Abstract
Preventive measures against oral carcinogenesis are urgently warranted to lower the high morbidity and mortality associated with this malignancy worldwide. Here, we investigated the chemopreventive efficacy of grape seed extract (GSE) and resveratrol (Res) in 4-nitroquinoline-1-oxide (4NQO)-induced tongue tumorigenesis in C57BL/6 mice. Following 8 weeks of 4NQO exposure (100 μg/mL in drinking water), mice were fed with either control AIN-76A diet or diet containing 0.2% GSE (w/w) or 0.25% Res (w/w) for 8 subsequent weeks, while continued on 4NQO. Upon termination of the study at 16 weeks, tongue tissues were histologically evaluated for hyperplasia, dysplasia and papillary lesions, and then analyzed for molecular targets by immunohistochemistry. GSE and Res feeding for 8 weeks, moderately decreased the incidence, but significantly prevented the multiplicity and severity of 4NQO-induced preneoplastic and neoplastic lesions, without any apparent toxicity. In tongue tissues, both 4NQO+GSE and 4NQO+Res treatment correlated with a decreased proliferation (BrdU labeling index) but increased apoptotic death (TUNEL-positive cells) as compared to the 4NQO group. Furthermore, tongue tissues from both the 4NQO+GSE and 4NQO+Res groups showed an increase in activated metabolic regulator phospho-AMPK (Thr172) and decreased autophagy flux marker p62. Together, these findings suggest that GSE and Res could effectively prevent 4NQO-induced oral tumorigenesis through modulating AMPK activation, and thereby, inhibiting proliferation and inducing apoptosis and autophagy, as mechanisms of their efficacy.
Keywords: Chemoprevention, head and neck squamous cell carcinoma, biomarkers, tumor progression, AMPK
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the 6th most common cancer and has a poor prognosis, resulting in the major morbidity and mortality [1] Risk factors for HNSCC are tobacco, smokeless tobacco products, betel nuts, alcohol, human papillomavirus (HPV) and poor hygiene [2,3]. All these risk factors cause both genetic and epigenetic alterations in tumor suppressor genes and/or oncogenes at different stages of HNSCC/oral cancer development [4,5]. These deregulated genes have been targeted with agents to improve the clinical outcomes, but exhibit limited success with enormous side-effects [6]. Similarly, the rate of development of secondary primary tumors in these patients has been reported to be higher than for any other malignancy, largely determining outcome of treatment among early-stage HNSCC patients [7]. Therefore, a better understanding of the neoplastic transformation and progression of HNSCC, and targeting at early stage with non-toxic cancer chemopreventive agents could be a more practical translational alternative [8]. In this regard a relevant model involves, the continuous exposure of a synthetic chemical carcinogen 4-nitroquinoline 1-oxide (4NQO) to mice that induces the oral (tongue) tumorigenesis that resembles the sequential progression of human oral cancer including hyperplasia, dysplasia, severe dysplasia, papilloma and squamous cell carcinoma (SCC) [9,10]. Furthermore, 4NQO-induced oral carcinogenesis in mice exhibit the molecular changes including the overexpression of the protein kinase B/mammalian target of rapamycin (mTOR) pathways, cyclin D1, p53 and H-ras mutation, as observed clinically in humans [10–13]. Thus, the 4NQO-induced oral tumorigenesis model, with well-defined histopathological and molecular alterations associated with disease progression, provides an excellent opportunity to investigate the efficacy of chemopreventive agents against premalignant lesions as well as SCC of oral mucosa [11,14–16].
With regard to non-toxic cancer chemopreventive agents, a meta-analysis has shown an inverse correlation between that low consumption of fiber and vitamins in the form of fresh fruit and vegetables and the etiology of HNSCC, and that the overall oral cancer risk is reduced by 50% with a daily intake of fruits or vegetables [17–19]. Together, these findings suggest that natural dietary and non-dietary phytochemicals are the excellent sources of effective preventive agents against HNSCC. Consistent with this suggestion, two of the natural dietary phytochemicals, namely grape seed extract (GSE) and resveratrol (Res) isolated from the grape seed and skin, respectively, have been widely investigated for their anticancer and cancer chemopreventive efficacy in various models [20–22]. Recent studies have shown a strong anticancer efficacy of both GSE and Res against HNSCC in preclinical models [22–25]. Both GSE and Res inhibit the invasiveness of human HNSCC cells, and reduce and/or prevent the toxicity of chemotherapeutic agents when used in combination [22,26–28]. However, their efficacy at different stages of tumor progression in experimentally-induced oral tumorigenesis has not yet been studied. Accordingly, here we assessed the chemopreventive efficacy of GSE and Res against 4NQO-induced oral tumorigenesis in C57BL/6 mice, and the ability of these two chemopreventive agents to modulate the expression of molecular regulators associated with proliferation, apoptosis, cellular metabolism, and autophagy.
MATERIAL AND METHODS
Chemicals and reagents
4NQO and Res were from Sigma-Aldrich Chemical Co. (St. Louis, MO). GSE sold as ActiVin and rich in oligomeric proanthocyandins was purchased from San Joaquin Valley Concentrates (Fresno, CA) [24]. Antibody for phospho-AMPK (Thr172) was from Cell Signaling (Beverly, MA). Anti-p62 was from Progen Biotek (Heidelberg, Germany). AIN-76A diet was from Dyets Inc. (Bethlehem, PA). Streptavidin, and biotinylated anti-mouse secondary antibody were from Dako (Carpinteria, CA), and biotinylated anti-rabbit secondary antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Dead End Colorimetric terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit was purchased from Promega (Madison, WI). 5-bromo-2′-deoxyuridine (BrdU) labeling reagent and BrdU detection kit were purchased from Invitrogen (Federick, MD).
Experimental protocol for 4NQO-induced oral carcinogenesis
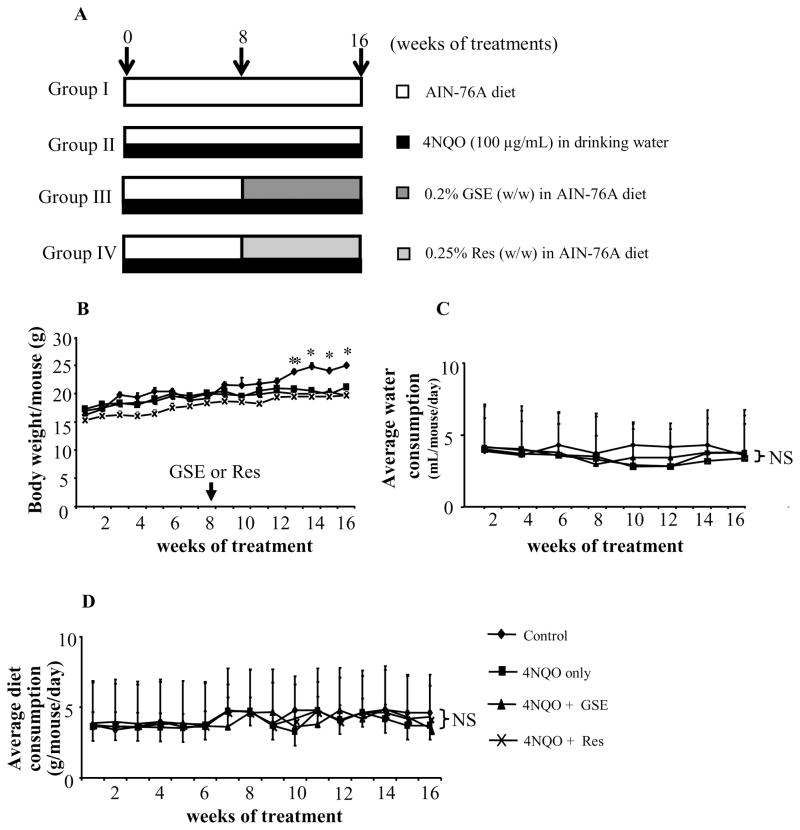
Six-week-old female C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were housed in animal care facility at standard laboratory conditions following the protocol approved by Institutional Animal Care and Use Committee (IACUC) of University of Colorado Denver. 4NQO (100 μg/mL) was administered to mice in drinking water and adequate precaution was taken to avoid the decomposition of 4NQO from light exposure [12]. Mice were divided into four groups; control (group I, n=5), 4NQO only (group II, n=6), 4NQO+GSE (group III, n=6), and 4NQO+Res (group IV, n=6) as shown in Figure 1A. The animals in group II–IV were given 4NQO (100 μg/mL) in drinking water for 16 consecutive weeks while animals in group I received normal tap water. Following 8 weeks of 4NQO exposure, animals in groups III and IV were switched to AIN-76A diet containing either GSE (0.2% w/w) or Res (0.25% w/w), and animals in groups I and II remained on normal (control) AIN-76A diet throughout the study period. The mice in each group were evaluated weekly for body weight and diet consumption, and biweekly for water consumption. The experiment was terminated at 16 weeks, animals were euthanized and tongue tissues were collected. The tongue tissues were fixed in buffered formalin for histopathological evaluation following hematoxylin and eosin (H & E) staining and immunohistochemical (IHC) analysis. Briefly, tongue tissues were collected from all the animals in each group (number of animals per group=6) and serial sectioning was performed. Approximately, 30 slides were prepared from the tongue tissue of each animal and each slide had three serial sections. In IHC analyses, 1 in each 15 slides were randomly selected from five randomly chosen animals (total 10 slides per group analyzed for each staining), and stained for different biomarkers.
Figure 1.
(A) Experimental protocol for chemopreventive studies of GSE and Res in 4NQO – induced oral tumorigenesis in mice. Animals were randomly divided into four groups: group I (normal AIN-76A diet and tap water), group II (normal AIN-76A diet and 4NQO in drinking water), group III (0.2% GSE w/w in AIN-76A diet and 4NQO in drinking water), and group IV (0.25% Res w/w in AIN-76A diet and 4NQO in drinking water). Mice in group II–IV were given 100 μg/mL of 4NQO in drinking water for 16 consecutive weeks. After 8 weeks of 4NQO exposure, mice in group III–IV were given GSE or Res in diet as indicated in ‘Materials and methods’ for 8 weeks while continued on 4NQO administration. Effect of GSE and Res on (B) body weight, (C) water consumption and (D) diet consumption. *, P<0.001; NS, not significant; GSE, grape seed extract; Res, resveratrol.
Histopathological analysis
The paraffin-embedded 5 μm-thick tongue tissue sections from different study groups were processed for H&E staining [29]. The sections were histopathologically evaluated as normal or classified as follows: a) epithelial hyperplasia with loss of normal arrangement of epithelial cells into layers, increased number of epithelial cells; b) epithelial dysplasia showing hyperchromatism, disturbed basement membrane, increased nuclear size, mitotic figures, and nuclear/cytoplasm ratio, and dyskeratosis; c) papilloma characterized by moderate or severe anaplastic epithelial lesions resulting in exophytic papillary projections; and, d) oral carcinoma characterized by epithelial invasion through the basement membrane and into the lamina propria and muscle layers of the tongue [12,30]. Since the grading of these lesions is likely to be interpreted subjectively, we elected not to provide the detailed description of “severity” of these histological lesions. Images of H&E-stained lesions were captured on an Olympus BX51 microscope equipped with a 4-megapixel Macrofire digital camera (Optronics, Goleta, CA, USA) using the PictureFrame Application 2.3 (Optronics).
Determination of in vivo proliferation by BrdU incorporation and IHC analyses
To examine the proliferative capacity of squamous epithelium of the tongue, the mice in all treatment groups were given a single intraperitoneal injection of BrdU labeling reagent (1 ml concentrated reagent/100 g of body weight) 2 hours prior to the euthanasia. The tongue tissue collected after necropsy were fixed in buffered formalin overnight and processed for IHC analysis as described earlier [24]. During IHC analysis, paraffin-embedded 5 μm-thick tumor tissue sections were selected and stained with primary antibody against BrdU, phospho-AMPK (Thr172) or p62, following the protocol published previously [23]. The immunoreactivity (represented by brown staining) was analyzed in five randomly defined areas for each tumor section and scored as 0+ (no staining), 1+ (weak staining), 2+ (moderate staining), 3+ (strong staining), 4+ (very strong staining). BrdU-positive cells were quantified by counting total 1000–1500 cells in 5 randomly selected areas versus brown-stained cells per slide at 400X magnification. All IHC and histological analysis were conducted using a Zeiss Axioskop 2 microscope (Carl Zeiss, Inc.), and photomicrographs were captured using a Carl Zeiss AxioCam MRC5 camera with Axiovision Rel 4.5 software.
In situ apoptosis detection by TUNEL staining
The 5 μm-thick sections were subjected to TUNEL staining as per vendor’s protocol, and apoptosis was evaluated by counting the TUNEL-positive cells (brown-stained) as well as the total number of cells in five randomly selected at 400X magnification. The apoptotic index was calculated as number of apoptotic cells × 100 / total number of cells (approximately 1000–1500 cells were examined in each area and in total 5 randomly selected areas were analyzed per slide at 400X magnification).
Statistical analysis
The statistical significance among the groups was tested by one-way ANOVA (Bonferroni) using Sigma Stat software version 2.03 (Jandel Scientific, San Rafael, CA), and two sided P<0.05 was considered significant.
RESULTS
General observations after 16 weeks of 4NQO exposure
We did not observe any abnormality during gross evaluation at necropsy. Mice in 4NQO group showed significant decrease in body weight 13 weeks onward compared to non-4NQO negative control group. The GSE and Res feeding did not significantly affect body weight when compared to 4NQO alone group (Figure 1B). Also, no significant change in water (measured biweekly) or diet consumption (measured weekly) profiles was observed among the mice in 4NQO, 4NQO+GSE and 4NQO+Res groups during the study period (Figures 1C and Figure 1D).
Effect of GSE and Res on 4NQO-induced tongue pre-neoplastic and neoplastic lesions
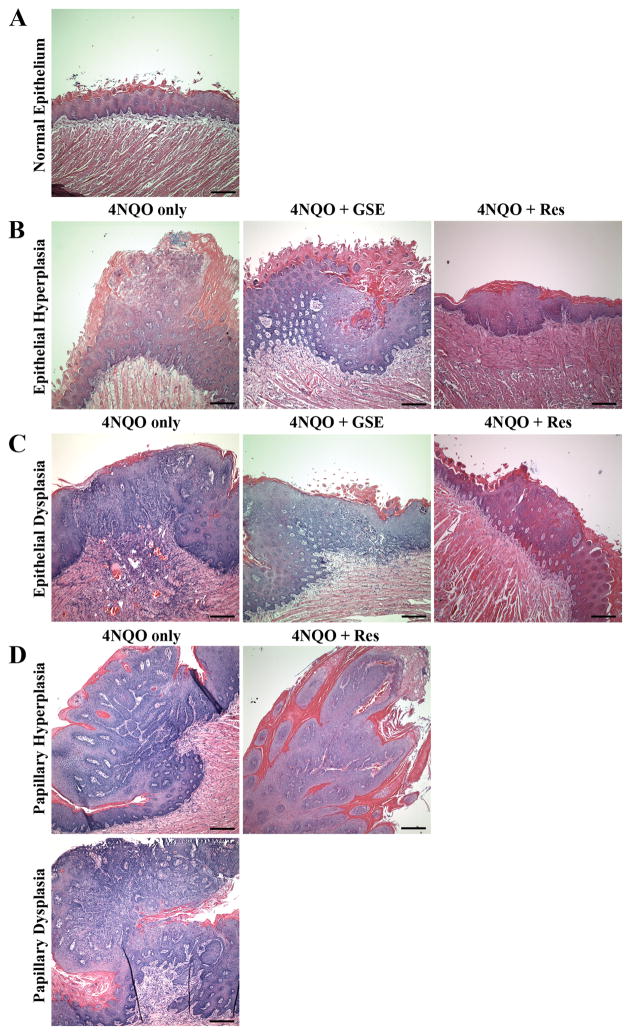
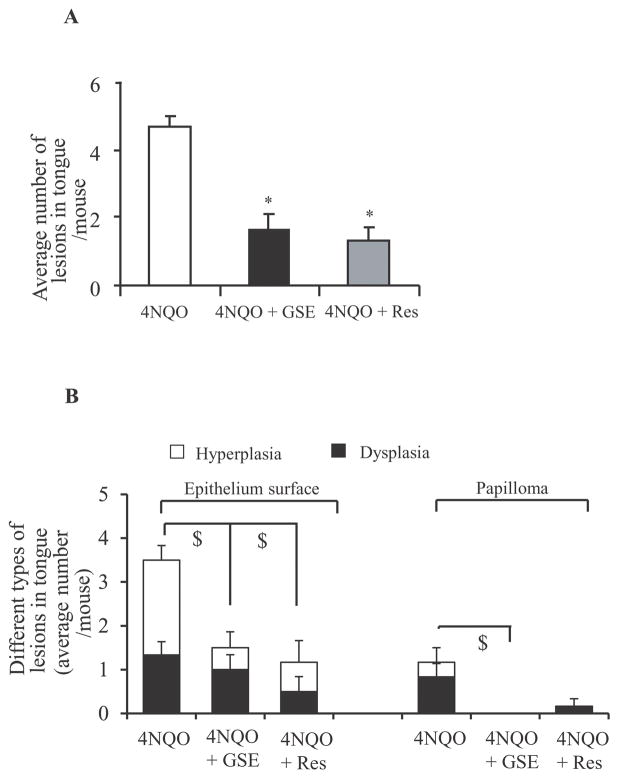
The survival rate of animals in control, 4NQO, 4NQO+GSE and 4NQO+Res was 100%. Similar to the study published earlier, 4NQO-induced oral lesions developed mostly at the posterior part of the tongue and were multifocal, i.e. the lesions developed at different sites of the tongue [12]. These lesions were categorized histologically to assess GSE and Res efficacy on the severity of lesions, which were graded based upon histological criteria described in Materials and methods. The histopathologic evaluation of tongue tissues revealed that the lesions developed after 16 weeks of 4NQO exposure in C57BL/6 mice were mostly hyperplasia (mild, moderate and severe), dysplasia (mild, moderate and severe), and papilloma, with no evidence of carcinoma. In agreement with our observation, Hasina et al., has previously reported that 16 weeks of 4-NQO exposure predominantly leads to the formation of hyperplasia and dysplasia [7]. Representative images of different types of oral lesions from 4NQO, 4NQO+GSE, and 4NQO+Res are shown in Figure 2. Furthermore, a lack of two panels (papillary hyperplasia and papillary dysplasia) for 4NQO+GSE and one panel (papillary dysplasia) for 4NQO+Res in Figure 2D is due to the fact that no such lesions were found in animals from these groups. In 4NQO group, 100% mice had oral lesions (hyperplasia, dysplasia and/or papilloma), compared to ~68% and 83% in 4NQO+GSE and 4NQO+Res groups, respectively. Whereas the difference in the incidence of oral lesions was not very strong between 4NQO and 4NQO+GSE or 4NQO+Res groups, there were striking differences in lesion multiplicity, size and degree of severity (Figures 3A and B). The lesions in the 4NQO group were higher in number, larger in size microscopically, and severe enough that it was difficult to represent in quantitative terms. In contrast, 4NQO+GSE and 4NQO+Res groups showed a significant decrease in the overall lesion burden as compared to 4NQO alone, which was evident by lesser number of lesions, smaller size microscopically, and less severity. At 16 weeks, the mice in 4NQO+GSE and 4NQO+Res groups showed 1.5 and 1.33 lesions/mouse (p<0.001), accounting for 68% and 71% decrease, respectively, compared to 4.66 lesions/mouse in 4NQO group (Figure 3A). Specifically, in 4NQO group, 28% lesions were epithelial hyperplasia, 47% epithelial dysplasia, 18% papilloma-hyperplasia and 7% papilloma-dysplasia with different degree of atypia, representing different stages of oral tumorigenesis (Figure 3B). In contrast, the frequency of epithelial hyperplasia in 4NQO+GSE and 4NQO+Res groups was 67% and 50% respectively (Figure 3B). Likewise, epithelial dysplasia in 4NQO+GSE and 4NQO+Res groups was 33% and 37%, respectively (Figure 3B). Importantly, there was no evidence of papilloma development (both hyperplasia and dysplasia) in 4NQO+GSE group compared to 25% (18% papillary hyperplasia, 7.1% papillary dysplasia) in 4NQO group, whereas in 4NQO+Res group, there was a significant decrease in the incidence of papilloma-hyperplasia (12.5%) with no incidence of papilloma-dysplasia (Figures 2D and 3B). Together, these results clearly demonstrated that both GSE and Res significantly decrease the progression of precancerous lesions to papilloma hyperplasia to papilloma dysplasia in 4NQO-induced oral tumorigenesis in mice.
Figure 2.
A detailed histopathological evidence of oral (tongue) tumorigenesis in mice after 4NQO exposure without or with GSE and Res treatment. The details of experimental protocol are provided in ‘Materials and methods’. After the termination of experiment, the tongue tissues were collected, formalin fixed and processed further for H & E staining. (A) Images of normal epithelium from control, (B) epithelial hyperplasia, and (C) epithelial dysplasia with marked loss of cell polarity and nuclear pleomorphism in tongue tissues from 4NQO, 4NQO+GSE and 4NQO+Res groups. (D) Papillary hyperplasia and dysplasia lesions in tongue tissues from 4NQO and 4NQO+Res groups. Bar in each panel represents 200 microns. GSE, grape seed extract; Res, resveratrol.
Figure 3.
Effect of GSE and Res on 4NQO-induced oral lesions multiplicity and severity. After the termination of experiment, the tongue tissues were collected, formalin fixed and processed further for H & E staining. The histological grading of the H & E sections was performed as mentioned in ‘Materials and methods’. (A) Total number of lesions per mouse from 4NQO, 4NQO+GSE and 4NQO+Res groups, and (B) incidence of different histology grades of lesions per mouse from 4NQO, 4NQO+GSE and 4NQO+Res groups. *, P<0.001; $, P<0.05; GSE, grape seed extract; Res, resveratrol.
Effect of GSE and Res feeding on proliferation and apoptosis in tongue tissues
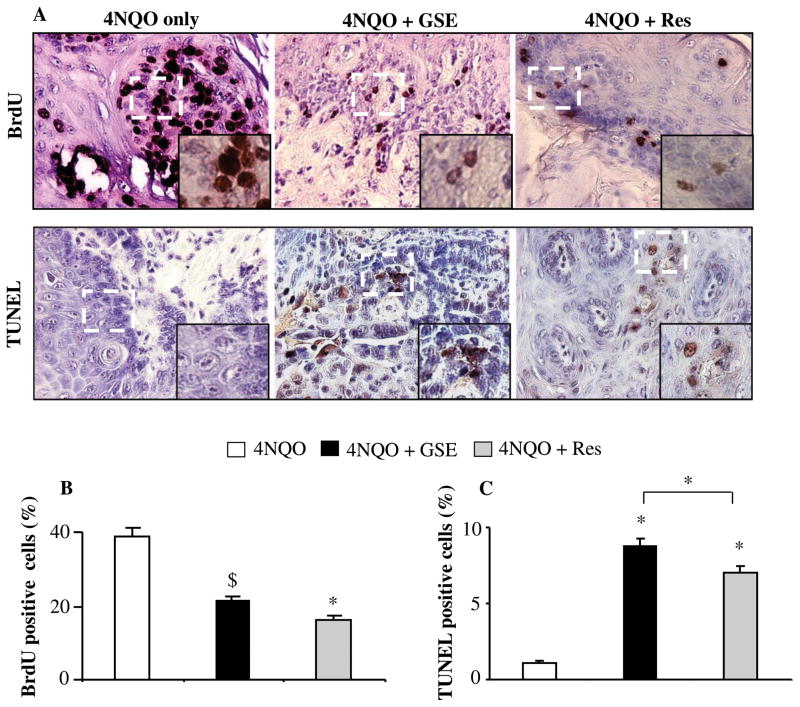
To study the mechanism and/or biomarkers underlying the preventive efficacy of GSE and Res, we next investigated the effect of GSE and Res on cell proliferation by assessing the BrdU uptake by proliferating cells in these lesions from each treatment groups. 4NQO-induced oral tumorigenesis was associated with increased expression of BrdU labeled cells at the parabasal layer of oral epithelium as reported previously [12], which were significantly less in the GSE- and Res-treated groups (Figure 4A, upper panel). Quantification of BrdU positive cells revealed that GSE and Res exhibit 45% (P<0.05) and 59% (P<0.001) decrease in proliferating cells, respectively, as compared to 4NQO group of mice; there was no significant difference between 4NQO+GSE and 4NQO+Res groups (Figure 4B). In addition, the anti-proliferative effects of GSE and Res in tongue tissue were not limited to any specific stage of lesions. Next, we evaluated the apoptotic indices in tongue tissues from 4NQO, 4NQO+GSE and 4NQO+Res groups by TUNEL staining (Figure 4A, lower panel). As shown in Figure 4C, compared to 4NQO group, we observed that GSE and Res significantly increase TUNEL positive cells (7.6 fold and 6.0 fold, P<0.001), respectively. In contrast, we didn’t observe any TUNEL positive cell in tongue tissue of control animals (data not shown), and there was slight but significant difference between 4NQO+GSE and 4NQO+Res groups, indicating that GSE had prominent pro-apoptotic effect as compared to Res (Figure 4B). Together, our results suggest that GSE and Res exert strong anti-proliferative and pro-apoptotic effects contributing towards their chemopreventive efficacy against 4NQO-induced tongue tumorigenesis.
Figure 4.
Effect of GSE and Res on cell proliferation and apoptosis in 4NQO-induced oral (tongue) tumorigenesis. (A) Representative photographs for BrdU and TUNEL staining from 4NQO, 4NQO+GSE and 4NQO+Res groups are presented at 400X; insets represent further magnification of a part of the photographs. The positive stained cells (brown colored) were quantified for (B) BrdU staining and (C) TUNEL staining in tongue tissues from 4NQO, 4NQO+GSE and 4NQO+Res groups following the procedures described in ‘Materials and methods’. The data shown are mean ± SEM of five individual tongue tissues from each group. *, P<0.001; $, P<0.05; GSE, grape seed extract; Res, resveratrol.
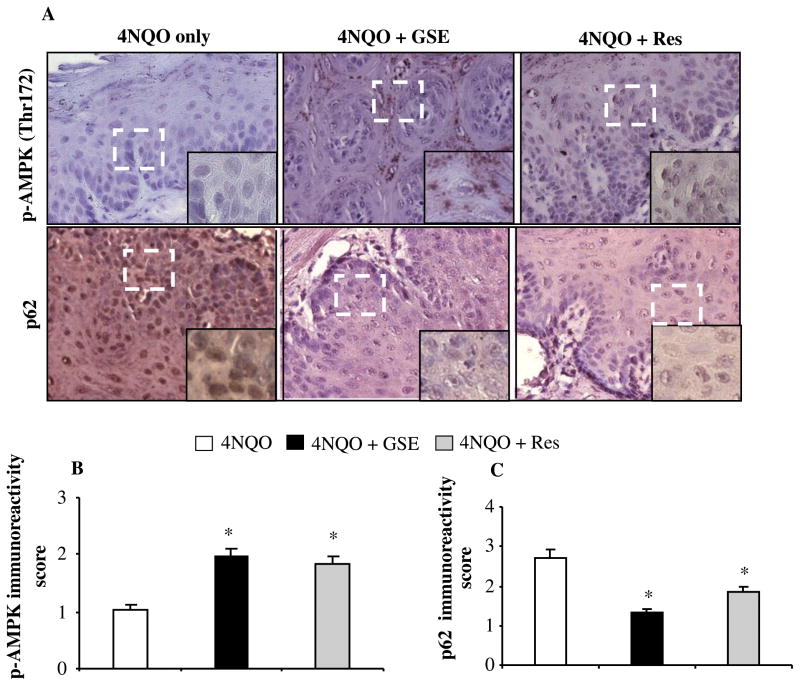
Effect of GSE and Res feeding on the expression of phospho-AMPK(Thr 172) and p62 in tongue tissues
The mTOR, a central regulator of cellular metabolism, is hyperactivated in head and neck cancer, and has been suggested to be a potential therapeutic target [31]. The activation of AMPK results in the inhibition of mTOR signaling, and the evidence of metabolic status in vivo can be assessed by IHC for phospho-AMPK (Thr172). In an attempt to examine whether these two agents also affect AMPK activation as a mechanism contributing in their chemopreventive efficacy against 4NQO-induced tongue tumorigenesis, next we analyzed tongue tissues from all groups for metabolic alteration (Figure 5A, upper panel). IHC analysis revealed a increase in the expression of phospho-AMPK (Thr 172) by about 2 fold (P<0.001) and 1.8 fold (P<0.001) in 4NQO+GSE and 4NQO+Res groups, respectively, compared to tongue tissues from 4NQO group, but there was no significant difference between 4NQO+GSE and 4NQO+Res groups (Figure 5B). AMPK is activated by phosphorylation when ATP synthesis is decreased and/or ATP consumption is increased, and such cellular metabolic conditions also induce autophagy [32,33]. Therefore, we also evaluated the expression of p62/SQSTM1, commonly described as a hallmark of autophagic flux [34], in the tongue tissues from 4NQO, 4NQO+GSE and 4NQO+Res groups by IHC analysis (Figure 5A, lower panel). In comparison to the 4NQO group, tissues from the 4NQO+GSE and 4NQO+Res groups showed a decreased p62 immunoreactivity by about 1.33 (P<0.001) and 1.83 (P<0.001), respectively, but there was no significant difference between 4NQO+GSE and 4NQO+Res groups, as compared to 2.71 in 4NQO group (Figure 5C). In general, the expression of both phospho-AMPK (Thr172) and p62 was heterogeneous in nature and not confined to the areas of the lesions in tongue tissues from 4NQO, 4NQO+GSE and 4NQO+Res treated mice (Figure 5A). Collectively, these results suggested that GSE and Res alter the metabolic status in vivo as a part of their efficacy against 4NQO-induced tongue tumorigenesis.
Figure 5.
Effect of GSE and Res on the expression of phospho-AMPK and p62 in 4NQO-induced oral (tongue) tumorigenesis. IHC analyses was performed in tongue tissues from 4NQO, 4NQO+GSE and 4NQO+Res groups, and (A) representative photographs for phospho-AMPK (Thr172), and p62 are presented at 400X; insets represent further magnification of a part of the photographs. The immunoreactivity for (B) phospho-AMPK (Thr172), and (C) p62 was quantified as described in ‘Materials and methods’. The quantitative data for immunoreactivity are shown as mean ± SEM of five individual tongue tissues from each group. *, P<0.001; GSE, grape seed extract; Res, resveratrol.
DISCUSSION
Head and neck cancer is mostly preceded by the development of precursor lesions, clinically identified as leukoplakia (hyperplasia and dysplasia) and erythroplakia (dysplasia), which are identified visually during oral examination, making early diagnosis possible [35]. Therefore, one of the most translationally relevant strategies to control oral cancer and improve survival in these patients is the prevention and/or delay in the progression of precancerous lesions to the advanced stage of the malignancy [35]. In this regard, 4NQO-induced oral carcinogenesis provides a good preclinical model representing various stages of oral cancer development with similar histological and molecular changes as in humans [7,10]. Employing this mouse model, herein, our results for the first time showed the strong chemopreventive efficacy of GSE and Res against 4NQO-induced oral (tongue) tumorigenesis and found the role of AMPK activation and associated biological responses in the in vivo activity of these two dietary agents.
Histological evaluations of lesions in our studies revealed that GSE and Res strongly inhibited 4NQO-induced hyperplasia and dysplasia (pre-neoplastic) lesions and attenuated the growth of papilloma, indicating their ability to inhibit tumor promotion and progression at various stages of oral tumor. These findings were consistent with previous studies where chemopreventive efficacy of GSE and Res in other chemical carcinogenesis models was associated with inhibition of tumor promotion and progression [36,37]. With regard to biological responses, unlimited proliferation and inhibition of apoptosis are considered the hallmarks of tumor development and many chemopreventive agents have been shown to inhibit proliferation and induce apoptosis to block the carcinogenic process [38]. Likewise, in the present study, both GSE and Res showed strong anti-proliferative and pro-apoptotic effects, providing further evidence that GSE and Res might be significantly contributing towards their chemopreventive efficacy against 4NQO-induced oral tumorigenesis. One of the long-term goals of chemoprevention must be the development of treatments that can be easily taken by at-risk individuals for prolonged periods of time with minimal side effects to achieve widespread acceptance and long-term compliance. This would be particularly important in the case of high-risk patients who have not yet developed their first malignancy. Furthermore, many of the chemopreventive agents currently under investigation, such as epidermal growth factor receptor tyrosine kinase inhibitors, however, the toxicities observed at the current prescribed dosages may preclude them from being used widely as chemopreventive agents. Therefore, the combination of one or both of these agents at lower doses in concert with other chemotherapeutic agents may reduce toxicities and improve overall efficacy. The data presented here show proof of principle that the induction of new blood vessel growth by premalignant cells may be one such phenotype that could be targeted.
Previously, we observed similar anti-proliferative and pro-apoptotic effects in human HNSCC cell lines in vitro and nude mouse xenografts in vivo treated with GSE and Res (22, 23). Together, these findings suggest that both GSE and Res could be beneficial at least in HNSCC patients with precancerous lesions; however, additional studies are needed under the clinical settings to support this assumption. In terms of toxicity and bioavailability of GSE and Res, numerous studies conducted in the past have shown that GSE and Res are very well tolerated, efficiently absorbed after oral administration further supporting their biological activities [39–42]. Besides, the doses of GSE (0.2% w/w in diet) and Res (0.25% w/w in diet) used in the current study equates to 300 mg/kg and 375 mg/kg daily doses respectively; and as per Hoh et al. [43] criterion, this extrapolates to approximately 1.8 gm GSE or 2.25 gm Res per person (~70 Kg weight) daily. Therefore, cancer chemopreventive efficacy of GSE and Res reported in the present study could be translated in humans without toxicity.
Emerging literature has shown that AMPK, an upstream target of mTOR (mammalian target of rapamycin), is a major sensor of cellular energy within cells [44,45]. The effect of AMPK activation on cellular proliferation and metabolism through inhibiting mTOR pathway has emerged to be a therapeutic target. mTOR is hyperactivated in both precancerous and cancerous dysplasia lesions as well as in advanced HNSCC [9,46,47]. Both of these metabolic sensors, AMPK and mTOR, have been targeted to cause the regression of the large squamous carcinomas and inhibition in the progression of premalignant lesions [9,13,45]. Consistent with these reports, for the first time, we found that both GSE and Res upregulated the expression of phospho-AMPK (Thr172) in 4NQO-induced oral cancer tissues. The exact mechanism of AMPK activation in our study needs further investigation, as AMPK is activated by phosphorylation at threonine 172 by upstream kinases such as liver kinase B1 (LKB1) and TGF-β-activated kinase (TAK1) [32,48]. Phosphorylated AMPK expression has been shown to correlate with survival in a subset of lung and colorectal cancer patients [49,50]. Therefore, we hypothesize that GSE- and Res may mediate activation of AMPK possibly leading to mTOR suppression and that could be responsible for the inhibition of 4NQO-induced oral tumorigenesis. The activation of AMPK also leads to autophagic response in the cancer cells by inhibiting mTOR pathway [46,51]. p62, marker of autophagic flux, is an important signaling hub frequently overexpressed in human tumors including HNSCC [51,52]. We observed that the tongue tissues from GSE- and Res-treated mice have decreased p62 expression as compared to 4NQO group, suggesting that GSE and Res induced autophagy in vivo. Different stimuli are known to cause autophagic cell death in cancer cells; however, autophagy induction can also be a cell survival mechanism [53]. Accordingly, more studies are needed to establish the role of GSE- and Res-induced autophagy in their overall anti-cancer and/or cancer chemopreventive activity.
In summary, our studies for the first time revealed that GSE and Res significantly inhibited tumor promotion and progression in 4NQO-induced tongue tumorigenesis model. Results also revealed that GSE and Res exert pleiotropic effects on cell proliferation, apoptosis, cellular metabolism, and autophagy revealing multiple possible mechanisms involved in their chemopreventive efficacy. Overall, GSE and Res appear to be ideal chemopreventive agents for future evaluation of their chemopreventive effectiveness against head and neck cancer.
Acknowledgments
This work was supported in part by NCI R01 grants CA91883 and CA140368.
List of abbreviations
- HNSCC
head and neck squamous cell carcinoma
- GSE
grape seed extract
- Res
resveratrol
- 4NQO
4-nitroquinoline 1-oxide
- SCC
squamous cell carcinoma
- SPT
secondary primary tumor
- HPV
human papilloma virus
- H&E stain
hematoxylin and eosin stain
- IHC
immunohistochemistry
- DAB
3,3′-diaminobenzidine
- AMPK
adenine monophosphate protein kinase
- BrdU
5-bromo-2′-deoxyuridine
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- LKB1
liver kinase B1
- TAK1
TGF-β-activated kinase1
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Goon PK, Stanley MA, Ebmeyer J, et al. HPV & head and neck cancer: a descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 4.Aruna DS, Prasad KV, Shavi GR, Ariga J, Rajesh G, Krishna M. Retrospective study on risk habits among oral cancer patients in Karnataka Cancer Therapy and Research Institute, Hubli, India. Asian Pac J Cancer Prev. 2011;12(6):1561–1566. [PubMed] [Google Scholar]
- 5.Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Reboiras-Lopez MD, Gandara Rey JM, Garcia-Garcia A. Genetic and molecular alterations associated with oral squamous cell cancer (Review) Oncol Rep. 2009;22(6):1277–1282. doi: 10.3892/or_00000565. [DOI] [PubMed] [Google Scholar]
- 6.Guntinas-Lichius O, Wendt T, Buentzel J, et al. Head and neck cancer in Germany: a site-specific analysis of survival of the Thuringian cancer registration database. J Cancer Res Clin Oncol. 2010;136(1):55–63. doi: 10.1007/s00432-009-0636-y. [DOI] [PubMed] [Google Scholar]
- 7.Hasina R, Martin LE, Kasza K, Jones CL, Jalil A, Lingen MW. ABT-510 is an effective chemopreventive agent in the mouse 4-nitroquinoline 1-oxide model of oral carcinogenesis. Cancer Prev Res (Phila) 2009;2(4):385–393. doi: 10.1158/1940-6207.CAPR-08-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas GR, Nadiminti H, Regalado J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol. 2005;86(6):347–363. doi: 10.1111/j.0959-9673.2005.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila) 2009;2(1):27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 10.Kanojia D, Vaidya MM. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006;42(7):655–667. doi: 10.1016/j.oraloncology.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Nauta JM, Roodenburg JL, Nikkels PG, Witjes MJ, Vermey A. Comparison of epithelial dysplasia--the 4NQO rat palate model and human oral mucosa. Int J Oral Maxillofac Surg. 1995;24(1 Pt 1):53–58. doi: 10.1016/s0901-5027(05)80857-4. [DOI] [PubMed] [Google Scholar]
- 12.Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10(1 Pt 1):301–313. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 13.Wong KK. Oral-specific chemical carcinogenesis in mice: an exciting model for cancer prevention and therapy. Cancer Prev Res (Phila) 2009;2(1):10–13. doi: 10.1158/1940-6207.CAPR-08-0234. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, Nishikawa A, Mori Y, Morishita Y, Mori H. Inhibitory effects of non-steroidal anti-inflammatory drugs, piroxicam and indomethacin on 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male ACI/N rats. Cancer Lett. 1989;48(3):177–182. doi: 10.1016/0304-3835(89)90115-8. [DOI] [PubMed] [Google Scholar]
- 15.Kawabata K, Tanaka T, Honjo S, et al. Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int J Cancer. 1999;83(3):381–386. doi: 10.1002/(sici)1097-0215(19991029)83:3<381::aid-ijc14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Yanaida Y, Kohno H, Yoshida K, et al. Dietary silymarin suppresses 4-nitroquinoline 1-oxide-induced tongue carcinogenesis in male F344 rats. Carcinogenesis. 2002;23(5):787–794. doi: 10.1093/carcin/23.5.787. [DOI] [PubMed] [Google Scholar]
- 17.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83(5):1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S77–81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macfarlane GJ, Zheng T, Marshall JR, et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer B Oral Oncol. 1995;31B(3):181–187. doi: 10.1016/0964-1955(95)00005-3. [DOI] [PubMed] [Google Scholar]
- 20.Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. 2009;139(9):1806S–1812S. doi: 10.3945/jn.109.106864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber HA, Hodges AE, Guthrie JR, et al. Comparison of proanthocyanidins in commercial antioxidants: grape seed and pine bark extracts. J Agric Food Chem. 2007;55(1):148–156. doi: 10.1021/jf063150n. [DOI] [PubMed] [Google Scholar]
- 22.Sun Q, Prasad R, Rosenthal E, Katiyar SK. Grape seed proanthocyanidins inhibit the invasiveness of human HNSCC cells by targeting EGFR and reversing the epithelial-to-mesenchymal transition. PLoS One. 2012;7(1):e31093. doi: 10.1371/journal.pone.0031093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Tyagi A, Gu M, Takahata T, et al. Resveratrol Selectively Induces DNA Damage, Independent of Smad4 Expression, in Its Efficacy against Human Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2011;17(16):5402–5411. doi: 10.1158/1078-0432.CCR-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrotriya S, Deep G, Gu M, et al. Generation of reactive oxygen species by grape seed extract causes irreparable DNA damage leading to G2/M arrest and apoptosis selectively in head and neck squamous cell carcinoma cells. Carcinogenesis. 2012;33(4):848–858. doi: 10.1093/carcin/bgs019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24(5A):2783–2840. [PubMed] [Google Scholar]
- 26.Hu FW, Tsai LL, Yu CH, Chen PN, Chou MY, Yu CC. Impairment of tumor-initiating stem-like property and reversal of epithelial-mesenchymal transdifferentiation in head and neck cancer by resveratrol treatment. Mol Nutr Food Res. 2012;56(8):1247–1258. doi: 10.1002/mnfr.201200150. [DOI] [PubMed] [Google Scholar]
- 27.Erdem T, Bayindir T, Filiz A, Iraz M, Selimoglu E. The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur Arch Otorhinolaryngol. 2011;269(10):2185–2188. doi: 10.1007/s00405-011-1883-5. [DOI] [PubMed] [Google Scholar]
- 28.Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47(6):1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Tyagi A, Raina K, Singh RP, et al. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Molecular cancer therapeutics. 2007;6(12 Pt 1):3248–3255. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 30.Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85(2):74. [PubMed] [Google Scholar]
- 31.Freudlsperger C, Burnett JR, Friedman JA, Kannabiran VR, Chen Z, Van Waes C. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas: attractive targets for molecular-oriented therapy. Expert Opin Ther Targets. 2010;15(1):63–74. doi: 10.1517/14728222.2011.541440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carling D, Sanders MJ, Woods A. The regulation of AMP-activated protein kinase by upstream kinases. Int J Obes (Lond) 2008;32(Suppl 4):S55–59. doi: 10.1038/ijo.2008.124. [DOI] [PubMed] [Google Scholar]
- 33.Meijer AJ, Codogno P. AMP-activated protein kinase and autophagy. Autophagy. 2007;3(3):238–240. doi: 10.4161/auto.3710. [DOI] [PubMed] [Google Scholar]
- 34.Puissant A, Fenouille N, Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012;2(4):397–413. [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrotra R, Gupta DK. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol. 2011;3:33. doi: 10.1186/1758-3284-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai ML, Lai CS, Chang YH, Chen WJ, Ho CT, Pan MH. Pterostilbene, a natural analogue of resveratrol, potently inhibits 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin carcinogenesis. Food Funct. 2012;3(11):1185–1194. doi: 10.1039/c2fo30105a. [DOI] [PubMed] [Google Scholar]
- 37.de Moura CF, Noguti J, de Jesus GP, et al. Polyphenols as a chemopreventive agent in oral carcinogenesis: putative mechanisms of action using in-vitro and in-vivo test systems. Eur J Cancer Prev. 2012 doi: 10.1097/CEJ.0b013e32835b6a94. in press. [DOI] [PubMed] [Google Scholar]
- 38.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215(2):129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Ray S, Bagchi D, Lim PM, et al. Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res Commun Mol Pathol Pharmacol. 2001;109(3–4):165–197. [PubMed] [Google Scholar]
- 40.Yamakoshi J, Saito M, Kataoka S, Kikuchi M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem Toxicol. 2002;40(5):599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 41.la Porte C, Voduc N, Zhang G, et al. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clinical pharmacokinetics. 2010;49(7):449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Sano A, Yamakoshi J, Tokutake S, Tobe K, Kubota Y, Kikuchi M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67(5):1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- 43.Hoh C, Boocock D, Marczylo T, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res. 2006;12(9):2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]
- 44.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitale-Cross L, Molinolo AA, Martin D, et al. Metformin prevents the development of oral squamous cell carcinomas from carcinogen-induced premalignant lesions. Cancer Prev Res (Phila) 2012;5(4):562–573. doi: 10.1158/1940-6207.CAPR-11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Advani SH. Targeting mTOR pathway: A new concept in cancer therapy. Indian J Med Paediatr Oncol. 2011;31(4):132–136. doi: 10.4103/0971-5851.76197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amornphimoltham P, Patel V, Sodhi A, et al. Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res. 2005;65(21):9953–9961. doi: 10.1158/0008-5472.CAN-05-0921. [DOI] [PubMed] [Google Scholar]
- 48.Gruzman A, Babai G, Sasson S. Adenosine Monophosphate-Activated Protein Kinase (AMPK) as a New Target for Antidiabetic Drugs: A Review on Metabolic, Pharmacological and Chemical Considerations. Rev Diabet Stud. 2009;6(1):13–36. doi: 10.1900/RDS.2009.6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baba Y, Nosho K, Shima K, et al. Prognostic significance of AMP-activated protein kinase expression and modifying effect of MAPK3/1 in colorectal cancer. Br J Cancer. 2012;103(7):1025–1033. doi: 10.1038/sj.bjc.6605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanjundan M, Byers LA, Carey MS, et al. Proteomic profiling identifies pathways dysregulated in non-small cell lung cancer and an inverse association of AMPK and adhesion pathways with recurrence. J Thorac Oncol. 2010;5(12):1894–1904. doi: 10.1097/JTO.0b013e3181f2a266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rikiishi H. Autophagic action of new targeting agents in head and neck oncology. Cancer Biol Ther. 2012;13(11):978–991. doi: 10.4161/cbt.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grander D, Panaretakis T. Autophagy: cancer therapy’s friend or foe? Future Med Chem. 2011;2(2):285–297. doi: 10.4155/fmc.09.155. [DOI] [PubMed] [Google Scholar]
- 53.Singletary K, Milner J. Diet, autophagy, and cancer: a review. Cancer Epidemiol Biomarkers Prev. 2008;17(7):1596–1610. doi: 10.1158/1055-9965.EPI-07-2917. [DOI] [PubMed] [Google Scholar]