Abstract
Thiamine dependent enzymes are diminished in Alzheimer’s disease (AD). Thiamine deficiency in vitro and in rodents is a useful model of this reduction. Thiamine interacts with cellular calcium stores. To directly test the relevance of the thiamine dependent changes to dynamic processes in AD, the interactions must be studied in cells from patients with AD. These studies employed fibroblasts. Mitochondrial dysfunction including reductions in thiamine dependent enzymes and abnormalities in calcium homeostasis and oxidative processes occur in fibroblasts from Alzheimer’s Disease (AD) patients. Bombesin-releasable calcium stores (BRCS) from the endoplasmic reticulum (ER) are exaggerated in fibroblasts from patients with AD bearing a presenilin-1 (PS-1) mutation and in control fibroblasts treated with oxidants. ER calcium regulates calcium entry into the cell through capacitative calcium entry (CCE), which is reduced in fibroblasts and neurons from mice bearing PS-1 mutations. Under physiological conditions, mitochondria and ER play important and interactive roles in the regulation of Ca2+ homeostasis. Thus, the interactions of mitochondria and oxidants with CCE were tested. Inhibition of ER Ca2+-ATPase by cyclopiazonic acid (CPA) stimulates CCE. CPA-induced CCE was diminished by inhibition of mitochondrial Ca2+ export (−60%) or import (−40%). Different aspects of mitochondrial Ca2+ coupled to CPA-induced-CCE were sensitive to select oxidants. The effects were very different when CCE was examined in the presence of InsP3, a physiological regulator of ER calcium release, and subsequent CCE. CCE under these conditions was only mildly reduced (20–25%) by inhibition of mitochondrial Ca2+ export, and inhibition of mitochondrial Ca2+ uptake exaggerated CCE (+53%). However, t-BHP reversed both abnormalities. The results suggest that in the presence of InsP3, mitochondria buffer the local Ca2+ released from ER following rapid activation of InsP3R and serve as a negative feedback to the CCE. The results suggest that mitochondrial Ca2+ modifies the depletion and refilling mechanism of ER Ca2+ stores.
Keywords: Calcium, Alzheimer’s disease, mitochondria, endoplasmic reticulum, oxidants, capacitative calcium entry, IP3, fibroblasts
INTRODUCTION
Thiamine dependent enzymes are diminished in Alzheimer’s disease (AD). Rodent thiamine deficiency (TD) has been used to model the mild impairment of metabolism that occurs in AD [Karuppagounder et al.,2009]. TD exaggerates plaque and tangle formation in mouse models [Karuppagounder et al.,2009] and elevating thiamine levels diminish plaques, tangles and memory deficits [Pan et al., 2010]. An understanding of the consequences of the reduction of thiamine dependent enzymes is important for understanding the pathophysiology of AD and for developing new therapies. Reduction of the thiamine dependent enzyme alpha-ketoglutarate dehydrogenase (KGDHC) either with an inhibitor or by genetic manipulation reveal that another consequence of diminished activity of a thiamine dependent enzyme is an alteration in the calcium stores in the endoplasmic reticulum. Thus, neurons taken from mice deficient in KGDHC have exaggerated stores of ER calcium whether the neurons are cultured from embryos or adults, just as in fibroblasts from patients with AD [Gibson et al., 2012].
Whether this change occurs and is important in AD is more difficult to answer. Since the calcium change is dynamic one cannot measure this property in autopsy brain. A commonly used model to study disease processes is cultured fibroblasts. Indeed, fibroblasts were used by Dr. Butterworth in pioneering studies in the 1980s in which he looked at thiamine dependent enzymes in Leigh’s disease in fibroblasts. Surprisingly, the same abnormalities in calcium homeostasis that we observed by reducing a thiamine dependent enzyme in mouse brains occurs in fibroblasts from AD patients. BRCS from the endoplasmic reticulum (ER) are exaggerated in fibroblasts from patients with AD bearing a presenilin-1 (PS-1) mutation [Ito et al., 1994] and in control fibroblasts treated with specific oxidants [Huang et al., 2005]. The two oxidants employed in these studies were: (1) tert-Butyl-hydroperoxide (t-BHP) which produces the radicals tert-butyloxyl (t-bu-OS) and t-butylperoxyl (t-bu-OOS) and (2) 3-morpholinosydnonimine (SIN-1), which is commonly used to produce various forms of nitrogen monoxides that react with O2.− to form peroxynitrite. A more detailed discussion is provided in [Huang et al., 2005]. The goal of the current study is to understand the consequences of these changes on cellular calcium regulation. Considerable research has been accomplished in understanding the increase in calcium in fibroblasts bearing presenilin-1 mutations leading to AD [Nelson et al., 2010]. However, these mechanisms only apply for patients bearing presenilin mutations. Thus, these interactions need be better understood in non-genetic forms of AD. The best cells to accomplish this are cells with a human genetic background (i.e., fibroblasts).
Calcium dynamics and the response of cells to oxidants are modified by thiamine [Huang, Chen and Gibson, 2010]. Specific oxidants can induce the same changes in calcium dynamics that occur in fibroblasts from patients with AD (an exaggeration of BRCS) [Huang, Chen and Gibson, 2005]. Further, the responses of cells to oxidants are modified by thiamine. Thiamine diminishes ER calcium stores, but does not alter CCE [Huang, Chen and Gibson, 2010].
The current studies test how these changes in ER calcium interact to regulate cellular calcium and the effects of oxidants on these responses. The mitochondria and ER are linked by proteins (mitochondrial associated proteins) that have been linked to proteins that underlie some genetic forms of AD [Area-Gomez et al., 2009]. In addition, to the interaction between the ER and mitochondria there is a link between these processes and the ability to fill cellular calcium pools. The depletion of ER Ca2+ content triggers Ca2+ influx through plasma membrane Ca2+ channels, a process known as capacitative calcium entry (CCE) [Putney and Ribeiro, 2000]. CCE is reduced in fibroblasts and neurons from mice bearing PS-1 mutations [Yao et al., 2000; Leissring et al., 2000]. Thus, genetic mutations that cause AD lead to increase intracellular calcium stores and an attenuation in the refilling mechanism CCE. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons [Chan et al., 2000]. Studies suggest that ER Ca2+ disruption in AD is related to an enhancement of ryanodine receptor regenerative process [Stutzmann et al., 2006]. The ryanodine receptor is one of the Ca2+ -release channels located on the ER which have been implicated in Ca2+ -induced Ca2+ release activity (CICR). In addition, oxidation of RyR results in activation of CICR [Hidalgo et al., 2000].
Mitochondrial dysfunction may underlie altered calcium homeostasis in AD. Mitochondria may have a fundamental role in regulating CCE through bidirectional Ca2+-dependent crosstalk between mitochondria and CCE [Glitsch et al., 2002]. Mitochondria are often located near Ca2+ release channels on the ER [Simpson et al., 1997] or Ca2+ influx channels in the plasma membrane and modulate store-operated or CCE [Hoth et al., 1997]. Thus, mitochondria contribute to cellular Ca2+ homeostasis in many cell types by taking up and releasing Ca2 which propagates and synchronizes Ca2+ signals [Pozzan et al., 1994]. Whether mitochondria act as a Ca2+ sink or as a Ca2+ relay mechanism, or whether the increase in mitochondrial Ca2+ an essential step to activate CCE is unclear [Glitsch et al., 2002].
The Ca2+ buffering capacities of the ER and mitochondria can regulate CCE [Malli et al., 2003]. InsP3-mediated pathways regulate CCE and Ca2+ uptake and release from mitochondria [Kojima and Ogata, 1989]. Inhibition of the mitochondrial Na+/Ca2+ exchanger with CGP37157 results in the increase of [Ca2+]mito and reduces the ability of mitochondria to buffer subplasmalemmal Ca2+ and leads to an increase of BK-sensitive Ca2+ channels activity and a decrease in CCE [Malli et al, 2003]. In addition, CGP37157 prevents complete Ca2+ refilling of the ER during cell stimulation with an InsP3-generate agonist [Malli et al., 2005].
Mitochondrial Ca2+ uptake is operated by a high-capacity, low-affinity Ca2+ uniporter, which can be inhibited specifically by Ru360. At sites where mitochondria are in close contact with the ER [Rizzuto et al., 1998], micro-domains of high cytosolic Ca2+ are generated by Ca2+ release from ER through InsP3 receptors [Rizzuto et al., 1999]. These high local cytosolic Ca2+ concentrations lead to increases in mitochondrial Ca2+ accumulation that correlate with increases in cytosolic Ca2+ [Rizzuto et al., 1993] [Rizzuto et al., 1998]. Ru360 has no effect on Ca2+ release from ER [Seguchi et al., 2005]. Partial inhibition of Ca2+ uptake by Ru360 increases the [Ca2+]i transient in ventricular myocytes, and propagates the Ca2+ release. This effect of Ru360 did not appear to be due to altered ER Ca2+ content but may contribute to local control of Ca2+ release [Huang et al., 2004].
The present studies tested the interactions of mitochondria, ER Ca2+ and CCE under control conditions as well as with oxidants or InsP3.
MATERIALS AND METHODS
The supplies were from the indicated companies: Cell culture reagents (GIBCO; Grand Island, NY); 3-morpholinosydnonimine (SIN-1) (Molecular Probes; Eugene, Oregon); bombesin, tert-butyl-hydroxyperoxide (t-BHP) (Sigma Chemical, St Louis, MO); CGP17157 and Ru360 (Calbiochem, San Diego, CA).
A human skin fibroblast cell line from a young male control (8399) was purchased from Coriell Cell Repository (Camden, NJ). Cells were maintained exactly as described in our published protocol [Stutzman et al., 2006].
Bombesin releasable calcium stores (BRCS)
Fluorescent intracellular calcium images were monitored as described previously [Huang et al., 2005; Huang et al., 2004]. Fibroblasts were loaded with 2 μM Fura-2 AM in BSS for one hr at room temperature, were rinsed twice with Ca2+-free BSS and [Ca2+]i was monitored on the stage of an inverted Olympus IX70 microscope at room temperature with a Delta Scan System from PTI (Photon Technology International, Lawrenceville, NJ). Excitation wavelengths were alternated between 350 and 378 nm (band pass 4 nm) and emission was monitored at 510 nm with a Hamamatsu C2400 SIT camera at 5 sec intervals. Each value was the average of 32 images taken within 5 sec. Standard images of Fura-2 solutions with minimum and maximum [Ca2+]i were taken at the end of each day’s experiment to calculate the intracellular calcium concentrations.
Capacitative Calcium Entry (CCE) measurement
After cells were preincubated in Ca2+-free media with or without bombesin and the ER Ca2+ -ATPase inhibitor cyclopiazonic acid (CPA), CaCl2 (2.5 mM) was added. In Ca2+-free media, bombesin will release ER Ca2+ from InsP3 sensitive stores, and CPA without bombesin will release InsP3 insensitive Ca2+ stores. The resulting increase in calcium is a measurement of CCE.
Statistical analysis
All data are expressed as mean ± SEM. A Student’s t-test was used to compare two variables. For multiple variable comparisons, data were analyzed by a one-way analysis of variance (ANOVA) followed by a Student Newman-Keul’s test.
RESULTS
Characterization of CPA activated CCE
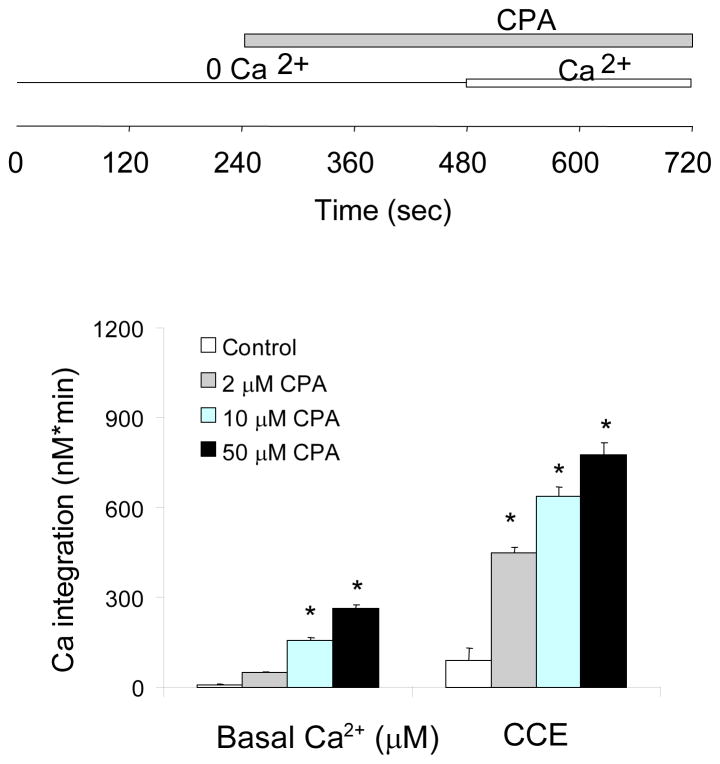
Fibroblasts were incubated in the absence of extracellular Ca2+, the ER Ca2+ was released with CPA, and then Ca2+ was added to initiate Ca2+ re-entry as a measure of CCE. CPA depletes ER Ca2+ stores by reversibly inhibiting ER Ca2+-ATPase that replenishes ER Ca2+. CPA gradually increased cytosolic Ca2+ in a dose-dependent manner by inhibiting ER Ca2+ uptake and allowing the Ca2+ leak from ER (Fig 1). Although 2 μM CPA did not elevate the cytosolic Ca2+ significantly, it activated subsequent CCE markedly (Fig 1). Thus, 2 μM CPA was used for the subsequent experiments most to minimize the complications form increases in cytosolic Ca2+.
Figure 1. Characterization of CPA activated CCE.
Typical temporal profiles of the CCE are shown in subsequent figures. Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. CPA (0, 2, 10, 50 μM) was added 4 min after basal [Ca2+]i measurement, and after an additional 4 min CaCl2 (2.5 mM) was added. The top panel shows experimental paradigms. The bottom panel shows the integrations of the [Ca2+]i peak over the 3 min interval after CPA (255–480 sec) and after calcium addition (495–720 sec). Data are means ± SEM (n=51–62 cells). Asterisks indicate values vary significantly (p<0.05) from the control group by ANOVA followed by Student Newman Keul’s test.
Blockade of mitochondrial Ca2+ exporter by CGP37157 diminished CCE
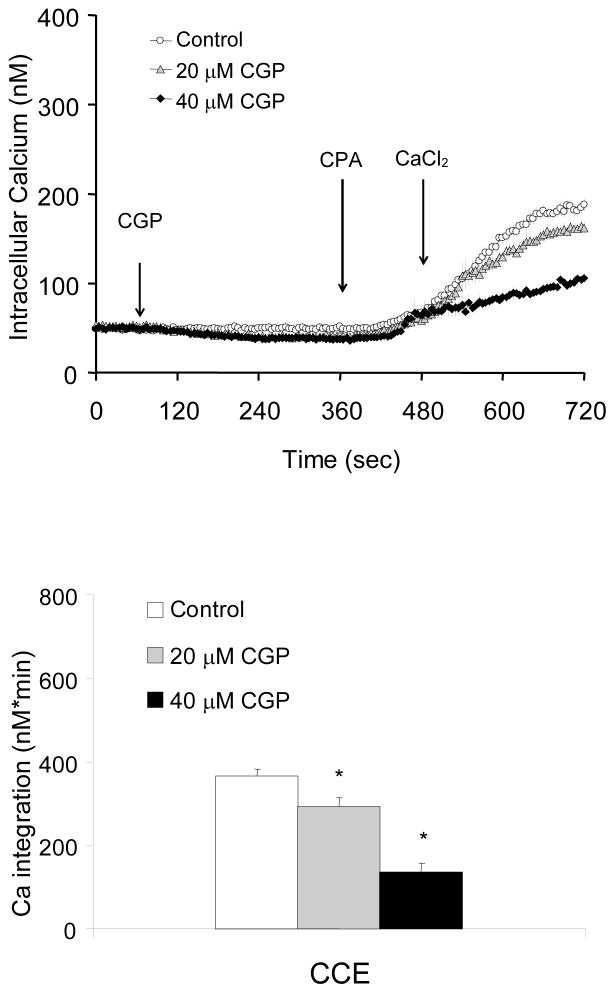
Fibroblasts were incubated with in the absence of extracellular Ca2+ prior to treatment with CGP37157 (0, 20, 40 μM) an inhibitor of the Na+/Ca2+ exchanger the enzyme responsible for mitochondrial calcium export. CPA was then added and subsequently Ca2+ was added to a final concentration of 2.5 mM (CCE). Blockade of the mitochondrial Ca2+ exporter by CGP37157 did not elevate cytosolic Ca2+ significantly, but reduced subsequent CCE following CPA treatment in a dose-dependent manner to 80% (20 μM) or 37% (40 μM) of control (Fig 2).
Figure 2. Inhibition of mitochondrial exporter by CGP37157 diminished CCE induced by CPA.
Blockade of mitochondrial Ca2+ exporter by CGP37157 diminished CCE. Fibroblasts were loaded with Fura 2AM for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. CGP37157 (20, 40 μM), an inhibitor of the mitochondrial Na+/Ca2+ exchanger blocker was added after 1 min of basal [Ca2+]i measurements, and CPA (2 μM) was added after 5 min. After an additional 2 min, CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 45–133 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 3 min interval after calcium addition. Data are means ± SEM (n=45–133 cells). Asterisks indicate values vary significantly (p<0.05) from the control group by ANOVA followed by Student Newman Keul’s test.
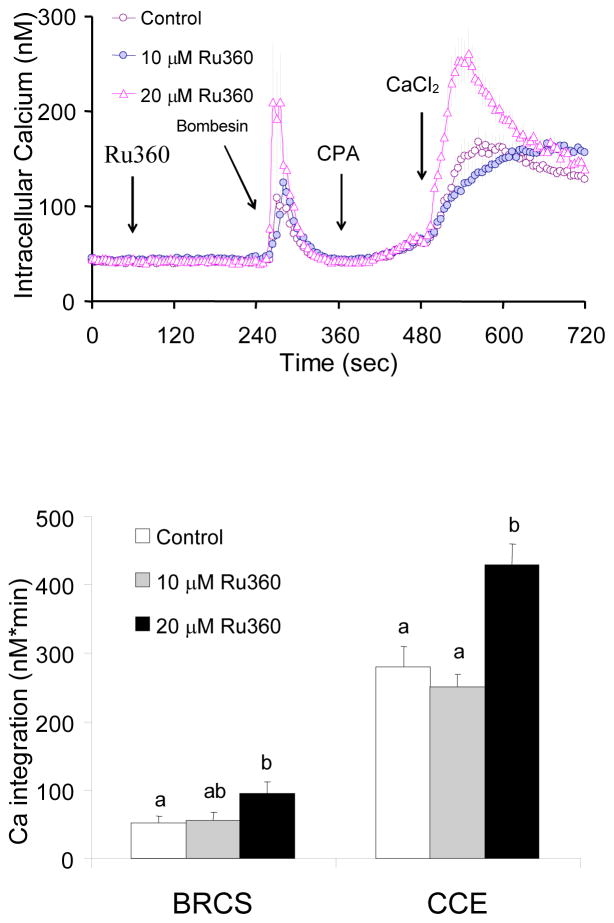
Inhibition of mitochondrial Ca2+ uptake by Ru360 impaired CCE
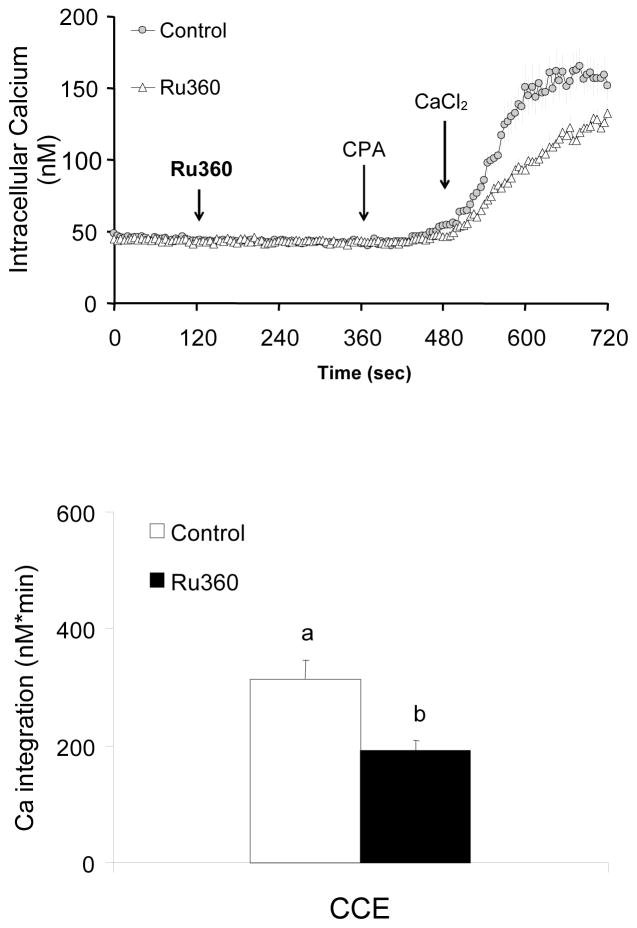
Whether mitochondrial Ca2+ uptake regulates ER Ca2+ and subsequent CCE were tested by using an inhibitor of the mitochondrial Ca2+ uptake Ru360. Fibroblasts were incubated in the absence of extracellular Ca2+ prior to treatment with the Ca2+ uptake inhibitor Ru360 (0, 20 μM). CPA was added and subsequently Ca2+ was added (final concentration of 2.5 mM). Ru360 did not alter basal calcium nor CPA-induced Ca2+ release, whereas it reduced the subsequent CCE (−39%) (Fig 3).
Figure 3. Inhibition of mitochondrial Ca2+ uptake by Ru360 diminished CCE induced by CPA.
Inhibition of mitochondrial Ca2+ uptake by RU360 impaired CCE. Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. The mitochondrial Ca2+ uptake blocker Ru360 (0, 20 μM) was added 2 min after basal [Ca2+]i measurements, and 4 min later CPA (2 μM) was added. Two min later CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 50–51 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 3 min interval after calcium addition (495–720 sec). Data are means ± SEM (n=50–51 cells). Different letters indicate values vary significantly (p<0.05) from the other groups by ANOVA followed by Student Newman Keul’s test.
t-BHP did not alter the CCE induced by CPA
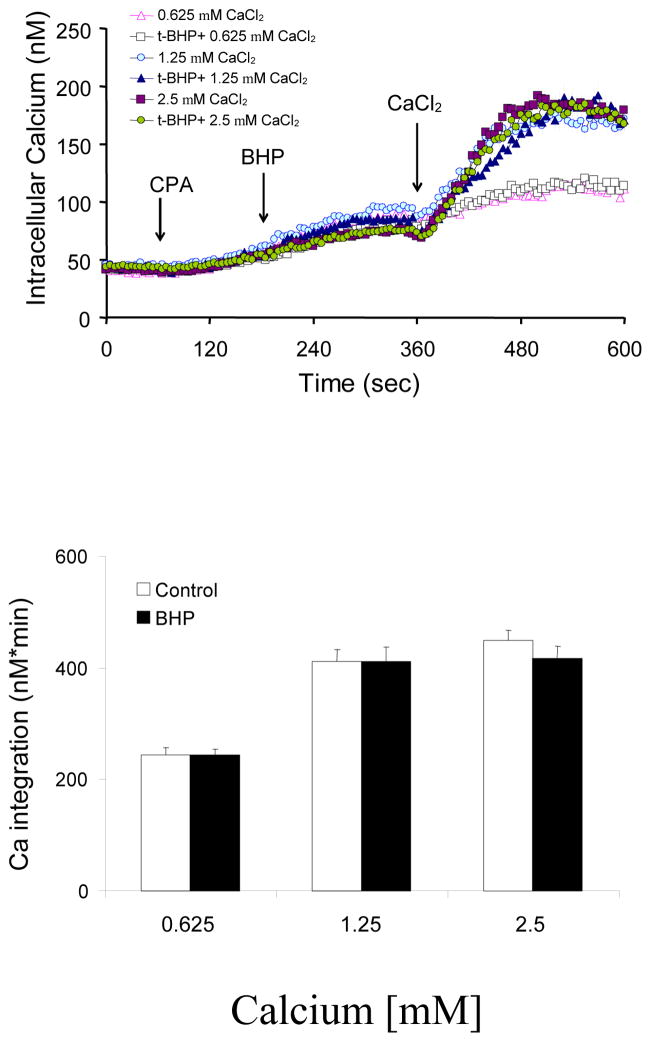
Fibroblasts were incubated in the absence of extracellular Ca2+. The ER Ca2+ was released with CPA (2 μM) prior to addition of t-BHP (100 μM), and then various concentrations of Ca2+ (0.625, 1.25 and 2.5 mM) were added to initiate CCE. The magnitude of CPA-induced elevation in CCE depended on the extracellular Ca2+ concentrations. However, CCE was not modified by t-BHP at any concentration of calcium (Fig 4).
Figure 4. t-BHP did not alter CCE induced by CPA.
Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. CPA (2 μM) was added after 1 min of basal [Ca2+]i measurement, and after an additional 3 min, t-BHP (100 μM) was added. Three min later different concentrations of CaCl2 (final concentrations of 0.625, 1.25 or 2.5 mM) were added. The top panel shows the tracings taken from 44–87 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 3 min interval after calcium addition (380 - 660 sec). Data are means ± SEM (n= 44–87 cells).
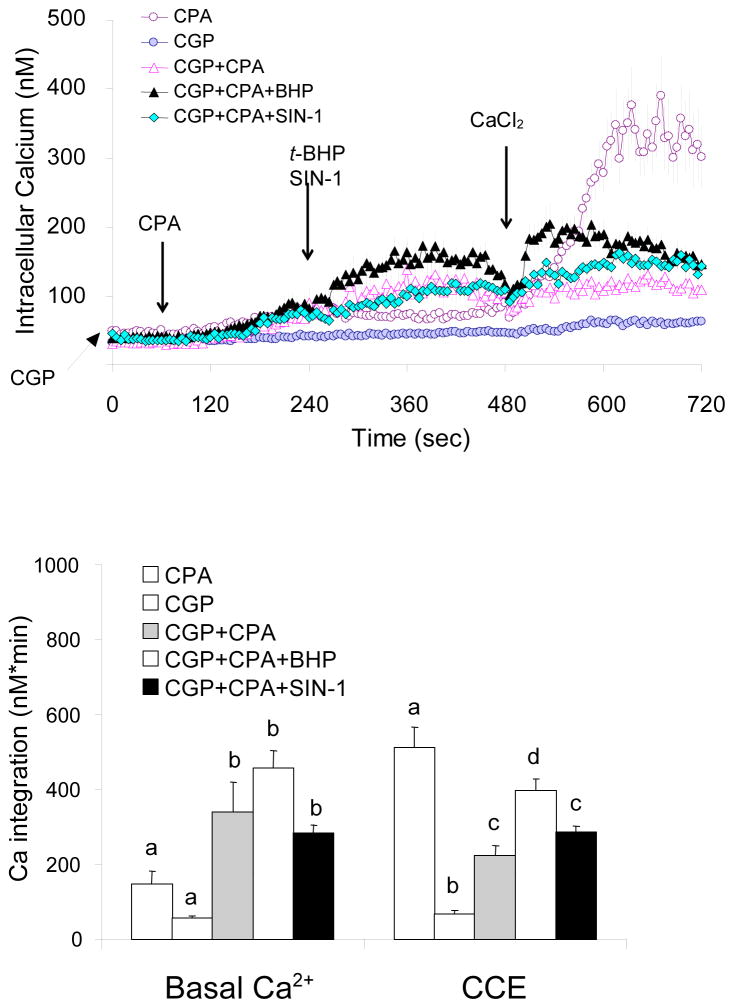
t-BHP but not SIN-1 attenuated the impairment of CCE by CGP37157
Previous studies demonstrated that tBHP increases BRCS just as occurs in AD [Huang, Chen and Gibson, 2005]. The current studies extended that to CCE. The selective effects of oxidants were tested with a higher concentration of CPA. As shown in Figure 1, higher concentrations of CPA caused a larger increase in CCE which provides more opportunity for experimental manipulation. Cells were incubated in the absence of extracellular Ca2+. CGP37157 was added 1 min prior to addition of CPA (10 μM). Oxidants (t-BHP or SIN-1) were then added, and finally Ca2+ was added to activate CCE. The mitochondrial Ca2+ exporter inhibitor CGP37157 enhanced CPA-induced release Ca2+ from ER (+129 % of CPA alone) and t-BHP further enhanced this Ca2+ release from ER by 209 % of CPA treatment but SIN-1 did not alter the reduction by CGP37157 treatment (Fig 5). However, activation of CCE after CPA treatment was reduced by blockade of mitochondria Ca2+ exporter by CGP37157 (−60%) (Figs 2, 5). This inhibitory effect of CGP was reversed by t-BHP (−22%) but not by SIN-1 (Fig 5).
Figure 5. t-BHP but not SIN-1 attenuated the impairment of CCE by CGP37157.
Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. The CPA (10 μM) was added 1 min after CGP37157 (40 μM), t-BHP (100 μM) or SIN-1 (500 μM) was added 3 min later. After an additional 2 min CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 21–29 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 5 min interval after CPA (10 μM) or 3 min after calcium addition (495–720 sec). Data are means ± SEM (n=21–29 cells). Different letters indicate values vary significantly (p<0.05) from the other groups by ANOVA followed by Student Newman Keul’s test.
Blockade of mitochondrial Ca2+ uptake reduced CPA-induced CCE
Fibroblasts were incubated in the absence of extracellular Ca2+. SIN-1 was added prior to addition of the mitochondrial Ca2+ uptake inhibitor Ru360, then CPA was added, and finally Ca2+ was added to activate CCE. The mitochondrial Ca2+ uptake inhibitor RU360 reduced activation of CCE by CPA treatment by 38%, whereas SIN-1 abolished this impairment and the CCE was same as the control group (Figs 3, 6).
Figure 6. Inhibition of CPA-induced mitochondrial Ca2+ uptake by RU360 was reversed by SIN-1.
SIN-1 abolished the inhibition of CPA-induced CCE by the mitochondrial Ca2+ uptake inhibitor RU360. Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. RU360 (0, 20 μM) was added 2 min after SIN-1 (500 μM). After an additional 4 min, CPA (2 μM) was added, and 2 min later CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 42–51 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 3 min interval after calcium addition (495–660 sec). Data are means ± SEM (n= 42–51 cells). Different letters indicate values vary significantly (p<0.05) from the other groups by ANOVA followed by Student Newman Keul’s test.
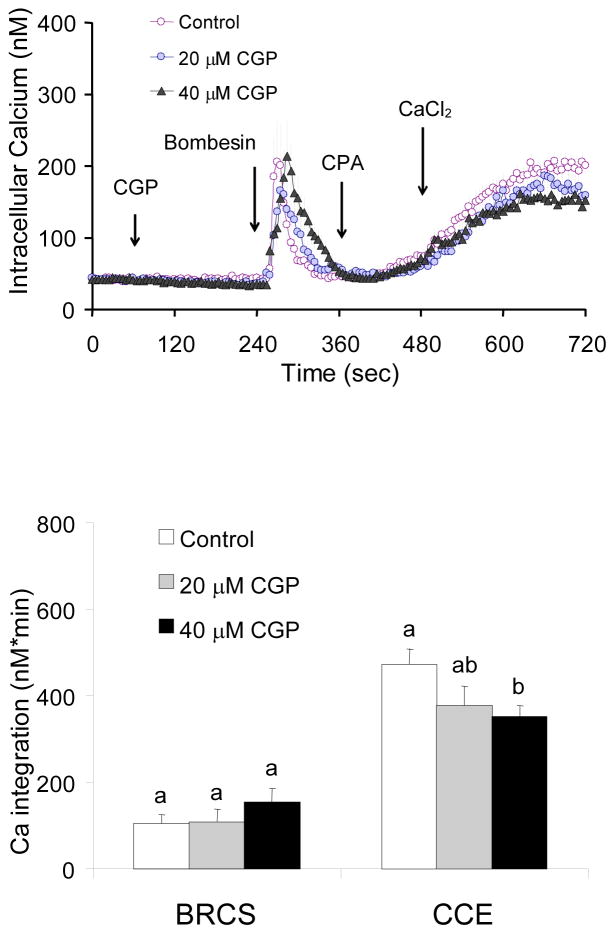
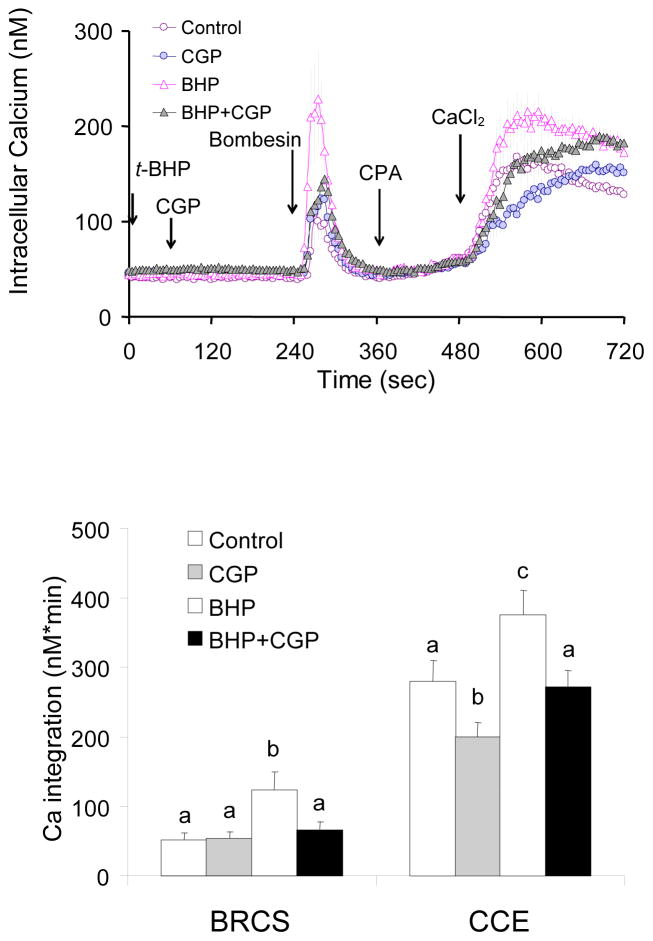
In the presence of InsP3, blockade of the mitochondrial Ca2+ exporter had no effect on BRCS but impaired CCE
Under physiological conditions, CCE is generally induced following depletion of ER calcium stores by agonists that induce InsP3 which binds to the InsP3 receptor to release Ca2+ from the ER. Fibroblasts were incubated in the absence of extracellular Ca2+. CGP37157, inhibitor of the mitochondrial Ca2+ release (Na+/Ca2+ exchanger) was added prior to addition of bombesin, which depletes ER Ca2+. CPA and then Ca2+ were added to activate CCE. CGP37157 (20 or 40 μM) did not alter basal Ca2+ or Ca2+ release by bombesin, but reduced subsequent CCE (20% and 25%) (Fig 7). Thus, the impairment of CCE by CGP37157 was much less in the presence of InsP3 (−20%; Fig 7) than that in the absence of InsP3 agonist (−63%; Fig 2). Thus, the impairment of CCE by a blocker of calcium export from the mitochondria was attenuated by InsP3.
Figure 7. In the presence of InsP3, blockade of mitochondrial Ca2+ release had no effect on BRCS but impaired CCE.
Fibroblasts were loaded with Fura 2AM for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. The CGP37157 (0, 20, 40 μM) was added after 1 min of basal [Ca2+]i measurements, and bombesin (2 μM) was added after 3 min. After an additional 3 min, CPA (4 μM) was added, and 3 min later CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 49–66 cells. The bottom panel shows the integrations of the [Ca2+]i peak over the 2–3 min interval after bombesin (255–480 sec) or calcium addition (495–720 sec). Data are means ± SEM (n = 49–66 cells). Different letters indicate values vary significantly (p<0.05) from the other groups by ANOVA followed by Student Newman Keul’s test.
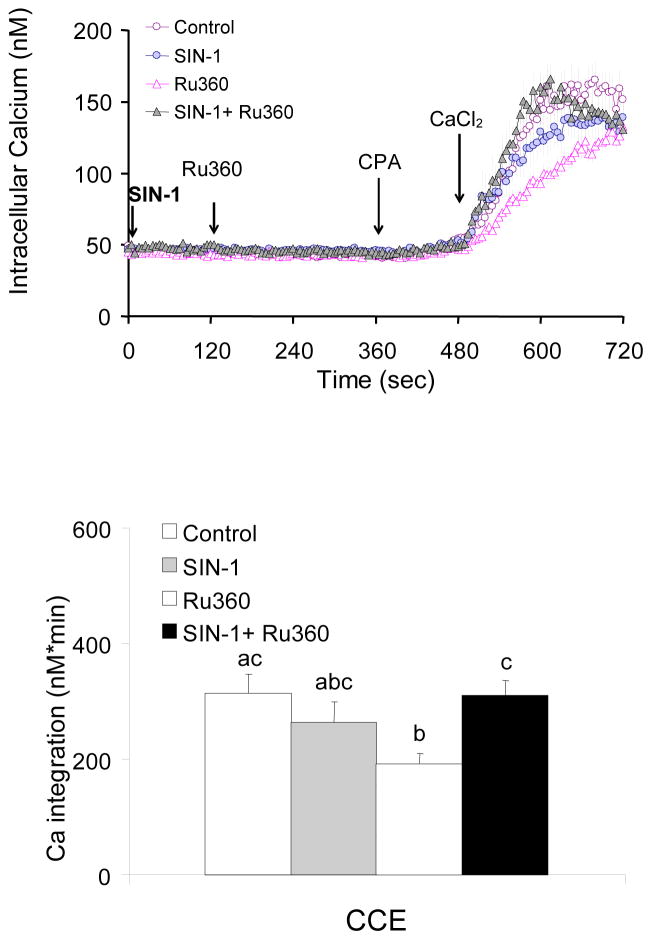
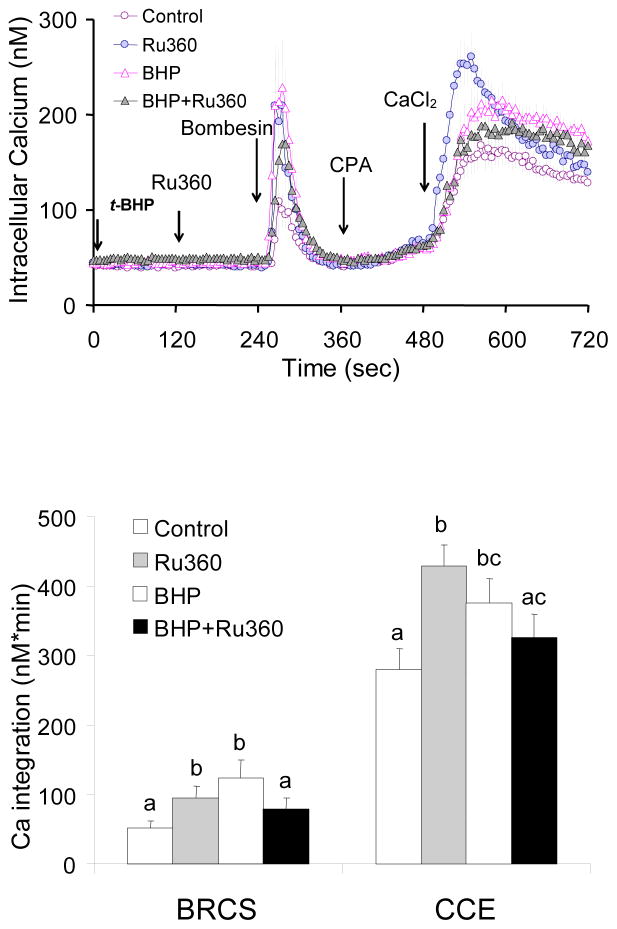
In the presence of InsP3, blockade of mitochondrial Ca2+ uptake exaggerated CCE
Fibroblasts were treated with the mitochondrial Ca2+ uptake inhibitor Ru360 prior to bombesin treatment, and subsequent re-addition Ca2+. Ru360 (10 or 20 μM) did not alter basal calcium (data not shown) but increased Ca2+ release by bombesin (+81%) and exaggerated the subsequent CCE significantly (+53%) (Fig 8). This is in striking contrast with the results in the absence of InsP3 where Ru360 diminished CCE (Fig 3).
Figure 8. In the presence of InsP3, blockade of mitochondrial Ca2+ uptake, exaggerated BRCS and subsequent CCE.
In the presence of InsP3, blockade of mitochondrial Ca2+ uptake by Ru360 exaggerated BRCS and subsequent CCE. Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. Mitochondria Ca2+ uptake blocker Ru360 (0, 10, 20 μM) was added after 1 min basal [Ca2+]i measurements, and bombesin (1 μM) was added after 3 min. After additional 3 min, CPA (2 μM) was added, and 3 min later CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 74–91 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 2–3 min interval after bombesin or calcium addition. Data are means ± SEM (n=74–91 cells). Different letters indicate values vary significantly (p<0.05) from the other groups by ANOVA followed by Student Newman Keul’s test.
In the presence of InsP3, impairment of CCE by blockade of mitochondrial Ca2+ release was attenuated by t-BHP
Fibroblasts were incubated in the absence of extracellular Ca2+ and were then treated with t-BHP one min prior to addition of inhibitor of mitochondria Ca2+ release. Bombesin was added to deplete ER Ca2+. CPA was added and finally Ca2+ was added to activate CCE. The experiments show the effect of t-BHP on the results in Fig 7. CGP37157 did not alter BRCS but diminished subsequent CCE 29% (Fig 9). Under the conditions, t-BHP enhanced BRCS (+139%) which replicates our previous studies [Huang et al., 2005]. t-BHP also exaggerated the CCE (+34%) (Fig 9) and this increase was also blocked by CGP37157 (Fig 9).
Figure 9. In the presence of InsP3, impairment of CCE by mitochondrial Ca2+ exporter inhibitor was attenuated by t-BHP.
Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. CGP37157 (20 μM) was added 1 min after t-BHP (100 μM) treatment, and bombesin (1 μM) was added after 3 min. After an additional 2 min, CPA (2 μM) was added, and 2 min later CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 69–116 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 3 min interval after bombesin or calcium addition. Data are means ± SEM (n=69–116 cells). Different letters indicate values vary significantly (p<0.05) from the other groups by ANOVA followed by Student Newman Keul’s test.
In the presence of InsP3, t-BHP attenuated the exaggeration of BRCS and CCE due to inhibition of mitochondrial Ca2+ uptake
Fibroblasts were incubated in the absence of extracellular Ca2+, and were treated with t-BHP one min prior to addition of inhibition of mitochondrial Ca2+ uptake. Bombesin was then added to deplete ER Ca2+. CPA was added and finally Ca2+ was added to activate CCE. The experiment shows the effects of oxidant on the results in Fig 8. Blockade of mitochondrial Ca2+ uptake exaggerated BRCS and CCE by 82% and 53% of control, respectively (Fig 8, 10). In addition, t-BHP exaggerated BRCS and CCE by 139 % and 34% of control, respectively (Fig 9, 10). Interestingly, in the presence of InsP3, the oxidant t-BHP abolished the exaggeration of BRCS and CCE due to the inhibition of mitochondrial Ca2+ uptake (Fig 10).
Figure 10. In the presence of InsP3, t-BHP diminished exaggeration of BRCS and CCE due to the inhibition of mitochondrial Ca2+ uptake.
Fibroblasts were loaded with Fura 2AM (2 μM) for 60 min. After loading, the media were changed to calcium free BSS, and 3 min later the calcium measurements were initiated. The mitochondrial Ca2+ uptake blocker Ru360 (20 μM) was added 2 min after t-BHP (100 μM), and bombesin (1 μM) was added after 2 min. After additional 2 min, CPA (2 μM) was added, and 2 min later CaCl2 (final concentration of 2.5 mM) was added. The top panel shows the tracings taken from 69–106 cells. The bottom panel shows the integration of the [Ca2+]i peak over the 3 min interval after bombesin or calcium addition. Data are means ± SEM (n = 69–106 cells). Different letters indicate values vary significantly (p<0.05) from the other groups by ANOVA followed by Student Newman Keul’s test.
DISCUSSION
The present studies demonstrated that CPA induced Ca2+ release from ER, activated CCE, and that this process was regulated by mitochondrial Ca2+ release and uptake. Under normal condition, ER Ca2+ ATPase activity balances the passive leak of ER Ca2+ into the cytosol. Inhibition of this Ca2+ pump by CPA causes the release of ER Ca2+ and increases cytosolic Ca2+. The concentration dependent increase in cytosolic calcium after CPA indicates that greater inhibition of the Ca2+ ATPase leads to greater release of ER calcium. The release of ER calcium activates CCE even in the absence of increases in cytosolic free calcium (i.e. at low CPA concentrations) (Fig 1). Under this condition, impairment of the mitochondrial Ca2+ export system (Fig 2) or uptake system (Fig 3) attenuated CCE. The inhibitory effect of CGP37157 on CCE suggests that mitochondrial Ca2+ release has a direct impact on the activation of CCE (Fig 2, Fig 11). The reduction of CCE by Ru360 (−40%) without an alteration in cytosolic calcium suggests that mitochondrial calcium uptake is directly linked to CCE (Fig 3). Thus, mitochondria play an important role in the regulation of the CCE following depletion of the ER Ca2+. The results suggest that Ca2+ release from mitochondria contributes to the activation of CCE either directly or indirectly.
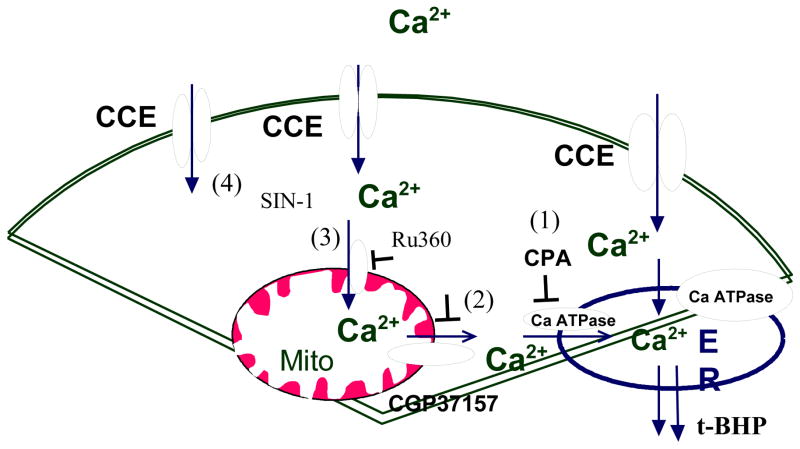
Fig 11. In the absence of InsP3 agonist.
In the absence of InsP3 producing agonist, CCE can be regulated by passive leak of ER Ca2+ (1), mitochondria Ca2+ release -refilling to ER (2), Ca2+ uptake by mitochondria (3) Ca2+ influx directly across plasma membrane (4).
CCE can be regulated at several possible sites (Figure 11. The –number after the figure number refers to the number on the figure): including passive leak of ER Ca2+ (Fig 11-1), mitochondrial Ca2+ release-refilling to ER (Fig 11-2), Ca2+ uptake by mitochondria (Fig 11-3), Ca2+ influx across plasma membrane (Fig 11-4). When the ER Ca-ATPase is inhibited, a reduction of mitochondrial Ca2+ release markedly enhanced cytosolic Ca2+ elevation and reduced subsequent CCE (− 56%). Thus, the results suggest that mitochondrial Ca2+ release serve as ER refilling mechanism (Fig 11-2). In other words, the mitochondria Ca2+ directly fill the ER stores, which is the signal for the CCE. Ru360 impaired the CCE (−40%) without altering cytosolic Ca2+. Thus suggests that mitochondria uptake Ca2+ influx directly affects CCE. Perhaps the mitochondria regulate localized calcium concentrations (Fig 11-3). However, in the absence of CPA, the addition of Ca2+ increased cytosolic free calcium also activated CCE (19.8 % of that with CPA), suggesting that Ca2+ influx is independent of either the mitochondria or the ER (Fig 11-4). Therefore, the results suggest that CCE can be regulated by passive leak of ER Ca2+ (Fig 11-1), mitochondrial Ca2+ release to refill ER calcium (Fig 11-2), Ca2+ uptake by mitochondria (Fig 11-3), Ca2+ influx across the plasma membrane (Fig 11-4).
Selective oxidants affect different sites of regulation of CCE. The present studies showed that t-BHP had no effect on CCE (Fig 4). These results suggest that the t-BHP must promote calcium entry either by facilitating calcium export from the mitochondria or by facilitating calcium entry into the ER from other sources. This effect is oxidant selective because SIN-1 is ineffective in this regard. These results are in agreement with our previous studies [Huang et al., 2005] which showed that t-BHP but not SIN-1, increased ER calcium stores. On the other hand, SIN-1 did overcome the inhibition of CCE that was caused by inhibition of mitochondrial calcium uptake (Fig 6). Taken together the results suggest that CCE regulated by mitochondrial Ca2+ release-refilling to ER (Fig 11-2) is sensitive to t-BHP, while CCE regulated by mitochondrial Ca2+ uptake can be modified by the action of SIN-1 (Fig 11-3). CCE regulated by ER Ca2+ passive leak pathway is not sensitive to t-BHP (Fig 4, Fig 11-1). The results suggest that Ca2+ release from mitochondria contributes to the activation of CCE either directly or indirectly and can be modified by select oxidants.
These interactions are modified in the presence of the InsP3 producing agonist bombesin (Figure 12). Impairment of mitochondrial Ca2+ release by CGP37157 impaired CCE (Fig 7), suggesting that mitochondrial Ca2+ play a role to regulate CCE. CGP37157 prevents Ca2+ refilling of the ER during cell stimulation with an InsP3-producing agonist [Malli et al., 2005]. The impairment of CCE by CGP37157 was much less in the presence of InsP3-producing agonist (−25 %) than in its absence (−56 %). In the absence of InsP3 producing agonist, ER Ca2+ was not up taken by mitochondria. However, mitochondria can uptake and release Ca2+ during cell stimulation with InsP3-produdcing agonists, thereby generating subplasmalemmal microdomains of low Ca2+ that sustain activity of CCE [Malli et al., 2005]. Thus, mitochondrial Ca2+ uptake from the local transient Ca2+ release from ER via activation of InsP3 receptor (Fig 12-1) is a major source for refilling ER by mitochondria Na+/Ca2+ exchanger (Fig 12-2). Other studies that directly measured mitochondrial Ca2 show that in the absence of InsP3 agonist, CGP37157 has no effect on mitochondrial Ca2+, while in the presence of InsP3 agonist, CGP37157 leads to a long-elevation of mitochondrial Ca2+ [Malli et al., 2005].
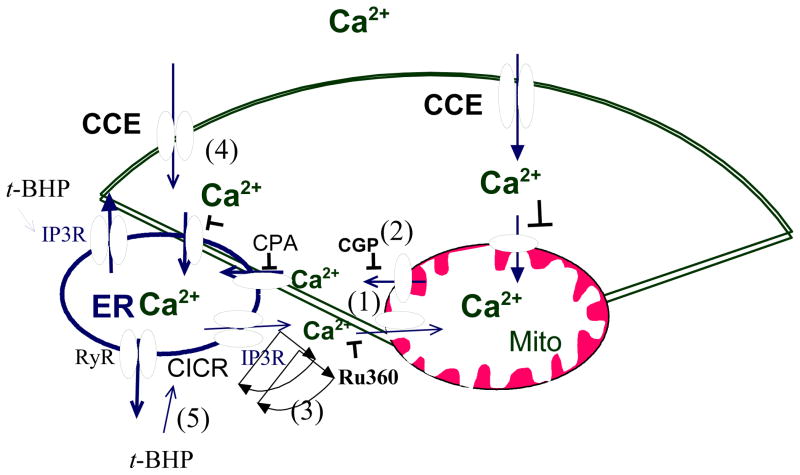
Fig 12. In the presence of InsP3 agonist.
In the presence of InsP3 producing agonist, activation of InsP3R evoked cytosolic Ca2+ release from ER that are transmitted to the mitochondria (1). The mitochondrial Ca2+ refills ER (2). If inhibition of the mitochondrial Ca2+ uptake by Ru360, the transient Ca2+ will facilitate rate of CICR and RyR(3) to empty more of the ER Ca2+ stores and activated more CCE (4).
The results show a cross talk between mitochondrial Ca2+, ER calcium and CCE. Impairment of mitochondrial Ca2+ uptake exaggerated Ca2+ release from ER and enhanced CCE activity following depletion of the ER Ca2+ by stimulation with InsP3 producing agonist (Figs 8, 10,11, 12). The results with Ru360 in the presence (Fig 11) and absence (Fig 12) of InsP3 producing agonist are opposite. Cytosolic Ca2+ signals elicited by activation of InsP3R or RyR are transmitted to the mitochondria [Rizzuto et al., 1998, Rizzuto et al, 1993; Hajnoczky et al., 1995] (Fig 12-1). Thus, calcium moves from the ER to the mitochondria without mixing with the cytosol. Furthermore, mitochondrial Ca2+ transport modulates the spatio-temporal pattern of cytosolic Ca2+ responses evoked by InsP3. As shown in Fig 12, mitochondrial Ca2+ uptake limits the rate of propagation of calcium induced calcium release (CICR) which leads to activation of RyR. Therefore, disabling mitochondrial Ca2+ uptake will increase the rate of CICR which requires Ca2+ released locally by InsP3 acting in the ER Ca2+ stores (Fig 12-3). The greatest emptying of ER Ca2+ occurred with the combination of InsP3R and CICR (Figs 8,10 as indicating by higher BRCS), and this produced higher CCE (Figs 8, 10; Fig 12-4). Thus, mitochondrial Ca2+ uptake is tightly coupled to Ca2+ release following activation of InsP3R-or ryanodine receptor. InsP3 binding protein suppresses the Ca2+ release mediated by the InsP3Rs that provides Ca2+ for mitochondrial Ca2+ uptake [Lin et al., 2005].
In the presence of InsP3, t-BHP reversed the abnormalities caused by the disruptions of uptake or release of mitochondrial Ca2+ (Fig 9, 10, Figure 12), suggesting t-BHP-induced ROS signaling regulated this mitochondrial and ER Ca2+ signals. Oxidant t-BHP exaggerated Ca2+ release from ER and elevated CCE replicated our previous studies [Huang et al., 2005]. t-BHP independently exaggerated the elevation of Ca2+ release from ER and impaired CCE by blocking mitochondrial Ca2+ release. The t-BHP-induced exaggeration in CCE may be due to an oxidant-induced exaggeration in the ability of bombesin to deplete InsP3 sensitive calcium stores (i.e. increased BRCS) and subsequent CICR. The ryanodine receptor channel is a thiols protein, which is S-nitrosylated and results to a progressive channel activation [Xu et al., 1998]. However, oxidation of additional thiols (or of another class of thiols) produces irreversible activation can lead to loss of control [Xu et al., 1998]. The disruption that ER Ca2+ regulation in AD has been suggested to be related to an enhancement of ryanodine receptor [Stutzmann et al., 2006]. In the presence of InsP3, the mitochondria buffer the elevation of Ca2+ by the InsP3 receptor, and refill the ER Ca2+. If the Ca2+ released by InsP3 is not up taken by mitochondria, Ca2+ will facilitate the rate of CICR. In addition, t-BHP facilitated InsP3 and RyR activation and compensated mitochondria-ER refilling process (Fig 12-5).
t-BHP interrupted cross talk between ER and mitochondria Ca2+ stores, which in turn altered CCE. Ca2+ signals between ER and mitochondria occur during conditions that increase InsP3. InsP3 receptors have been identified as an important component of ER-mitochondria interactions [Boehning et al., 2003]. Cytochrome c, after its initial Ca2+ dependent release from the mitochondria translocates to the ER and interacts with InsP3 receptors, which further augment Ca2+ release. The diminished ER Ca2+ augments CCE. During stimulation, mitochondria release cytochrome C and InsP3 promotes release of ER calcium and together they activate apoptosis [Boehning et al., 2003]. Phospholipase C and polyphosphoinositides are required for the activation of the CCE [Broad et al., 2001].
Summary.
An understanding of the interaction of cellular calcium stores with oxidants and InsP3 is necessary to determine how these are altered in AD, and to develop therapies directed at the calcium dysregulation in AD. Mitochondrial Ca2+ plays an important role in the regulation of the CCE following depletion of the ER, and the mechanisms differ in the presence and absence of InsP3 producing agonist. Inhibition of mitochondrial Ca2+ uptake impaired the CCE in the absence of InsP3 producing agonist, whereas it enhanced CCE activity in the presence of InsP3 producing agonist. The results suggest that in the presence of InsP3, mitochondria buffer the local Ca2+ released from ER following rapid InsP3Ca2+ induced release, and serve as a negative feedback to the CCE. In the presence of InsP3, blocking mitochondrial calcium release and uptake impaired and exaggerated CCE, respectively and these abnormalities could be reversed by t-BHP. On the other hand, impaired CCE by inhibition of mitochondrial Ca2+ uptake in the absence of InsP3 could be diminished by SIN-1. Thus, selective oxidants regulated the interactions of mitochondria with CCE whether or not the InsP3 producing agonist is present. Now that these interactions have been defined in a control line, the changes with AD can be delineated and strategies to ameliorate the abnormalities can be developed..
Acknowledgments
The work was supported by AG14930, AG14600, AG19589 and Burke Medical Research Institute.
Abbreviations
- BRCS
Bombesin-releasable calcium store
- BSS
balanced salt solution
- CCE
capacitative calcium entry
- CGP37157
7-chloro-3,5-dihydro-5-phenyl-1H-4,1-benzothiazepine-2
- CPA
cyclopiazonic acid
- [Ca2+]i
cytosolic free calcium concentration
- DMEM
Dulbecco’s modified Eagle’s medium
- ER
endoplasmic reticulum
- Fura-2AM
fura-2-acetoxymethyl ester
- PBS
phosphate-buffered saline
- Ru360
ruthenium amino binuclear complex
- SIN-1
3-morpholinosyndnonimine
- t-BHP
tert-butyl-hydroxyperoxide
Reference List
- Area-Gomez E, de Groof AJC, Boldogh I, Bird TD, Gibson GE, Koehler CM, Yu WH, Duff KE, Yaffe MP, Pon LA, Schon EA. Presenilins Are Enriched in Endoplasmic Reticulum Membranes Associated with Mitochondria. Amer J Path. 2009;175:1810–1816. doi: 10.2353/ajpath.2009.090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau A, Butterworth RF, Ngo T, Breton G, Melançon S, Shapcott D, Geoffroy G, Lemieux B. Pyruvate metabolism in Friedreich’s ataxia. The Canadian J Neurol Sci Le J Canadien des Sci Neurologiques. 1976;3:379–388. doi: 10.1017/s0317167100025634. [DOI] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–61. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Broad LM, Braun FJ, Lievremont JP, Bird GS, Kurosaki T, Putney JW., Jr Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem. 2001;276:15945–52. doi: 10.1074/jbc.M011571200. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Chen HL, Xu H, Qiu L, Xu Z, Denton TT, Shi Q. Deficits in the mitochondrial enzyme α-ketoglutarate dehydrogenase lead to Alzheimer’s disease-like calcium dysregulation. NeurobiolAging. 2012;33:1121.e1113–1121.e1124. doi: 10.1016/j.neurobiolaging.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Hirsch JA, Cirio RT, Jordan BD, Fonzetti P, Elder J. Abnormal thiamine-dependent processes in Alzheimer’s Disease. Lessons from diabetes. Molec Cell Neurosci. 2013;55:17–25. doi: 10.1016/j.mcn.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002;21:6744–54. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–24. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Hidalgo C, Bull R, Marengo JJ, Perez CF, Donoso P. SH oxidation stimulates calcium release channels (ryanodine receptors) from excitable cells. Biol Res. 2000;33:113–24. doi: 10.4067/s0716-97602000000200011. [DOI] [PubMed] [Google Scholar]
- Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–48. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HM, Chen HL, Gibson GE. Thiamine and Oxidants Interact to Modify Cellular Calcium Stores. Neurochem Res. 2010;35:2107–2116. doi: 10.1007/s11064-010-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HM, Chen HL, Xu H, Gibson GE. Modification of endoplasmic reticulum Ca2+ stores by select oxidants produces changes reminiscent of those in cells from patients with Alzheimer disease. Free Rad Biol Med. 2005;39:979–89. doi: 10.1016/j.freeradbiomed.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Huang HM, Zhang H, Ou HC, Chen HL, Gibson GE. alpha-Keto-β-Methyl-n-valeric acid diminishes reactive oxygen species and alters endoplamic reticulumn Ca(2+) stores. Free Rad Biol Med. 2004;37:1779–89. doi: 10.1016/j.freeradbiomed.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ito E, Oka K, Etcheberrigaray R, Nelson TJ, McPhie DL, Tofel-Grehl B, Gibson GE, Alkon DL. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci (USA) 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Tepikin AV, Erdemli G. Role of mitochondria in Ca(2+) homeostasis of mouse pancreatic acinar cells. Cell Calcium. 2002;32:59–69. doi: 10.1016/s0143-4160(02)00091-x. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Xu H, Shi Q, Chen LH, Pedrini S, Pechman D, Baker H, Beal MF, Gandy SE, Gibson GE. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Neurobiology Aging. 2009;(30):1587–1600. doi: 10.1016/j.neurobiolaging.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I, Ogata E. Na-Ca exchanger as a calcium influx pathway in adrenal glomerulosa cells. Biochem Biophys Res Commun. 1989;158:1005–12. doi: 10.1016/0006-291x(89)92822-2. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, LaFerla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. Journal of Cell Biology. 2000;149(4):793–8. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Varnai P, Csordas G, Balla A, Nagai T, Miyawaki A, Balla T, Hajnoczky G. Control of calcium signal propagation to the mitochondria by inositol 1,4,5-trisphosphate-binding proteins. J Biol Chem. 2005;280:12820–32. doi: 10.1074/jbc.M411591200. [DOI] [PubMed] [Google Scholar]
- Malli R, Frieden M, Osibow K, Zoratti C, Mayer M, Demaurex N, Graier WF. Sustained Ca2+ transfer across mitochondria is Essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem. 2003;278:44769–79. doi: 10.1074/jbc.M302511200. [DOI] [PubMed] [Google Scholar]
- Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem. 2005;280:12114–22. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- Meador K, Loring D, Nichols M, Zamrini E, Rivner M, Posas H, Thompson E, Moore E. Preliminary findings of high-dose thiamine in dementia of Alzheimer’s type. J Geriatr Psychiatry Neurol. 1993;6:222–9. doi: 10.1177/089198879300600408. [DOI] [PubMed] [Google Scholar]
- Nelson O, Supnet C, Liu H, Bezprozvanny I. Familial Alzheimer’s Disease Mutations in Presenilins: Effects on Endoplasmic Reticulum Calcium Homeostasis and Correlation with Clinical Phenotypes. J Alzheimer’s Dis. 2010;21:781–793. doi: 10.3233/JAD-2010-100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, Sun X, Zhao L, Yu M, Xu Z, Dong W, Qin Y, Fei G, Zhong C, Xu T-L. Powerful beneficial effects of benfotiamine on cognitive impairment and β-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010;133:1342–1351. doi: 10.1093/brain/awq069. [DOI] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Putney JW, Jr, Ribeiro CM. Signaling pathways between the plasma membrane and endoplasmic reticulum calcium stores. Cell Mol Life Sci. 2000;57:1272–86. doi: 10.1007/PL00000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–7. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–9. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–6. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Seguchi H, Ritter M, Shizukuishi M, Ishida H, Chokoh G, Nakazawa H, Spitzer KW, Barry WH. Propagation of Ca2+ release in cardiac myocytes: role of mitochondria. Cell Calcium. 2005;38:1–9. doi: 10.1016/j.ceca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Simpson PB, Mehotra S, Lange GD, Russell JT. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J Biol Chem. 1997;272:22654–61. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, Oddo S, LaFerla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged alzheimer’s disease mice. J Neurosciences. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–7. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, Saunders AJ, Ding K, Frosch MP, Tanzi RE, Kim TW. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27:561–72. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]