Abstract
Augmenting hippocampal neurogenesis represents a potential new strategy for treating depression. Here we test this possibility by comparing hippocampal neurogenesis in depression-prone ghrelin receptor (Ghsr)-null mice to that in wild-type littermates and by determining the antidepressant efficacy of the P7C3 class of neuroprotective compounds. Exposure of Ghsr-null mice to chronic social defeat stress (CSDS) elicits more severe depressive-like behavior than in CSDS-exposed wild-type littermates, and exposure of Ghsr-null mice to 60% caloric restriction fails to elicit antidepressant-like behavior. CSDS resulted in more severely reduced cell proliferation and survival in the ventral dentate gyrus (DG) subgranular zone of Ghsr-null mice than in that of wild-type littermates. Also, caloric restriction increased apoptosis of DG subgranular zone cells in Ghsr-null mice, although it had the opposite effect in wild-type littermates. Systemic treatment with P7C3 during CSDS increased survival of proliferating DG cells, which ultimately developed into mature (NeuN+) neurons. Notably, P7C3 exerted a potent antidepressant-like effect in Ghsr-null mice exposed to either CSDS or caloric restriction, while the more highly active analog P7C3-A20 also exerted an antidepressant-like effect in wild-type littermates. Focal ablation of hippocampal stem cells with radiation eliminated this antidepressant effect, further attributing the P7C3 class antidepressant effect to its neuroprotective properties and resultant augmentation of hippocampal neurogenesis. Finally, P7C3-A20 demonstrated greater proneurogenic efficacy than a wide spectrum of currently marketed antidepressant drugs. Taken together, our data confirm the role of aberrant hippocampal neurogenesis in the etiology of depression and suggest that the neuroprotective P7C3-compounds represent a novel strategy for treating patients with this disease.
INTRODUCTION
Despite the multitude of antidepressant drugs available to patients, major depression remains a significant cause of morbidity and mortality in our society. Thus there is a great need to further understand the mechanisms underlying depression in order to develop new treatments. To that end, we have been investigating the relationship between the orexigenic gut hormone ghrelin and depression. Ghrelin induces feeding by activating growth hormone secretagogue receptors (Ghsr; ghrelin receptor) in the hypothalamus, caudal brainstem and elsewhere in the central nervous system.1-4 Within the hippocampus and ventral tegmental area, Ghsr mediates ghrelin’s enhancement of reward-related behaviors,5-8 cue-potentiated feeding,5,9 hippocampal spine synaptic density and memory retention.10 Ghrelin additionally confers neuroprotective efficacy in models of kainic acid hippocampal toxicity, spinal cord motor neuron excitotoxicity, dopaminergic neuron toxicity and oxygen glucose deprivation in hypothalamic and cortical neurons.11-17 Notably, ghrelin also exerts antidepressant efficacy in rodent models of depression.18-21 In mice, raising ghrelin levels either directly through acute injection or indirectly via caloric restriction elicits an antidepressant response in the forced swim test (FST), a common screening tool for new candidate antidepressants.21 Additionally, elevated plasma ghrelin levels occur in mice exposed to chronic social defeat stress (CSDS), a model of prolonged psychosocial stress featuring aspects of major depression and posttraumatic stress disorder, and Ghsr-null mice exhibit more severe depressivelike behavior after CSDS than wild-type littermates.7,21,22 Besides exaggerated depressive-like behavior, CSDS-exposed GHSR-deficient mice lack the hyperphagia and conditioned place preference for high-fat diet otherwise present in CSDS-exposed wild-type mice.7,21,23 Ghrelin is also elevated in other models of acute and chronic stress in animals, as well as in a model of psychosocial stress in people.23-30 Furthermore, Ghsr polymorphism has been associated with major depression in humans, and administration of ghrelin improves mood in some patients with major depression.18,31 Thus, stress-associated activation of ghrelin signaling may help protect against depression, while aberrant ghrelin signaling may confer increased sensitivity to stress-induced depression as well as changes to the usual metabolic and food reward behavioral responses to stress.
The mechanism by which ghrelin confers antidepressant efficacy has previously eluded the field, although clues in the literature point to involvement of hippocampus neurogenesis. For example, changes in hippocampal neurogenesis and cell survival in the dentate gyrus (DG) have been correlated with depressive-like behavior,32-35 and antidepressants and environmental factors that elevate mood, such as exercise, environmental enrichment and social interaction, increase the net magnitude of hippocampal neurogenesis.36-39 By contrast, negative regulators of neurogenesis and cell survival, such as chronic stress, old age, drugs of abuse and social isolation, are associated with depressed mood.21,38,40,41 Additionally, ablation of neurogenesis decreases the efficacy of some antidepressant drugs in rodents.42-44 It has also been reported that ghrelin potently stimulates hippocampal neurogenesis within the DG.45-48 The antidepressant efficacy of compounds initially categorized as neuroprotective and/or proneurogenic, however, has not yet been described. In considering the convergence of evidence linking neurogenesis with depressive-like behavior, as well as the role of ghrelin in hippocampal neurogenesis and hippocampal distribution of Ghsr,49 we hypothesized that the antidepressant efficacy of ghrelin might relate to its proneurogenic effect. If so, both depression-prone Ghsr-null mice and even wild-type mice with intact ghrelin signaling might be protected from stress-induced depression by pharmacologically augmenting hippocampal neurogenesis. Here, we have tested these hypotheses by utilizing the P7C3-series of neuroprotective compounds and Ghsr-null mice.
MATERIALS AND METHODS
For full Material and Methods, refer to Supplementary Materials and Methods online.
Animals
Male Ghsr-null and wild-type littermates on pure C57/BL/6J genetic background were generated and housed as described previously.7,50 All procedures were performed according to the protocols approved by The University of Texas Southwestern Medical Center Institutional Animal Care and Use of Committee guidelines.
P7C3 compounds
P7C3 was from Asinex (Moscow, Russia). P7C3-A20 was prepared as described.51
Behavioral testing
Behavioral tests were performed as described previously.7,21 P7C3 compounds were administered intraperitoneally at 0900 and 1700 hours daily, and bromodeoxyuridine (BrdU) was administered at 0900 hours. Body weights were unaffected by compounds (Supplementary Figure S7).
CSDS
Focal cranial irradiation
Refer to online Methods. To achieve hippocampal focused cranial irradiation, the anterior portion of the collimator was aligned and centered to where the caudal portion of orbits met squamosal bone (Bregma~0.0 to 0.5 mm), providing an approximate irradiation field from Bregma 0.5 to −5.5 mm.
Caloric restriction
Sixty percent calorie restriction protocol was performed as previously described.21 Mice were provided with 60% of their usual daily calories, resulting in an 18–20% body weight loss in both Ghsr-null and wild-type littermates (Supplementary Figure S7), as observed previously.21 Mice received twice-daily injections of either P7C3 (20 mg kg−1 day− 1 in divided doses) or vehicle 5 days before and during 10 days of calorie restriction or ad libitum feeding. On Day 16, the FST was performed for each mouse, as done previously.21
Immunohistochemistry and stereology
Immunohistochemistry and quantification were performed as described previously.7,39,51
Quantitative reverse transcriptase-PCR
Brain punch collection and quantitative PCR was performed as previously described.5,7
Determination of P7C3 levels in brain tissue
Refer to online Methods.
Comparison of P7C3 compounds and antidepressant drugs
Proneurogenic efficacies of P7C3 and P7C3-A20 were compared with vehicle (artificial cerebrospinal fluid) and several antidepressants (Sigma-Aldrich, St Louis, MO, USA), according to established methods.51-53
Statistical analyses
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for statistical analysis. Significance was defined as P < 0.05. Data are presented as mean ± s.e.m.
RESULTS
Chronic stress severely reduces DG cell proliferation and survival in Ghsr-null mice
The CSDS model of chronic psychosocial stress and major depression exposes male mice to repeated bouts of social subordination by an older and larger aggressor mouse for 5 min per day over 10 days.7,21,22,54 Afterwards, the stressed mouse typically shows significantly reduced social interaction with a novel mouse, reflected by decreased time spent in the interaction zone with a novel mouse and/or increased time spent in the corners of the testing chamber, mimicking stress-induced social avoidance and depression in humans.7,22,54 CSDS is a valuable model of depression, because the social avoidance behavioral manifestation of depressive-like behavior is long-lasting and responsive to chronic, but not acute, antidepressant administration.54,55 Exposure of male Ghsr-null mice to CSDS elicited significantly more time in the corners and less time interacting with a novel mouse, compared with wild-type littermates (Supplementary Figure S1), consistent with our previous reports.7,21
To determine whether the more severe depressive phenotype in Ghsr-null mice correlated with effects on hippocampal neurogenesis, we harvested brain tissue from both CSDSexposed and non-CSDS-exposed mice and performed immunohistochemistry for Ki67, a marker of proliferating cells, in the DG subgranular zone where newborn neural precursor cells proliferate. We separately analyzed the dorsal 2/3 (septal and intermediate regions) and ventral 1/3 (temporal region) of the hippocampus based on reported differences between these regions in neuronal projections, connectivity and gene expression profiles, as well as evidence differentially linking the ventral hippocampus to mood regulation and emotion-based learning and the dorsal hippocampus to spatial learning and memory.56-62 More specifically, adult neurogenesis in the ventral DG has been correlated with an antidepressant effect.63-65 In addition, ghrelin delivery to the ventral hippocampus stimulates food intake, whereas delivery to the dorsal hippocampus does not.9 Ghrelin administration to the ventral hippocampus also induces various motivational and learning-related eating behaviors (operant responding for sucrose reward and cue-potentiated feeding).9 Although both wild-type and Ghsr-null mice displayed an equally significant reduction in the number of Ki67+ cells in the dorsal DG following CSDS (Figure 1a), there was significantly greater reduction in Ki67+ cells in the ventral DG of Ghsr-null mice than in wild-type littermates (Figures 1b–f).
Figure 1.
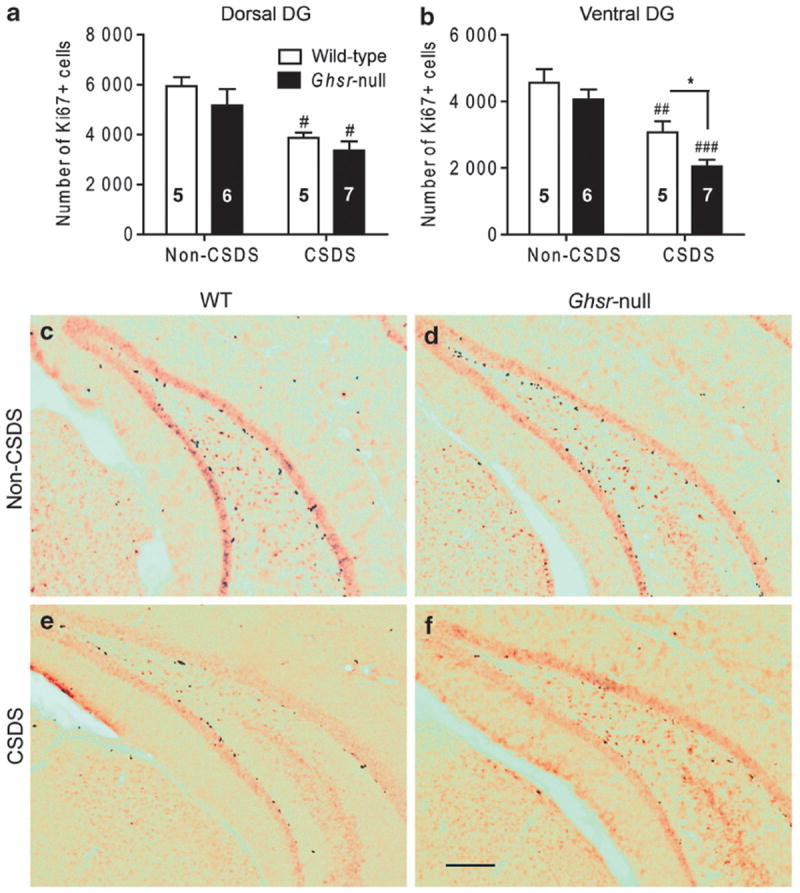
Effect of chronic social defeat stress (CSDS) on cellular proliferation in the dentate gyrus (DG) of Ghsr-null and wild-type littermates. (a, b) Ki67+ cell counts in the subgranular zone of the dorsal (a) and ventral (b) DG of CSDS-exposed or non-CSDS-exposed control mice. Group sizes (n) indicated within bars. (c–f) Representative photomicrograph images of Ki67-immunolabeled brain sections in the ventral DG of CSDS-exposed and non-CSDS-exposed wild-type and Ghsr-null mice. Legend in panel (a) pertains to panel (b). Scale bar in panel (f) (300 μm) pertains to panels (c–f). *P < 0.05, comparing genotypes. #P < 0.05, ##P < 0.01, ###P < 0.001, comparing treatment.
Because the vast majority of newborn hippocampal neural precursor cells in mice die by apoptosis, and pharmacologically inhibiting apoptosis augments hippocampal neurogenesis,51 we wondered whether apoptosis of newborn neural precursor cells might also be affected by CSDS. Therefore, we immunohistochemically stained adjacent tissue sections for activated caspase 3 (AC3), a marker of apoptotic cells. Although CSDS had no effect on AC3+ cell numbers in the dorsal DG (Figure 2a), the ventral DG of all animals displayed significantly more AC3+ cells after CSDS. Furthermore, significantly more AC3+ cell numbers were observed in CSDS-exposed Ghsr-null mice than in CSDS-exposed wild-type littermates (Figures 2b–l).
Figure 2.
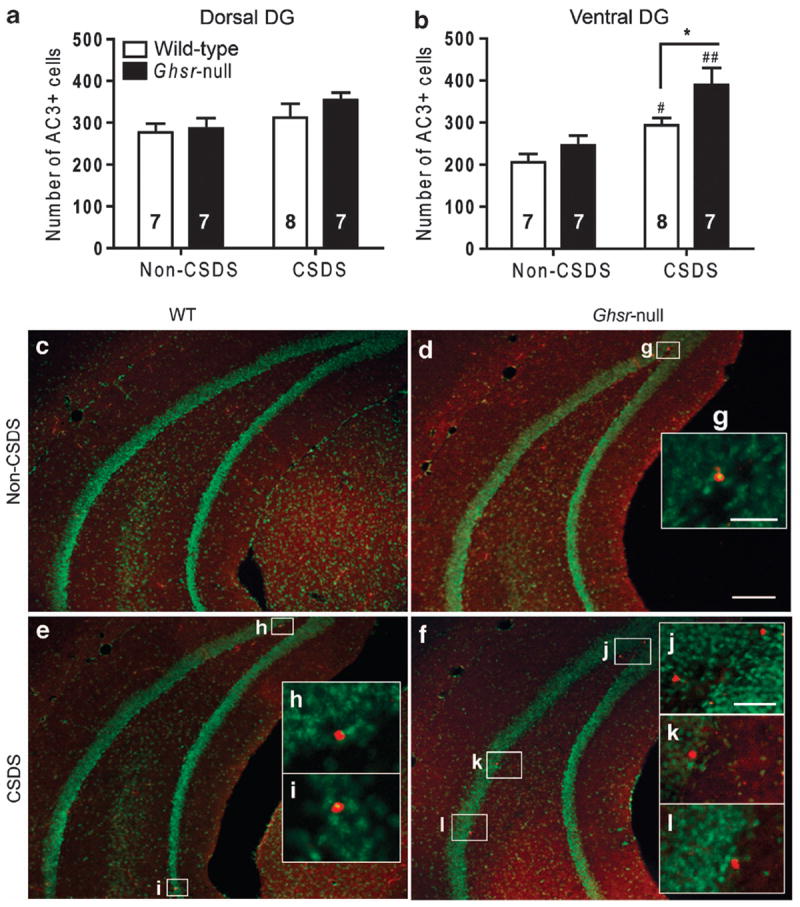
Effect of chronic social defeat stress (CSDS) on apoptosis in the dentate gyrus (DG) of Ghsr-null and wild-type littermates. (a, b). Activated caspase 3-positive (AC3+) cell counts in the subgranular zone of the dorsal (a) and ventral (b) DG of CSDS-exposed or non-CSDS-exposed mice. Group sizes indicated. (c–f) Representative photomicrograph images of the ventral DG from each study group. (g–l) Magnified images of AC3+ cells. Legend in panel (a) pertains to panel (b). Scale bar in panel (d) (300 μm) pertains to panels (c–f), in panel (g) (50 μm) pertains to panels (g–i) and in panel (j) (75 μm) pertains to panels (j–l). *P < 0.05, comparing genotypes. #P < 0.05, ##P < 0.01, comparing treatment.
Taken together, these results demonstrate that the pool of proliferating cells contributing to hippocampal neurogenesis throughout the DG is decreased in both wild-type and Ghsr-null littermates after CSDS, with the negative effect on hippocampal neurogenesis further exacerbated in the ventral DG by virtue of additionally elevated rates of cell death. This net reduction in ventral DG cell survival is significantly more pronounced in Ghsr-null mice and thus parallels the exacerbated depression-like behavior observed after CSDS in Ghsr-null mice as compared with wild-type littermates. Localization of this ghrelin effect to the ventral DG may be related to the hypothesized ventral DG role in regulating mood63-65 and to a higher baseline Ghsr expression within the ventral DG relative to the dorsal DG, as demonstrated here by quantitative reverse transcriptase-PCR of ventral and dorsal hippocampal tissue punches taken from wild-type mice (Supplementary Figure S2).
P7C3 compounds augment DG neurogenesis in chronic stress-exposed mice
We next investigated whether protecting neural precursor cells from CSDS-associated apoptosis might help protect against the depressive-like phenotype. To test this hypothesis, we utilized P7C3, a neuroprotective aminopropyl carbazole that elevates hippocampal neurogenesis by blocking apoptosis of DG neural precursor cells without affecting the number of glial cells.51-53 P7C3 and its active analogs have been shown to enhance hippocampal-dependent spatial learning in young rats that have undergone blunt-traumatic brain injury and in aged rats, and they have also shown potent protective efficacy in rigorous preclinical models of neurodegenerative disease.51,52,66,67 The neuroprotective effect of P7C3 is thought to involve protection of mitochondrial membrane integrity, although the precise molecular target of this novel class of molecules has not yet been identified.51 A twicedaily schedule of intraperitoneal P7C3 injections (20 mg kg−1 day−1 in divided doses) was initiated 2 days before CSDS and continued throughout the 10-day procedure for both wild-type and Ghsr-null littermates. Throughout the 10 days of CSDS, the thymidine analog BrdU (50mg kg−1 intraperitoneal) was administered daily to label newborn cells (Figure 3a). Liquid chromatography–tandem mass spectrometry analysis of plasma and brain P7C3 concentrations revealed that similar P7C3 levels were achieved in wild-type and Ghsr-null mice (Supplementary Figure S3).
Figure 3.
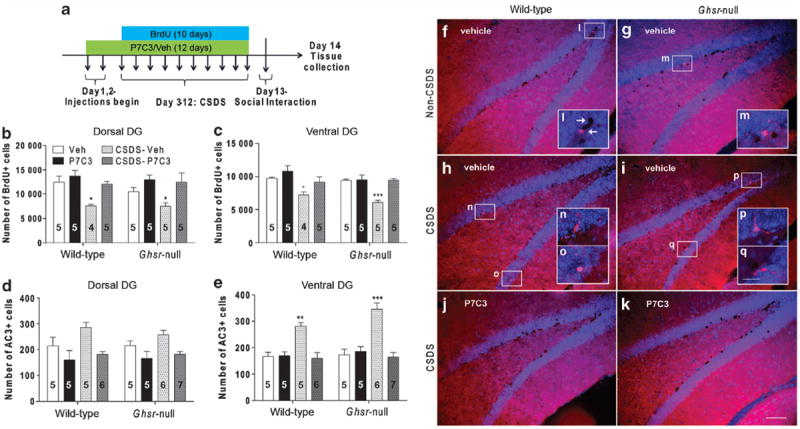
Effects of P7C3 on dentate gyrus (DG) cellular proliferation and apoptosis following chronic social defeat stress (CSDS). (a) Protocol schematic. (b–e) Bromodeoxyuridine-positive (BrdU+) and activated caspase 3-positive (AC3+) cell counts in the dorsal and ventral DG of CSDS-exposed and non-CSDS-exposed mice treated with P7C3 vs vehicle. Group sizes indicated. (f–k) Representative photomicrograph images of double-immunolabeled (BrdU and AC3) ventral DG sections. (l–q) Magnified images of immunoreactive cells (AC3+, leftward-facing arrow; BrdU+, rightward-facing arrow). Scale bar in panel (k) (100 μm) pertains to panels (f–k) and in panel (q) (25 μm) pertains to panels (l–q). *P < 0.05, **P < 0.01, ***P < 0.001, as compared with respective non-CSDS-exposed, vehicle-treated control group.
Similar to the above Ki67 results, immunohistochemical examination of brain tissue from these mice revealed that CSDS reduced the number of cells with BrdU incorporation (a marker of dividing cells and/or cell survival) in both vehicle-treated wild-type and Ghsr-null mice throughout the DG, with a more significant reduction in the ventral DG of Ghsr-null mice (Figures 3b, c, f–i, l–q). In both wild-type and Ghsr-null mice, P7C3 administration during CSDS blocked CSDS-induced reductions in BrdU+ cells throughout both dorsal and ventral regions of the DG (Figures 3b, c, f–k). In parallel, vehicle-treated mice displayed an elevation of AC3+ cells only in the ventral DG after CSDS, an outcome associated with a stronger P-value in Ghsr-null mice (Figures 3d–e) and blocked by P7C3 administration (Figures 3e–k). Thus, treatment with P7C3 blocked the elevated programmed cell death usually observed in the DG after CSDS. Dual-label immunohistochemistry for both BrdU and NeuN, which is a marker of mature neurons, revealed that P7C3 administration during CSDS resulted in significantly more BrdU+/NeuN+ cells than vehicle administration, reflective of increased survival of proliferating DG neural precursor cells that develop into mature neurons68 (Supplementary Figure S4). This, combined with the observed decreases in DG subgranular zone AC3+ cells, supports a net effect of increased hippocampal neurogenesis in mice receiving P7C3 during CSDS exposure.
P7C3 compounds reduce depression in chronic stress-exposed mice
Next, we used P7C3 and two P7C3 chemical analogs to investigate whether P7C3-mediated preservation of ventral DG neurogenesis during CSDS might result in an antidepressant-like effect. The analog P7C3-A20 substitutes a fluoride at the hydroxyl position in the linker region, conferring significantly greater neuroprotective efficacy than P7C3,52 as demonstrated in animal models of Parkinson’s disease,52 amyotrophic lateral sclerosis and traumatic brain injury.66,67 Conversely, the analog P7C3-S184 replaces bromines on the carbazole moiety with chlorines, and the aniline moiety with a naphthyl amine, leaving the analog devoid of neuroprotective activity.52,53 In CSDS-exposed wild-type mice, P7C3 treatment had no significant effect on depression-like behavior (Figures 4a and c). However, in CSDS-exposed Ghsr-null mice, both P7C3 and P7C3-A20 showed antidepressant efficacy, reflected by reduced time spent in the corners of the testing chamber and increased time spent in the interaction zone (Figures 4b and d). The more highly active analog P7C3-A20 also showed antidepressant efficacy in CSDS-exposed wild-type mice, as reflected by significantly reduced time spent in the corners of the testing chamber (Figure 4a). The inactive analog P7C3-S184 had no effect on stress-induced depressive-like behavior in either genotype (Figures 4a–d). Neither P7C3 nor the two tested analogs influenced social interaction test performance in non-CSDSexposed control mice (Supplementary Figure S5). Thus, P7C3 displayed antidepressant efficacy in the stressed, depressionprone Ghsr-null mice, while the more highly active analog P7C3-A20 exhibited antidepressant efficacy both in stressed Ghsr-null mice and in their more resilient, stressed wild-type littermates. Lack of an antidepressant effect of P7C3-S184, which lacks neuroprotective activity, supports the notion that the antidepressant efficacy of P7C3 and P7C3-A20 is due to neuroprotection.
Figure 4.
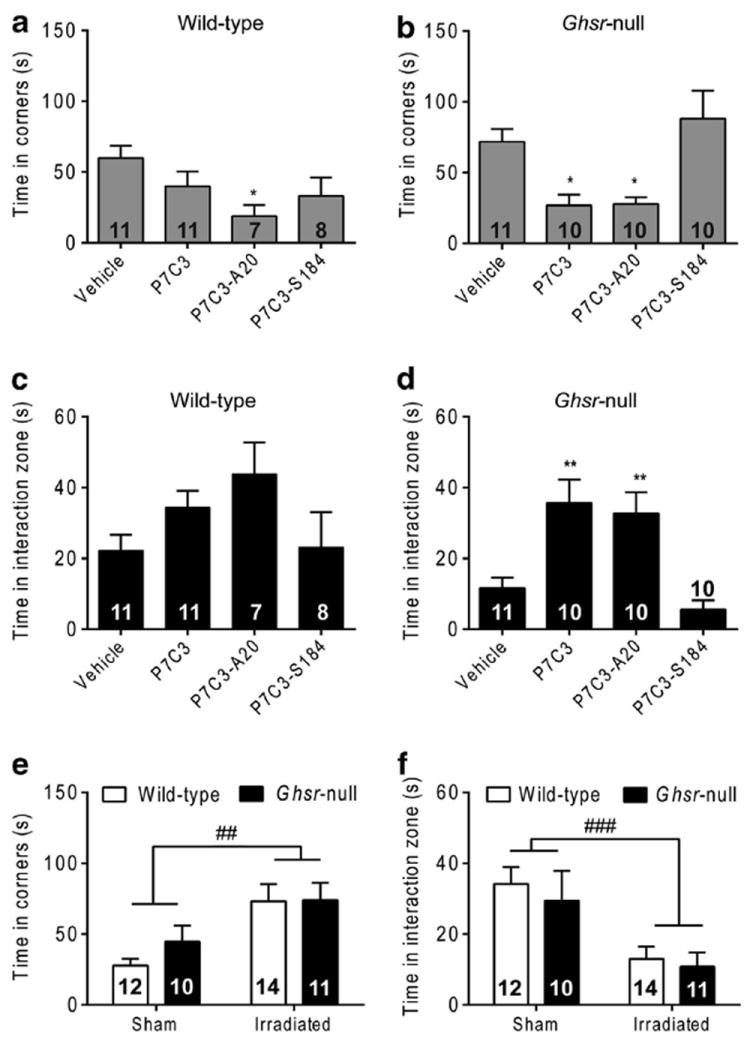
Effects of P7C3 on chronic social defeat stress-induced depressive-like behavior, as measured by the social interaction test. (a, b) Time spent in the corners with target present for wild-type (a) and Ghsr-null (b) mice. (c, d) Time spent in the interaction zone by wild-type (c) and Ghsr-null (d) mice. Group sizes indicated. (e, f) Time spent in the corners (e) or interaction zone (f) for sham or irradiated mice. *P<0.05, **P < 0.01, as compared with respective vehicle-treated control group; ##P < 0.01, ###P < 0.001 treatment effect of irradiation.
We also tested the antidepressant efficacy of P7C3-A20 using the CSDS protocol in mice that received focal irradiation targeting the hippocampus as compared with non-irradiated sham controls. Hippocampus-directed cranial irradiation was performed at a dose of 15 gray, which ablates proliferating cells without causing significant mRNA elevations in inflammatory markers at 1 month postirradiation.69,70 Administration of P7C3-A20 to irradiated Ghsr-null and wild-type mice during CSDS resulted in significantly more time spent in the corners and less time in the interaction zone as compared with that observed in P7C3-A20-administered sham mice (Figures 4e and f). Therefore, cranial irradiation-induced ablation of proliferating DG cells resulted in a loss of P7C3-A20′s antidepressant efficacy, further suggesting that the antidepressant efficacy of P7C3 and P7C3-A20 is due to their ability to augment the net magnitude of hippocampal neurogenesis by blocking death of proliferating neural precursor cells.
P7C3 blocks apoptosis and restores the antidepressant response to caloric restriction in Ghsr-null mice
Prolonged caloric restriction in wild-type mice elicits an antidepressant-like response in the FST (decreased immobility), whereas this effect is not observed in Ghsr-null mice.21 We first confirmed this finding21 (Figures 5d and e) and then investigated its relationship to differences in DG cell survival, similar to what we observed following CSDS. We exposed Ghsr-null and wild-type littermates to 60% caloric restriction for 10 days vs ad libitum food access to control mice. Five days before and throughout the 10 days of restricted feeding, mice received either P7C3 or vehicle. On Day 16, FST performance was assessed, followed by quantification of AC3+ cells in the DG (Figure 5a). Surprisingly, the number of AC3+ cells after caloric restriction was reduced throughout the DG in wild-type mice, yet increased in Ghsr-null mice (Figures 5b, c, f–p). Whereas P7C3 treatment did not further reduce the number of AC3+ cells in caloric-restricted wild-type mice (Figures 5b and c), it did block the increase in apoptosis observed after caloric restriction in Ghsr-null mice (Figures 5b and c). Notably, this effect correlated with significantly decreased immobility of Ghsr-null mice in the FST, with no behavioral effect in wild-type littermates (Figures 5d and e). Thus, the neuroprotective efficacy of P7C3 in Ghsr-null mice restores the antidepressant-like effect of caloric restriction to that normally observed in wild-type mice (Figures 5d and e).
Figure 5.
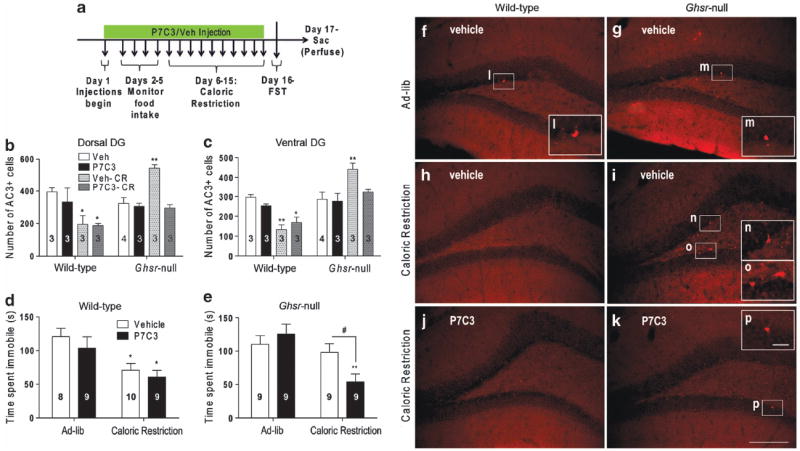
Effects of P7C3 on caloric restriction-induced dentate gyrus (DG) cell survival and antidepressant-like behavior, as measured by forced swim test (FST). (a) Protocol schematic. (b–c) Activated caspase 3-positive (AC3-immunoreactive cell counts in the DG of calorierestricted and ad libitum-fed mice treated with P7C3 vs vehicle. (d, e) FST immobility time. Group sizes indicated. (f–k) Representative photomicrograph images of AC3-immunolabeled ventral DG sections. (l–p) Magnified images of AC3-immunoreactive cells. Scale bar in panel (k) (150 μm) pertains to panels (f–k) and in panel (p) (25 μm) pertains to panels (l–p). *P < 0.05, **P < 0.01, as compared with respective ad libitum-fed, vehicle-treated control group. #P < 0.05, comparing compound treatment.
P7C3-A20 has greater proneurogenic efficacy than current antidepressants
We next compared the proneurogenic efficacy of P7C3 and P7C3- A20 to several currently marketed antidepressants using screening conditions identical to those by which P7C3 was discovered.51 Continuous and direct intracerebroventricular infusion of P7C3 or P7C3-A20 over a 1-week period of time into the left lateral ventricle markedly augmented BrdU labeling in the contralateral hemisphere by about 100% or 160%, respectively (Supplementary Figure S6). By contrast, only 4 out of the 14 antidepressants significantly increased DG BrdU labeling over vehicle. In particular, the norepinephrine–dopamine reuptake inhibitor bupropion, the monoamine oxidase inhibitor phenelzine and the tricyclic antidepressants clomipramine and desipramine increased BrdU labeling by about 49, 52, 75 and 102%, respectively. Of those, only clomipramine and desipramine were statistically as effective as P7C3 in elevating BrdU+ cell number, and none was statistically as effective as P7C3-A20. The remaining antidepressants tested, including the selective serotonin reuptake inhibitors paroxetine, citalopram, fluoxetine and sertraline, the serotonin– norepinephrine reuptake inhibitor venlafaxine, the noradrenergic and specific serotonergic antidepressant mirtazapine, the monoamine oxidase inhibitor tranylcypromine and the tricyclic antidepressants nortriptyline and imipramine, did not affect BrdU labeling. Thus the proneurogenic efficacy of the P7C3 class of compounds is superior to a wide spectrum of antidepressants representing the major classes currently prescribed. If indeed, as the current studies suggest, augmentation of hippocampal neurogenesis is crucial for the manifestation of antidepressant efficacy of endogenously generated substances (such as ghrelin) or exogenously administered pharmacologic agents, then the magnitude of proneurogenic efficacy offered by highly active members of the P7C3 class suggests that this chemical scaffold may serve as a basis for developing a new and improved class of antidepressants.
DISCUSSION
In the current study, we have identified impaired hippocampal neurogenesis as a general contributing factor to the depression associated with chronic psychosocial stress in our mouse models. Furthermore, effects seen in Ghsr-null mice suggest that hippocampal neuroprotection is a primary mechanism by which stress-induced elevations in ghrelin protect against what would otherwise be worsened stress-associated depression. These new insights led us to determine the antidepressant efficacy of the P7C3 class of neuroprotective compounds in our animal models of depression. Indeed, P7C3 blocked both the decrease in hippocampal neurogenesis and the exacerbated depression-like behavior observed in CSDS-exposed Ghsr-null mice. Notably, even depression-like behavior observed in CSDS-exposed wild-type mice was minimized by P7C3-A20, a more highly active analog of P7C3. Absence of a behavioral response to P7C3-S184, a P7C3 analog that lacks neuroprotective efficacy, further supports specificity of the neuroprotective properties of P7C3 and P7C3-A20 in conferring antidepressant efficacy. Furthermore, the lack of these behavioral effects in mice exposed to hippocampus-directed cranial irradiation even more strongly links neuroprotection of P7C3 compounds to the observed antidepressant response.
Interestingly, in the current study, P7C3 administration did not significantly increase the number of BrdU+ cells in the DG of non-stressed control animals, a finding that differs from the original manuscript reporting the discovery of P7C351 (Figures 3a–d). This difference may be attributed to changes in experimental technique. In particular, the original screen by which P7C3 was discovered utilized singly-housed 12-week-old adult male mice completely deprived of environmental enrichment. This design was used to maintain basal neurogenesis at a consistently low level for purposes of the discovery screen.51 By contrast, the current group of non-stressed control animals consisted of younger (8-weeks-of-age) mice that were group housed (in the same cage with a member of the same strain across a perforated divider) under conditions of normal environmental enrichment. Both younger age and social activity with group housing are associated with higher rates of baseline hippocampal neurogenesis36,40 and thus may have masked the effect of P7C3—a less active member of the P7C3 class of neuroprotective chemicals—in the non-stressed control mice. Nonetheless, here, P7C3 was observed to potently augment neurogenesis in pathological conditions associated with elevated cell death of newborn hippocampal neural precursor cells.
Effects of ghrelin and P7C3 on hippocampal neurogenesis and depression-like behavior and a mechanistic link between the two were observed not only in mice exposed to CSDS but also in mice exposed to 10-day caloric restriction, which also elevates circulating ghrelin. Similar to what was observed in CSDS-exposed Ghsr-null mice, the inability of Ghsr-null mice to mount the usual antidepressant response to caloric restriction was associated with acquired deficits in DG cell survival, as both the increase in apoptotic DG cells and the depression-like FST behavior observed in Ghsr-null animals were normalized by treatment with P7C3.
That said, the differential changes in hippocampal neurogenesis induced by the 10-day 60% caloric restriction protocol in Ghsr-null mice vs wild type littermates were slightly different than those induced by CSDS. In particular, the number of AC3+ cells after caloric restriction was reduced throughout the DG in wild-type mice, yet increased in Ghsr-null mice. CSDS, though, increased AC3 + hippocampal cell numbers in the ventral DG of both wild-type and Ghsr-null mice, with significantly more AC3+ cells observed in Ghsr-null mice as compared with wild-type littermates. The different degrees of CSDS-induced social avoidance observed in Ghsr-null and wild-type littermates suggest a dosage effect of defective hippocampal neurogenesis in driving depression-like behavior. However, if indeed hippocampal neurogenesis alone drives antidepressant-like responses, then an unchanged number of AC3+ DG cells (instead of an increased number) might have been expected in the calorically restricted Ghsr-null mice, which showed similar immobility times compared with ad libitum-fed Ghsr-null mice. P7C3 nonetheless was able to prevent the caloric restriction-induced elevation in AC3+ DG cell numbers in Ghsr-null mice, which we believe resulted in the restoration of the usual antidepressant-like behavioral response to the caloric-restriction protocol.
Altogether, the more severe depressive-like behavior and exaggerated decrease in hippocampal neurogenesis observed in stress-exposed Ghsr-null mice, the depressive-like behavior and increased hippocampal cell death in caloric-restricted Ghsr-null mice and the prevention of those effects by administration of active P7C3 compounds suggest a key protective role of ghrelin’s inherent proneurogenic capacity in mediating mood responses to chronic stress and moderate caloric restriction. In addition, these findings further demonstrate the impact of DG neurogenesis on regulation of depressive-like behavior, with protection against exacerbated neurogenic loss conferring beneficial effects for depressive behavior.
It is also important to mention that, whereas some of the antidepressant agents tested here previously had been shown to enhance neurogenesis, the same was not observed in our assay. This lack of neurogenic efficacy potentially may be accounted for by differences in animal species, route of administration, dose and administration duration. Regarding administration duration, previous studies demonstrated increased hippocampal BrdU labeling in response to fluoxetine after 14 days39,71 and 28 days of administration but not after 1 day or 5 days.39 Also, enhanced adult hippocampal neurogenesis was observed after 14 days of paroxetine and 21 days of tranylcypromine.39,72 Although we have not directly compared the effects of P7C3 and P7C3-A20 administration on neurogenesis to those of marketed antidepressants after 2 weeks of administration, we believe that the rapidity in which the P7C3 compounds affected neurogenesis in addition to the magnitude of their effect potentially could contribute to their superiority over current antidepressant agents.
Our results suggest that individuals with depression associated with insufficient ghrelin or ghrelin resistance might be particularly responsive to treatment with neuroprotective agents, as embodied by the P7C3 class. For instance, individuals who have undergone Roux-en-Y gastric bypass weight loss surgery have a higher rate of suicide than the general population.73 As some studies have demonstrated decreased plasma ghrelin levels following Roux-en-Y gastric bypass,74 neuroprotection by the P7C3 class of compounds may help protect from depression by counteracting the impact of aberrant ghrelin signaling on hippocampal neurogenesis. Future studies are needed to assess the role of impaired hippocampal neurogenesis as a contributory factor to other forms of depression besides those associated with chronic psychosocial stress or defective ghrelin signaling. Also, although we demonstrated that blocking loss of hippocampal neurons by administering P7C3 compounds during CSDS protects against the usual depressive-like behavioral response, future studies will be needed to determine whether the P7C3 compounds also will diminish CSDS-induced depression when administered after CSDS. Our hope is that the chemical scaffold represented by P7C3 and P7C3-A20 will provide a basis for optimizing and advancing a new class of antidepressants.
Supplementary Material
Acknowledgments
These studies were made possible through funding from the NIH (1R01MH085298 and 1R01DA024680 to JMZ; T32DA007290 to AKW through AJE, DA016765 and DA023555 to AJE and 1RO1MH087986 to AAP and Steven L McKnight), institutional funds from University of Iowa Carver College of Medicine to AAP, an International Research Alliance with the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen (to JMZ), the Edward N and Della C Thome Memorial Foundation (to JMR), the Welch Foundation (I-1612; to JMR), NASA (NNX12AB55G to AJE) and an unrestricted endowment provided to Steven L McKnight by an anonymous donor. We also thank Lauren Peca and Shari Birnbaum from the UTSW Medical Center Behavior Core for technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 3.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 4.Horvath TL, Diano S, Tschop M. Ghrelin in hypothalamic regulation of energy balance. Curr Top Med Chem. 2003;3:921–927. doi: 10.2174/1568026033452230. [DOI] [PubMed] [Google Scholar]
- 5.Walker AK, Ibia IE, Zigman JM. Disruption of cue-potentiated feeding in mice with blocked ghrelin signaling. Physiol Behav. 2012;108:34–43. doi: 10.1016/j.physbeh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perello M, Zigman JM. The role of ghrelin in reward-based eating. Biol Psychiatry. 2012;72:347–353. doi: 10.1016/j.biopsych.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang JC, Perello M, Sakata I, Osborne-Lawrence S, Savitt JM, Lutter M, et al. Ghrelin mediates stress-induced food-reward behavior in mice. J Clin Invest. 2011;121:2684–2692. doi: 10.1172/JCI57660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine-and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology. 2010;211:415–422. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry. 2013;73:915–923. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 11.Lim E, Lee S, Li E, Kim Y, Park S. Ghrelin protects spinal cord motoneurons against chronic glutamate-induced excitotoxicity via ERK1/2 and phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3beta pathways. Exp Neurol. 2011;230:114–122. doi: 10.1016/j.expneurol.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Chung H, Kim E, Lee DH, Seo S, Ju S, Lee D, et al. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007;148:148–159. doi: 10.1210/en.2006-0991. [DOI] [PubMed] [Google Scholar]
- 13.Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells. J Endocrinol. 2008;198:511–521. doi: 10.1677/JOE-08-0160. [DOI] [PubMed] [Google Scholar]
- 14.Moon M, Kim HG, Hwang L, Seo JH, Kim S, Hwang S, et al. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease by blocking microglial activation. Neurotox Res. 2009;15:332–347. doi: 10.1007/s12640-009-9037-x. [DOI] [PubMed] [Google Scholar]
- 15.Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057–14065. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, Chung H, Yoo YS, Oh YJ, Oh TH, Park S, et al. Inhibition of apoptotic cell death by ghrelin improves functional recovery after spinal cord injury. Endocrinology. 2010;151:3815–3826. doi: 10.1210/en.2009-1416. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Lim E, Kim Y, Li E, Park S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J Endocrinol. 2010;205:263–270. doi: 10.1677/JOE-10-0040. [DOI] [PubMed] [Google Scholar]
- 18.Kluge M, Schussler P, Dresler M, Schmidt D, Yassouridis A, Uhr M, et al. Effects of ghrelin on psychopathology, sleep and secretion of cortisol and growth hormone in patients with major depression. J Psychiatr Res. 2011;45:421–426. doi: 10.1016/j.jpsychires.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Carlini VP, Machado DG, Buteler F, Ghersi M, Ponzio MF, Martini AC, et al. Acute ghrelin administration reverses depressive-like behavior induced by bilateral olfactory bulbectomy in mice. Peptides. 2012;2:160–165. doi: 10.1016/j.peptides.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Lutter M, Elmquist J. Depression and metabolism: linking changes in leptin and ghrelin to mood. F1000 Biol Rep. 2009;1:63. doi: 10.3410/B1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson ZR, Khazall R, Mackay H, Anisman H, Abizaid A. Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology. 2013;154:1080–1091. doi: 10.1210/en.2012-1834. [DOI] [PubMed] [Google Scholar]
- 24.Raspopow K, Abizaid A, Matheson K, Anisman H. Psychosocial stressor effects on cortisol and ghrelin in emotional and non-emotional eaters: influence of anger and shame. Horm Behav. 2010;58:677–684. doi: 10.1016/j.yhbeh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, et al. The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology. 2007;32:693–702. doi: 10.1016/j.psyneuen.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Ochi M, Tominaga K, Tanaka F, Tanigawa T, Shiba M, Watanabe T, et al. Effect of chronic stress on gastric emptying and plasma ghrelin levels in rats. Life Sci. 2008;82:862–868. doi: 10.1016/j.lfs.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Kristenssson E, Sundqvist M, Astin M, Kjerling M, Mattsson H, Dornonville de la Cour C, et al. Acute psychological stress raises plasma ghrelin in the rat. Regul Pept. 2006;134:114–117. doi: 10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74:143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- 29.Patterson ZR, Ducharme R, Anisman H, Abizaid A. Altered metabolic and neurochemical responses to chronic unpredictable stressors in ghrelin receptor-deficient mice. Eur J Neurosci. 2010;32:632–639. doi: 10.1111/j.1460-9568.2010.07310.x. [DOI] [PubMed] [Google Scholar]
- 30.Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin–growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry. 2013 Oct 15; doi: 10.1038/mp.2013.135. advance online publication, (e-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakashima K, Akiyoshi J, Hatano K, Hanada H, Tanaka Y, Tsuru J, et al. Ghrelin gene polymorphism is associated with depression, but not panic disorder. Psychiatr Genet. 2008;18:257. doi: 10.1097/YPG.0b013e328306c979. [DOI] [PubMed] [Google Scholar]
- 32.Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 33.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisch AJ, Petrik D. Depression and hippocampal neurogenesis: a road to remission? Science. 2012;338:72–75. doi: 10.1126/science.1222941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrik D, Lagace DC, Eisch AJ. The neurogenesis hypothesis of affective and anxiety disorders: are we mistaking the scaffolding for the building? Neuropharmacology. 2012;62:21–34. doi: 10.1016/j.neuropharm.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 37.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 38.Van Bokhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ, Spijker S. Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci. 2011;33:1833–1840. doi: 10.1111/j.1460-9568.2011.07668.x. [DOI] [PubMed] [Google Scholar]
- 39.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- 42.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 43.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 44.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 45.Johansson I, Destefanis S, Aberg ND, Aberg MA, Blomgren K, Zhu C, et al. Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells. Endocrinology. 2008;149:2191–2199. doi: 10.1210/en.2007-0733. [DOI] [PubMed] [Google Scholar]
- 46.Li E, Chung H, Kim Y, Kim DH, Ryu JH, Sato T, et al. Ghrelin diectly stimulates adult hippocampal neurogenesis: implications for learning and memory. Endocr J. 2013;60:781–789. doi: 10.1507/endocrj.ej13-0008. [DOI] [PubMed] [Google Scholar]
- 47.Moon M, Kim S, Hwang L, Park S. Ghrelin regulates hippocampal neurogenesis in adult mice. Endocr J. 2009;56:525–531. doi: 10.1507/endocrj.k09e-089. [DOI] [PubMed] [Google Scholar]
- 48.Li E, Kim Y, Kim S, Park S. Ghrelin-induced hippocampal neurogenesis and enhancement of cognitive function are mediated independently of GH/IGF-1 axis: lessons from the spontaneous dwarf rats. Endocr J. 2013;60:1065–1075. doi: 10.1507/endocrj.ej13-0045. [DOI] [PubMed] [Google Scholar]
- 49.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Jesus-Cortes H, Xu P, Drawbridge J, Estill SJ, Huntington P, Tran S, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc Natl Acad Sci USA. 2012;109:17010–17015. doi: 10.1073/pnas.1213956109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacMillan KS, Naidoo J, Liang J, Melito L, Williams NS, Morlock L, et al. Development of proneurogenic, neuroprotective small molecules. J Am Chem Soc. 2011;133:1428–1437. doi: 10.1021/ja108211m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 55.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 56.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 57.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maggio N, Segal M. Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus. J Neurosci. 2009;29:8633–8638. doi: 10.1523/JNEUROSCI.1901-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanti A, Rainer Q, Minier F, Surget A, Belzung C. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology. 2012;63:374–384. doi: 10.1016/j.neuropharm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 60.Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19:20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- 61.Hock BJ, Jr, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18:7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson CL, Pathak SD, Jeromin A, Ng LL, MacPherson CR, Mortrud MT, et al. Genomic anatomy of the hippocampus. Neuron. 2008;60:1010–1021. doi: 10.1016/j.neuron.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 63.Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 64.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kheirbek MA, Hen R. Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood. Neuropsychopharmacology. 2011;36:373–374. doi: 10.1038/npp.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaya MO, Bramlett HM, Naidoo J, Pieper AA, Dietrich WD. Neuroprotective efficacy of a proneurogenic compound after traumatic brain injury. J Neurotrauma. 2013;31:476–486. doi: 10.1089/neu.2013.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tesla R, Wolf HP, Xu P, Drawbridge J, Estill SJ, Huntington P, et al. Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2012;109:17016–17021. doi: 10.1073/pnas.1213960109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordberg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 69.Moravan MJ, Olschowka JA, Williams JP, O’Banion MK. Cranial irradiation leads to acute and persistent neuroinflammation with delayed increases in T-cell infiltration and CD11c expression in C57BL/6 mouse brain. Radiat Res. 2011;176:459–473. doi: 10.1667/rr2587.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, et al. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein LJ, et al. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J Neurotrauma. 2011;28:259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu G, Helmeste DM, Samaranayake AN, Lau WM, Lee TM, Tang SW, et al. Modulation of the suppressive effect of corticosterone on adult rat hippocampal cell proliferation by paroxetine. Neurosci Bull. 2007;23:131–136. doi: 10.1007/s12264-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 74.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


