scholar]
- 32.Vairetti M, Griffini P, Pietrocola G, Richelmi P, Freitas I. Cold-induced apoptosis in isolated rat hepatocytes: protective role of glutathione. Free radical biology & medicine. 2001;31:954–961. doi: 10.1016/s0891-5849(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 33.Laidler KJ, Peterman BF. Temperature effects in enzyme kinetics. Methods in enzymology. 1979;63:234–257. doi: 10.1016/0076-6879(79)63012-4. [DOI] [PubMed] [Google Scholar]
- 34.Reim M, Althoff C, von Mulert B. Effect of low temperatures on the metabolism of corneal cultures. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1988;226:353–356. doi: 10.1007/BF02172966. [DOI] [PubMed] [Google Scholar]