Abstract
Interest in osteochondral repair has been increasing with the growing number of sports-related injuries, accident traumas, and congenital diseases and disorders. Although therapeutic interventions are entering an advanced stage, current surgical procedures are still in their infancy. Unlike other tissues, the osteochondral zone shows a high level of gradient and interfacial tissue organization between bone and cartilage, and thus has unique characteristics related to the ability to resist mechanical compression and restoration. Among the possible therapies, tissue engineering of osteochondral tissues has shown considerable promise where multiple approaches of utilizing cells, scaffolds, and signaling molecules have been pursued. This review focuses particularly on the importance of scaffold design and its role in the success of osteochondral tissue engineering. Biphasic and gradient composition with proper pore configurations are the basic design consideration for scaffolds. Surface modification is an essential technique to improve the scaffold function associated with cell regulation or delivery of signaling molecules. The use of functional scaffolds with a controllable delivery strategy of multiple signaling molecules is also considered a promising therapeutic approach. In this review, we updated the recent advances in scaffolding approaches for osteochondral tissue engineering.
Keywords: Osteochondral repair, interfacial tissue, tissue engineering, scaffold design, therapeutic functions
Introduction
Osteochondral tissue is hard to regenerate after injuries or degenerative diseases. So far, clinical treatments such as chondral shaving, abrasion arthroplasty, subchondral drilling, microfracturing, mosaicplasty, and prosthetic joint replacement have been available for the patients suffering from osteochondral pain.1,2 However, these treatments are still challenging due to their own disadvantages, such as unsuitable donor tissue availability, donor site morbidity, implant loss, and limited durability of prosthetics.3–5
Alternatively, tissue engineering approaches of using various types of bioavailable scaffolds, proper cell sources, and/or bioactive signaling molecules have recently emerged to substitute and replace the patient-painful treatments. Among the tissue engineering components, scaffolds play significant roles in providing three-dimensional (3D) environments for cells to populate on and to differentiate into proper lineages.6,7 These tissue-engineering scaffolds can be readily supplied and easily prepared without the need to consider immune/disease problem, costs, and availability. Above all, the design and properties of scaffolds should be importantly considered in order to induce satisfactory cell functions and to maximize therapeutic roles of bioactive molecules which are involved in the osteochondral repair processes.
In this topical review, we update strategies of osteochondral repair and regeneration, with particular emphasis on the recent designs and technologies in the development of scaffolds. For this, we begin with a brief explanation of the osteochondral tissue hierarchy and repair process, sketch the cells and signaling factors involved in, and focus on the functional design and modification of scaffolds for ideal tissue-engineered constructs.
Osteochondral tissue and repair
The osteochondral interfacial tissue extended from the superficial cartilage to the underlying subchondral bone is composed of stratified zones. Figure 1 illustrates the structural hierarchy of osteochondral tissues. Each zone of interfacial tissue is divided into four distinct cartilage zones (i.e. superficial, middle, deep, and calcified cartilage), and the subchondral zone has different components defined by a unique composition and organization of cells and extracellular matrix (ECM). The superficial articular cartilage, referring to the hyaline cartilage, is the smooth, shock-absorbent tissue that forms a layer of approximately 3–4 mm thick on the articular surface. The articular cartilage represents approximately 60–80 wt% of fluids, and the remainder is composed of type II collagen and glycosaminoglycans (GAGs).8 The first of cartilage zones is the superficial or tangential zone occupying the upper 10%–20% of the articular cartilage, and has a small amount of proteoglycans and low permeability.9 Specialized proteins that facilitate the frictional characteristics of the tissue are secreted by the cells that reside in the superficial zone.10 The next middle zone occupying the following 40%–60% down is rich in proteoglycans but has a low number of cells. In addition, the arch-shaped middle zone has oriented collagen fibers, and is highly compressive, allowing recovery from articular surface impacts.11 The deep zone has oriented collagen fibrils and cells perpendicular to the surface of the articular cartilage, and the fibrils are anchored on to the subchondral bone.9 The deep zone is also highly compressive as with the middle zone, but has fewer proteoglycans and the least cells.12 The osteochondral interface is structurally connected between a hyaline cartilage layer and an underlying bone plate,13 and plays a critical role in maintaining the cartilage integration. The osteochondral interface contains hypertrophic chondrocytes embedded in a mineralized cartilage matrix.14 Subchondral bone tissue comprises water, type I collagen, and hydroxyapatite (HA) crystals.
Figure 1.
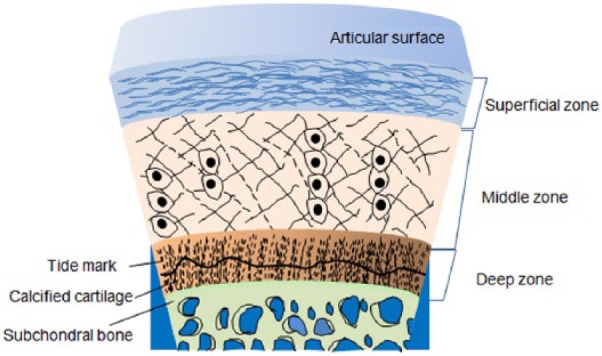
Cross section of osteochondral tissue.
These different yet stratified distributions of physical and chemical traits account for the unique mechanical and biological functions of the osteochondral complex tissues. In fact, the osteochondral repair process relies on progenitor cells or stem cells from the bone marrow to regenerate cartilage and bone tissue by allowing cell recruitment into the defect region.15 While an osteochondral injury that crosses vascular subchondral bones is spontaneously repaired by cell invasion into the lesion, the full-thickness defect repair of the osteochondral interface is transient because the articular cartilage contains few blood vessels and chondrocytes. According to the injury depth, osteochondral lesions are classified into partial-thickness defect, full-thickness defect, and osteochondral defect. Owing to their poor self-repair capacity, most severe osteochondral lesions lead to the insurgence of severe pain, joint deformity, and lack of joint motion.
Therefore, appropriate surgical treatments and technological developments such as grafting and tissue engineering are in high demand. Currently, common approaches for the treatment of osteochondral injury mostly rely on surgical techniques, as summarized in Table 1. As an invasive technique, patellar resurfacing currently used in total knee replacements showed anterior knee pain and cartilage wear.26 Similarly, microfracture or subchondral drilling stimulates the underlying bone marrow while contacting with the injured zone, and forms clots and fibrocartilage that contains a high quantity of type I collagen.27 These invasive techniques cause severe donor site morbidity and the pain thereby returns. In order to circumvent these shortcomings, transplantation of artificial or natural tissue grafts was introduced into osteochondral injured sites.28 Osteochondral tissue grafts used in mosaicplasty have been used to treat large osteochondral injured sites. One study showed that the mosaicplasty efficiency increased with the use of hybrid fixation such as metal screws.29 Several clinical and animal studies have shown that, while the mid-term outcome of mosaicplasty was acceptable, reliability in the long-term outcome is still questionable.30
Table 1.
Current surgical techniques for osteochondral repair.
| Surgical procedure | Invasive degree | Features | Reference |
|---|---|---|---|
| Mosaic-type osteochondral autologous transplantation (OAT) and microfracture (MF) | HIGH | Patient clinical improvement in OTA and MF Superior articular cartilage repair in OTA to MF |
Gudas et al.16 |
| MF-combined osteochondral paste | MODERATE | Poor Safranin-O and type II collagen staining in MF group High GAG content and the DNA-normalized GAG High expression of type II collagen and aggrecan |
Xing et al.17 |
| MF with hole geometry | HIGH | No significant effect in osteochondral repair | Kok et al.18 |
| Mix-mosaicplasty | VERY HIGH | Fibrocartilage-like tissue Small chondrocyte-like cells Full-thickness defect regeneration |
Leng et al.19 |
| Mosaicplasty grafting | MODERATE | Improved mean pain score Alternative to MF Repair deterioration from 12 months post-operation |
Solheim et al.20 |
| ACI | HIGH | Hypertrophy of transplant Insufficient regenerative cartilage and delamination |
Niemeyer et al.21 |
| MODERATE | Effective in younger patients Suitable for meniscus injuries Cruciate ligament and malalignment of cartilage |
Ossendorf et al.22 |
|
| MACI | MODERATE | Limited effective over 24 months Alternative to MF |
Basad et al.23 |
| Arthroscopic autogeneous osteochondral mosaicplasty | HIGH | Multiple surgery procedure Fibrocartilage formation near donor site |
Hangody et al.24 |
| Total joint replacement | VERY HIGH | Metal toxicity and sensitivity Bone necrosis and changes in vascular supply |
Evans et al.25 |
ACI: autologous chondrocyte implantation; MACI: matrix-induced autologous chondrocyte implantation; GAG: glycosaminoglycan.
Alternatively, cell-based treatments of osteochondral injury have emerged to overcome the aforementioned limitations. For example, autologous chondrocyte implantation (ACI) and matrix-induced ACI (MACI) are routinely used for cartilage repair in clinical applications. While ACI is a quick and simple surgery with a high success rate, it is limited by instability or loss of chondrocytes under in vitro culture. In fact, autologous chondrocytes have often been shown to lose cartilaginous phenotype during the in vitro cell expansion after their isolation from cartilage tissue,31 even though the sufficient number of cells is obtained from the individual harvested site. To address this challenge, an extra step of cartilage tissue fragmentation was introduced to the existing ACI method. Their results showed stable cell migration and growth, and indicated that fragmented cartilage tissue acted as a scaffold equal to a native ECM for chondrocyte redistribution.32 MACI is a next generation product that can also overcome the limitation of ACI, and has the potential to become an established method. The matrix used for the chondrocytes was type I/III collagen scaffold and fibrin glue was introduced to secure the lesion. Clinical results have been revealed on a large and deep osteochondral implantation via minimally invasive methods without noticeable graft-related inflammation.33 However, these techniques often resulted in donor site morbidity over long periods after treatment.34 Therefore, tissue-engineering approaches are in demand as a promising technological advance for osteochondral repair without risks that induce donor site morbidity, host immune responses, and disease transmission.
As the key components comprising tissue engineering, cells, signaling molecules, and scaffolds have shown great advances in terms of their basic knowledge and their biological interactions with each other. We briefly overview the cell sources and signaling molecules in the following section, and then focus further on the scaffolds with their recent technological developments and on the combinatorial designs with cells and signaling molecules; this is considered to be a promising approach to engineering functional tissues and mimicking tissue-equivalents.
Cell sources for osteochondral repair
Cells migrate into defect areas and play an important role in repair processes by secreting ECM proteins, resulting in new tissue formation. However, full-thickness defects require surgical procedures and tissue grafts. Along with scaffolds, the source of cells beneficial to osteochondral tissue can provide a potential for osteochondral repair. Therefore, the selection of an ideal cell source for osteochondral repair is significantly important to improve repair efficiencies. A desired cell source must have no limitations in the amounts available and be easy to maintain in vitro in terms of cell manipulation. In addition, cells without risks such as host immune responses and disease transmission must be used in tissue engineering. As listed in Table 2, two common cell resources, including stem cells and tissue-specific cells, are currently used for osteochondral tissue engineering. Various stem cells have recently gained considerable attention and are an attractive resource to meet the criteria mentioned above. Of such stem cells, adult mesenchymal stem cells (MSCs) are the most widely studied in the field of osteochondral tissue engineering and could be appropriate due to their availability and high capacity for proliferation and differentiation. Despite their promise, undifferentiated cells have often shown limited/unsatisfactory capacity for osteochondral repair due to the multi-potency and difficulty in cellular fate control. Thus, pre-differentiated cell lineages have been used. Along with MSCs, pluripotent stem cells (PSCs), particularly those induced from adult/patient cells, that is, induced PSCs (iPSCs), have also been considered as a potential therapeutic cellular tool for cartilage regeneration although they are still in emerging state for clinical availability and relatively less documented. Ko et al.41 have recently reported the implantation of chondrocyte-based human iPSCs into cartilage defects with improved cartilage repair capacity. However, more studies on iPSCs for osteochondral repair are considered to follow in the near future. Meanwhile, tissue-specific cells, such as osteoblasts and chondrocytes, have also been introduced as promising cell resources for bone and cartilage tissue regeneration in osteochondral repair, respectively. Nonetheless, without the use of proper osteochondral inductive factors, the synthesis of ECMs representing each specific tissue has been difficult to achieve, and requires appropriate extra therapeutic systems.
Table 2.
Cell resources currently used in osteochondral tissue engineering.
| Cell resource | Cell type | Features | Reference |
|---|---|---|---|
| Stem cells | Umbilical cord MSCs | Facilitate scaffold and tissue integration. | Wang et al.35 |
| Amniotic fluid–derived stem cells |
Differentiation into osteoblasts and chondrocytes encapsulated in 2% of alginate Non-requirement for osteochondral medium and production of protein-rich ECM |
Rodrigues et al.36 | |
| Synovium-derived MSCs |
Characteristics of MSCs with an ability to differentiate into osteoblasts and chondrocytes. Integration into the surrounding cartilage |
Koga et al.37 | |
| Bone marrow-derived MSCs |
Development toward both chondro- or osteo-lineages without fibrous tissue formation Complete tissue integration and low host immune response |
Betsch et al.38 | |
| Tissue-specific cells | Chondrocytes |
Complete mechanical stability High tissue integration into the surrounding tissue Clinically and histologically stable |
Horas et al.39 |
| Osteoblasts | High SPP1 mRNA expression Up-regulation of RUNX2 |
Innes et al.40 | |
MSCs: mesenchymal stem cells; ECM: extracellular matrix; RUNX2: Runt-related transcription factor 2.
Osteochondral scaffolds and functional designs
Scaffolds are a fundamental factor in cell-based tissue engineering. For the success of osteochondral tissue engineering, the primary requirements of scaffolding materials include biocompatibility, biodegradability, mechanical stability, and pore structure. Furthermore, the types of scaffolds, such as hydrogel, porous foam, and fibrous network, determine the physical and biological properties of the scaffold itself as well as the way how cells and signaling molecules are utilized. Scaffolds enable cells to adhere, migrate, grow, and differentiate into chondrogenesis and osteogenesis, consequently helping the formation of new osteochondral tissue. The following section discusses the considerations required for the successful fabrication of osteochondral scaffolds.
Choice of biocompatible and degradable materials
It is well known that scaffolds must be fabricated from biocompatible materials which do not elicit immune responses or foreign body reactions. In addition, the biodegradation of scaffolds during in vivo treatment should closely match tissue growth rates. The selection of proper materials to meet these requirements is thus the first consideration, and the compositions currently used for osteochondral scaffolds range from natural and synthetic polymers, metallic materials, or inorganic materials, to their composites.
Natural or synthetic polymers have excellent flexibility to adapt their shape to wanted forms via various molding and casting techniques. Beneficial molecules for cells are rich in natural biopolymers, such as GAGs, collagen, and GAG-like polysaccharides, and thus, these natural biopolymers have been easily introduced into synthetic biopolymers, resulting in improving the biological affinity of the scaffold to the host tissue. For example, gelatin-conjugated calcium-alginate scaffolds have improved cell adhesion and proliferation, and showed differentiation of MSCs into chondrogenic and osteogenic cell lineages.42 While the cell compatibility and bioactivity of natural polymers are generally superior to those of synthetic polymer, they have relatively weak mechanical properties and the degradation rate of them is not easy to control. On the other hand, biodegradable synthetic polymers, including aliphatic polyesters have been applied to osteochondral scaffolds with a broad spectrum of chemistries and processes to control their intrinsic characteristics such as the degradation rates. Depending on the synthesis conditions the mechanical properties can also be easily tunable. However, the poor surface activity and cell affinity have generally been the weakness of the synthetic polymers. To improve this, blending or copolymerization approach between different polymers has been made. For example, polyvinyl alcohol (PVA) was introduced into polycaprolactone (PCL) nanofibrous scaffolds to improve the biocompatibility and tensile properties of the scaffold. As a consequence, enhanced proliferation and chondrogenic differentiation of MSCs were shown in comparison with pure PCL scaffolds.43
However, typical polymeric scaffolds are still insufficient to sustain mechanical stress originating from the joint movement or biodegradation and further from the subchondral bone region. Therefore, these polymeric scaffolds have been hybridized with inorganic materials to improve their mechanical properties. Examples include metals and ceramics such as bioactive glasses that can participate in the bone part of the osteochondral scaffold. As a bone part, a small content of metallic materials such as titanium, stainless steels, cobalt, and their alloys helped to improve the capability of sustaining mechanical loads and ability to bond to weaker or softer scaffold materials for the cartilage area. For example, a biphasic construct, titanium-bound polyethylene glycol (PEG) hydrogel, was produced for cartilage and bone growth and integration in adjacent tissues.44 However, these metallic materials in tissue engineering show a lack of biodegradation over time and may cause tissue abrasion or corrosion.45 Thus, care must be taken when metallic materials are used for osteochondral scaffold design.
Bioactive ceramics such as calcium phosphates or bioactive glass are known to be biocompatible and even biodegradable, and have been primarily used as the bone part of osteochondral scaffolds. Particularly, their role in improving biomineralization is a promising aspect for bone formation. However, owing to their stiff nature possibly leading to brittleness, composites with biodegradable polymers have been popularly approached. For example, the stratified scaffold composed of agarose hydrogel and a composite polylactide-co-glycolide (PLGA)/bioactive glass improved mineralization by virtue of bioactive glass and resulted in integrative osteochondral repair.46 Accordingly, while the bioactive ceramics themselves are brittle, they can provide a favorable stiffness level for bone formation when incorporated within a polymeric phase.
Consideration of mechanical properties
Further understanding of mechanical properties of osteochondral tissue can contribute to the effective design of osteochondral scaffolds. Osteochondral interfacial tissue has different mechanical strengths depending on the property at each stratified layer. Mismatched viscoelastic properties of osteochondral tissue lead to stress disparities between cartilage tissues. Superficial cartilage can withstand a local compressive stress of 0.08– 2 MPa, tensile modulus of 5–25 MPa, and equilibrium shear modulus of 0.05–0.25 MPa.47 These differences arise from the biological and chemical composition and thereby from mechanical strengths in each zone. In order to optimize resistance in osteochondral tissue, superficial collagen exists parallel to the shear direction, while collagen in the deep zone is perpendicular to the surface. Owing to this highly organized structure and its properties, artificial recreation of this tissue is still challenging. Intensive progress on remodeling cartilage has been made using transforming growth factor-β1 (TGF-β1) and mechanical stimulation to improve its tensile modulus up to more than 3.4 MPa,48,49 which, however is, much lower than the native tissue tensile values mentioned above. Recent studies emphasized the mechanical properties of osteochondral tissue by arraying and cross-linking collagen organization.
Furthermore, osteochondral tissue operates as a frictionless bearing while transferring loads to the bone to prevent local stress. Although the phenomena of the onset of osteochondral degeneration and its progress have already been studied extensively, the exact mechanisms on the mechanical environments of osteochondral tissue are yet to be elucidated. This is an essential issue in the fabrication of scaffolds with a low-friction surface that could integrate with surrounding tissue and maintain the mechanical stabilities of the implant. The lubricated property of cartilage arises from the complex combination of squeeze film lubrication, elasto-hydrodynamic lubrication, boundary lubrication, interstitial fluid pressurization, and a migrating contact area.47 Without the friction (coefficient ~0.005) property of the cartilage, contact shear would result in considerable abrasion.50 Shi and Xiong51 developed low-friction cartilage scaffolds (coefficient values ~0.05) composed of PVA/polyvinyl pyrrolidone (PVP) hydrogels that have biocompatibility and excellent weight-bearing properties. Despite progressive challenges, osteochondral scaffolds that meet mechanical properties of the native cartilage tissue mentioned have not yet been reported.
Design of pore structure
In most scaffolds, the pore structures affect the cell responses and their further organization in the tissue, regulating cell invasion, vascularization, and tissue regeneration. While the hydrogel type characterizes water-filled channels and polymer networks, the space-open channels are the general feature of scaffolds. The porous structure relies primarily on the fabrication process. Conventional methods for these pore-structured scaffolds include fiber bonding, solvent casting/particulate leaching, gas foaming, and phase separation.52 The porous scaffolds processed using these techniques have controlled pore size and porosity suitable for tissue engineering. For example, the interconnected pore structure can meet the demand of the cell behaviors on the scaffold. Particularly, the region participating in the bone part of the osteochondral scaffolds should mimic bone morphology, structure, and function to optimize integration into the adjacent tissue. Native bone creates a porous environment with 50%–90% porosity,53 and its pore sizes are typically measured in the order of 1 mm in diameter.54 In practice, however, in several studies on the effects of pore size, the scaffold structure composed of porosity higher than 50% and pores larger than 300 µm is recommended to achieve direct osteogenesis with enhanced vascularization.55 On the contrary, small pores for the scaffold have been suggested for favorable chondrogenesis on 90–120 µm pores, where MSCs proliferate and form cartilage tissue on the scaffold.56
Fibrous scaffolds, particularly with nanofibrous morphology, have recently shown considerable promise for the repair of tissues, such as osteochondral tissue. This nanofiber morphology can be easily achieved by an electrospinning technique.57 A polymer solution is ejected through a needle into fibers under a strong electric field. The nanofibrous network facilitates cell adhesion and guides growth and tissue-specific differentiation.58 In addition, nanofibrous scaffolds can be easily modified by virtue of a tunable process, which enables gradient composition and tuning of fiber and pore geometry. When the nanofiber structure was combined with a microfiber scaffold, the cell seeding efficiency and 3D networking were improved. MSC differentiation was enhanced more on the microfibrous large-pored scaffold than that achieved on the nanofibrous micro-pored scaffold.59 Furthermore, an example of alternating bilayers composed of PCL microfibers and PCL nanofibers showed the promotion of osteogenesis and osteochondral ossification, respectively.60 Here, excellent MSC attachment was shown on nanofibers, but cell infiltration through nanofibers was still limited. However, these studies were performed in very small pore sizes of less than 30 µm, and thus were not appropriate for evaluating osteochondral tissue regeneration in terms of the pore size. A number of studies have been carried out on the optimal pore structures of electrospun osteochondral scaffolds by utilizing the inherent advantages of fiber types. Zhang et al.61 reported the advantageous effect of a biphasic scaffold composed of collagen and electrospun polylactic acid (PLA) nanofibers with pore sizes of 50 ~ 300 µm. Despite the prominent progress, the conventional electrospun fibrous scaffold with pores smaller than 300 µm may still hamper osteogenesis. Therefore, specific processing to make pores larger than the 300 µm of fibrous biphasic scaffolds is required for osteogenesis in osteochondral repair. For example, in order to produce a larger pore size, laser processing with femtosecond pulses was attempted and resulted in improvement of the pore size by up to 500 µm.62 In another case of electrospun fibrous scaffolds, a combination with a salt leaching method showed an approach that can increase pores, but resulted in reduced mechanical stability.57
Recently, attention has been paid to the rapid prototyping (RP) technique to produce optimal osteochondral scaffolds with highly accurate control over pore geometry by a computer-aided design/computer-aided manufacturing system and a layer-by-layer process. Unlike the conventional approaches previously described, this technique enables easy fabrication into well-defined 3D interconnected pore structures while possessing appropriate mechanical and biochemical properties.63 Hutmacher64 developed a fused deposition modeling (FDM) technique to fabricate highly porous PCL scaffolds for bone tissue engineering. This technique based on computer-guided 3D plotting is used to supply a pre-formed fiber through a heated nozzle and rollers. The scaffold fabricated by FDM has a narrow processing window and complex geometries, and the use of a pressurized syringe instead of a heated nozzle has made it possible to achieve a wide processing capability independently of the molten polymer temperature. As a modified FDM technique, a 3D fiber deposition (3DF) technique developed by Woodfield et al.65 was reported to fabricate highly porous osteochondral scaffolds with interconnected pore channels and an average pore size (415 µm). The 3DF fabrication is processed by molten polymer deposition from a motor-driven syringe on a stationary stage under a constant pressure.66 It was reported that the average interconnecting pore sizes of the scaffold fabricated by a 3DF technique can be increased by up to 1650 µm.67 It is assumed that a scaffold with this pore structure is suitable for the application of osteochondral tissue engineering. Furthermore, Bian et al.68 developed a combined technique of lithography and gel-casting to fabricate biphasic scaffolds comprised of β-tricalcium phosphate and type I collagen. The pore size and porosity in the bone phase of the ceramic biphasic scaffolds were 700–900 µm and 50%–65%, respectively. Moreover, a strong physical interlock between the cartilage phase and bone phase produced an appropriate binding force to recreate a biomimetic transitional structure.
Hydrogel scaffolds
Hydrogels comprise hydrophilic polymeric networks filled with a large amount of water. Owing to the ability of water uptake and swelling, hydrogels have been considered as the 3D matrices that mimic the 3D gel-like native ECMs, including cartilage. Therefore, for the culture of cells and tissue engineering, hydrogels have been widely employed as scaffolding materials. As with other scaffolds, hydrogel scaffolds should also support structural integrity as well as mechanical robustness to enable appropriate tissue formation.69 In addition, the hydrogel has the ability to safely incorporate drugs and growth factors and then deliver them in a controlled manner. For these reasons, viscoelastic hydrogels, such as type I collagen, gelatin, or GAG components, have been used for the repair of irregular cartilage lesions. However, these hydrogels, composed of a single phase, are definitely inadequate to define complex structures of interface tissue and to sustain osteochondral interface tissue with a wide range of motion because their mechanical stability is too weak.70 Furthermore, although these hydrogels somewhat enable the flexibility of cell migration and transfer of nutrients and oxygen to the cells, it is evident that cell behaviors and responses guided by these physicochemical properties and a single composition are vastly inferior to those shown in the typical porous scaffolds. As a result, cell penetration into each phase and finally cell distribution are restricted within the viscoelastic hydrogels.
Therefore, interest in hydrogel-based biphasic scaffolds to define osteochondral tissue properties has been increasing. Moreover, viscoelastic hydrogels have been used for the controlled release of growth factors that are beneficial for osteochondral repair by forming delivery vehicles such as microspheres. For example, biphasic composite hydrogels comprising PEG and gelatin microspheres to load growth factors were fabricated via a two-step cross-linking procedure.71 Here, gelatin microspheres in the biphasic scaffolds served as enzymatically digestible porogen as well as a delivery vehicle. While excellent release kinetics of growth factors in the scaffold were shown in vitro and in vivo, the mechanical stability of the scaffold with the pore structure attributed to the microspheres used as a porogen was still not satisfactory for osteochondral tissues. Hybridization of inorganic components with hydrogel-based biphasic scaffolds can alleviate the issue of their mechanical stability. Moreover, the gradient inorganic component of a hybrid biphasic scaffold is thought to induce an osteochondral-like transition in the ECM synthesis and cell phenotype. Munoz-Pinto et al.72 reported the fabrication of PEG hydrogels cross-linked to gradient polydimethylsiloxane using a photolithographic technique. As a result, the mechanical properties of the scaffold were enhanced and transdifferentiation of osteoblasts into chondrocyte-like cells was observed with increasing scaffold inorganic content, as indicated by the increased synthesis of ECMs such as chondroitin sulfate and type II collagen and by the upregulated sox9, a chondrocyte differentiation-associated transcription factor. Although a number of attempts to fabricate hydrogel-based biphasic scaffolds were evaluated as “promising,” the interfacial stabilities between the two phases are still questionable due to the feeble shear forces of hydrogels.
Biphasic structure and interfacial integration
Conventional monophasic scaffolds were inadequate to replace defective interfacial cartilage-to-bone tissue that possesses anistropic structural properties and functions. A variety of multiphasic scaffolds for the osteochondral interface have thus been suggested. Multiphasic scaffolds should provide an optimal environment to direct the communications of cell/cell and cell/matrix. Also, the integrated materials can transfer physical or chemical events from the cartilage to the bone layers, because the interface tissue is exposed to shear forces over a large range of motion.
Cell interaction with the scaffold makes possible the interfacial integration between the cartilage and bone phases of the osteochondral scaffold; in fact, cell-laden scaffolds with a gradient of phases showed a greater osteochondral tissue formation after implantation than the acellular scaffolds.73 Therefore, homogeneous or heterogeneous cell composites with osteochondral scaffolds with gradient phases have been developed to improve mechanical and functional integrity. Yunos et al.74 reported interface-integrated biphasic scaffolds, composed of PLA fibers as a cartilage phase and PLA-coated bioactive glass scaffold as a bone phase, by distributing homogeneous single cells within the two phases. Similarly, heterogeneous cell-laden biphasic scaffolds composed of chondrocyte-laden agarose hydrogel and osteoblast-laden microsphere composites with bioactive glasses have also been developed.46 Here, the interaction of cells contained in the biphasic scaffold avoided delamination of each layer, and improved mechanical stability. In another case, the dynamic culture of MSCs on biphasic scaffolds incubated in bioreactors improved the mechanical properties of the scaffold due to the shear stress generated in the bioreactor.75 Moreover, the enhanced cell viability on or in the scaffold cultured in the bioreactor resulted in tissue growth in vivo due to the supply of a homogeneous medium solution and shear stress.76 Figure 2 illustrates how the cells laden onto the biphasic scaffolds contribute to improving mechanical functions and interface integration.
Figure 2.
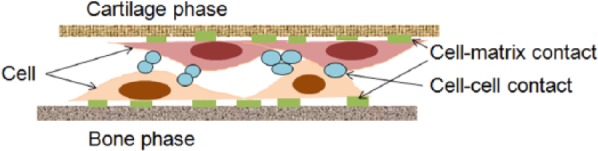
Schematic illustration of cell–cell contact and cell–matrix contact in the cell-laden biphasic scaffold resulting from the strategy used to stabilize the mechanical property and to integrate the interfaces of each phase.
Scaffolds delivering signaling molecules
Interest in signaling molecules and their consequences in the field of tissue engineering has grown with delivery strategies from the scaffold, affecting tissue repair efficiency. Examples of signaling molecules include growth factors and therapeutic drugs or genes. Likewise, osteochondral tissue engineering has utilized these strategies to facilitate cell growth and tissue formation. These strategies have facilitated smart biphasic scaffolds in osteochondral tissue engineering. The inductive conditions using signaling molecules influence cells on the ECM formation of osteochondral tissue, and cells may continuously secrete ECM proteins to ensure a comfortable scaffold environment while residing on the scaffolds. However, there is a subsequent challenge to manage spatiotemporal control of specific signaling molecules within the stratified scaffold. Therefore, along with scaffold design, effective carriers are needed to efficiently synthesize the osteochondral ECM. These considerations must be accompanied by comprehensive knowledge on the unique property of each matrix and of the physicochemical relationship between the carriers and molecules in terms of the efficiency of loading and release. This section explores the recent designs of the elaborate delivery techniques of osteochondral biphasic scaffolds, where critical factors such as spatiotemporal release and dose control are strongly considered. Table 3 summarizes the scaffolding system for osteochondral repair with a delivery potential of therapeutic molecules, such as growth factors, drugs, and genes.
Table 3.
Growth factors currently used in osteochondral tissue engineering.
| Signaling molecule | Carrier | Observations | Reference | |
|---|---|---|---|---|
| Gene | BMP-2 | Porous PLGA |
Increased GAG content in treated groups Low level of type I collagen and increased type II collagen shown in the treated group |
Chen et al.77 |
| Collagen sponge reinforced by PEG |
Increased ALP activity in test groups Stable complex formation with plasmid DNA through electrostatic interaction while showing increased stability |
Hosseinkhani et al.78 |
||
| VEGF/BMP-2 |
Hollow cylindrical PLGA scaffold |
Regeneration proximal and distal ends of the bony defects by co-expression |
Lin et al.79 |
|
| hIGF-I gene–modified BMSCs | 1.2 wt/% calcium-alginate solution | hIGF-I gene effective expression with high subchondral bone and cartilage integration High ECM production and distribution |
Leng et al.80 | |
| Growth factor | TGFβ-1/BMP-2 |
PLGA microspheres, dispersed in an alginate matrix |
High gene expression in the treated groups High quality of cartilage and tissue integration |
Reyes et al.81 |
| TGF-β3/IGF-I |
Gelatin microspheres in oligo-PEG fumarate hydrogel |
Excellent formation of subchondral bone and GAG Negligible effects of TGF-β3 in cartilage morphology and histological scoring |
Yi et al.82 |
|
| BMP-2 |
Hyaluronic hydrogel system |
Healing with fibrocartilage-like tissue formation. Severe ectopic bone formation observed in test group affecting joint functionality |
Aulin et al.83 |
|
BMP: bone morphogenetic protein; GAG: glycosaminoglycan; ALP: alkaline phosphatase; PLGA: polylactide-co-glycolide; VEGF: vascular endothelial growth factor; PEG: polyethylene glycol; BMSCs: bone marrow mesenchymal stem cells; ECM: extracellular matrix; hIGF-I: human insulin-like growth factor I; TGF: transforming growth factor.
In terms of the signaling molecules for osteochondral repair, the currently available growth factors are listed in Table 4. Moreover, chemical drugs or genes have been intensively explored for additive or synergetic effects of osteochondral repair. Here, we briefly summarize the osteochondral effects of various growth factors. Among these, fibroblast growth factors (FGFs) or TGF-β family enhance cartilage ECM formation under the culture condition of chondrogenic cells, while bone morphogenetic proteins (BMPs) induce osteogenesis of cells. Therefore, the use of these growth factors provides osteochondral inductive effects. The construction of osteochondral tissue-like scaffolds has been attempted in various studies using this strategy prior to implantation. MSC-seeded fibrin hydrogel/collagen-type I/III biphasic scaffold incorporating TGF-β improved cartilage ECM synthesis, owing to sustained release of TGF-β up to 7 days, which aided in inducing MSCs’ chondrogenesis.96 While the use of growth factors shows effects on osteochondral repair, gaining more sustainable and controllable release is a key issue, which can be facilitated by the fine design of scaffolds.
Table 4.
Potential delivery of therapeutic molecules including growth factors, drugs, or genes from the scaffold for osteochondral repair.
| Growth factor | Osteochondral effects | Reference |
|---|---|---|
| TGF-β1 | Synchronized development of cartilage and subchondral bone. Earlier modulator for cartilage repair before BMP-2 action with hyaline-like cartilage formation. |
Scherer et al.84 and Reyes et al.85 |
| FGF-2 | Hyaline-like cartilage and subchondral bone. Increased stiffness of the osteochondral construct. |
Maehara et al.86 and Nakayama et al.87 |
| BMP-2 | High-quality cartilage and tissue integration. In vivo BMP-2 causes osteochondral differentiation of MSCs even with short exposure. Combination of BMP-2 further enhanced osteochondral repair effects. |
Noel et al.88, Sakata et al.89 and Jung et al.90 |
| BMP-7 | Formation of mineralized tissue and ectopic bone. Combination of other growth factors enhanced chondrogenesis and osteogenesis. |
Dormer et al.91 and Tiwary et al.92 |
| IGF-I | Superior growth morphology and surface architecture of the neotissue. Hyaline cartilage formation. Increased chondrocyte viability. Single delivery of IGF-I showed higher subchondral bone morphology. |
Wang et al.93, Teng et al.94 and Fransès et al.95 |
| VEGF | Critical factor for chondrocytes survival. High osteochondral vascular density. |
Sakata et al.89 and Fransès et al.95 |
BMP: bone morphogenetic protein; VEGF: vascular endothelial growth factor; PEG: polyethylene glycol; IGF-I: insulin-like growth factor I; TGF: transforming growth factor; FGF: fibroblast growth factor.
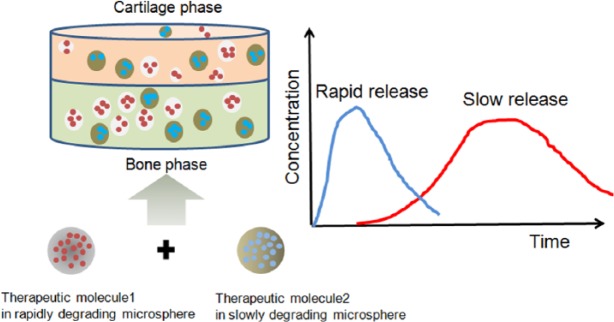
One promising approach is to encapsulate signaling molecules such as therapeutic growth factors or drugs in microspheres, which are then incorporated into biphasic scaffolds. Therapeutic molecules can be either surface-tethered to or incorporated within the microspheres. For the former case, the surface chemistry required to link molecules and drugs should be properly controlled.97 Meanwhile, for the latter case, the drug molecules can be incorporated during the microsphere preparation, and thus, a larger number of drug molecules can be loaded. The use of microspheres is versatile because they can be utilized as a scaffold as well as a delivery vehicle. This is particularly a good model for the repair of osteochondral tissue that requires a variety of signaling molecules. A spatiotemporal delivery strategy for osteochondral tissue engineering is depicted in Figure 3. For example, precise control over the spatiotemporal release kinetics of multiple growth factors is able to participate in damaged tissue healing.98 The sequential growth factor delivery system was designed to produce different profiles of growth factor release from the bilayered matrices with different physicochemical properties.100 Analogously, two types of microspheres with different degradation rates have been suggested to deliver two functional growth factors after the incorporation of the microspheres into a scaffold.99 These systems enable sequential and tighter control of release profiles. In practice, Wang et al.93 introduced a two-carrier system into porous polymer scaffolds to load different combinations of BMP-2 for osteogenesis and insulin-like growth factor I (IGF-I) for chondrogenesis with spatial distribution and temporally controlled release. Both growth factors were loaded onto PLGA/silk microspheres that have the ability to sustain the release of BMP-2 but are inefficient in the release of IGF-I due to the low loading efficiency. Although PLGA/silk microspheres showed inefficient MSC chondrogenesis, it is significant that this system offered a new option for the co-delivery of growth factors by spatiotemporal control.
Figure 3.
Spatiotemporal delivery from a biphasic scaffold containing therapeutic molecule–loaded microspheres with different degradation rates.98,99
In case of gene delivery techniques for osteochondral repair, non-viral cationic polymers or liposomes have been intensively used as carriers to promote gene transfection efficiency. For example, lipid-based endogenous gene delivery of FGF-2 and/or IGF-I101 was achieved to culture gene-modified chondrocytes on osteochondral scaffolds. It was reported that IGF-I stimulated the proliferation of chondrocytes102 and their synthesis of type II collagen103 and proteoglycan.104 Madry et al.105 reported that IGF-I expressing chondrocytes led to enhanced cartilage repair in vivo in osteochondral defect model. Recently, gene delivery system has been advanced to immobilize the carriers onto scaffolding matrices to effectively control cellular differentiation by specific target genes. For example, Chen et al.106 reported that the gene-activated matrix-based delivery of two plasmids encoding TGF-β1 and BMP-2 in MSCs immobilized into the biphasic scaffold was conducted to differentiate chondrogenesis and osteogenesis, respectively. Excellent MSC differentiation into chondrocytes and osteoblasts was shown in the biphasic scaffold composed of chitosan-gelatin layer for cartilage and HA for bone. The authors suggested that multi-tissue regeneration was performed in the biphasic scaffold by the gene delivery system. Although the study explored the gene delivery system for the osteochondral tissue repair, the delivery efficiency in the system is still not satisfactory because delivery of naked genes without any carriers is known to show a low efficiency. Theoretically, the spatiotemporal delivery of specific genes from the biphasic scaffold may direct cellular differentiation, facilitating the osteochondral regeneration. However, successful studies on the basis of this ideal strategy have not yet been reported. Instead of using the non-viral gene carriers, recombinant baculoviruses expressing BMP-2 were seeded into the osteochondral scaffolds containing de-differentiated chondrocytes, and then cultured in a rotating bioreactor for up to 3 weeks, which consequently led to the regeneration of hyaline cartilages.77
Perspective and concluding remarks
We reviewed the recent update of considerations required in osteochondral tissue engineering by discussing the previously published studies. Considering that interfacial tissue has a stratified structure depending on the unique property, theoretically, osteochondral scaffolds need to have multiphase structures to mimic the structure of the native stratified tissue. However, the multiphasic structures are too complex to control the properties of each phase, for example, degradation rates of each phase and shear forces between the phases. Therefore, the biphasic scaffold divided into a cartilage phase and a bone phase, which is simpler than the multiphasic scaffold, could be one of the most optimal osteochondral scaffolds to define interfacial tissue. Nonetheless, the mechanical stability between the two phases of the biphasic scaffold is still questionable. This weak stability could be because the integration of these two phases has been performed by physically combining the two phases after the individual fabrication of each phase. Although few studies have been reported that circumvent this limitation by using cell-substratum contact, the shear force between the two phases of the biphasic scaffold would still become weaker during its degradation after implantation in vivo. In order to overcome this, a one-pot method is required to fabricate a biphasic scaffold that can resist the shear force between the two phases.
In addition, it is still doubtful whether adequate chondrogenesis and osteogenesis can be mediated on each phase of the biphasic scaffold. This is because the respective cells on each phase could function as unexpected modulators affected by the inadequate release of signaling molecules. In order to overcome this challenge, a spatiotemporal targeting delivery system needs to be suggested. Using this system, the respective cells cultured on each phase would recognize the specific signaling molecules beneficial for the individual regeneration of each tissue. Therefore, we propose that future studies make full use of a spatiotemporal targeting delivery strategy in the biphasic scaffold that can withstand the shear force between the two phases.
Acknowledgments
Seog-Jin Seo and Chinmaya Mahapatra contributed equally to this work. This work was supported by the Priority Research Centers Program (grant#: 2009-0093829), National Research Foundation (NRF), Republic of Korea.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
References
- 1. Redman S, Oldfield S, Archer C. Current strategies for articular cartilage repair. Eur Cell Mater 2005; 9: 23–32. [DOI] [PubMed] [Google Scholar]
- 2. Grande DA, Breitbart AS, Mason J, et al. Cartilage tissue engineering: current limitations and solutions. Clin Orthop Relat Res 1999; 367: S176–S185. [DOI] [PubMed] [Google Scholar]
- 3. Patel KH, Nayyer L, Seifalian AM. Chondrogenic potential of bone marrow-derived mesenchymal stem cells on a novel, auricular-shaped, nanocomposite scaffold. J Tissue Eng 2013; 4: 2041731413516782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen G, Sato T, Ushida T, et al. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Eng 2004; 10: 323–330. [DOI] [PubMed] [Google Scholar]
- 5. Dai W, Kawazoe N, Lin X, et al. The influence of structural design of PLGA/collagen hybrid scaffolds in cartilage tissue engineering. Biomaterials 2010; 31: 2141–2152. [DOI] [PubMed] [Google Scholar]
- 6. Kim HJ, Park S-H, Durham J, et al. In vitro chondrogenic differentiation of human adipose-derived stem cells with silk scaffolds. J Tissue Eng 2012; 3: 2041731412466405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin S-H, Purevdorj O, Castano O, et al. A short review: recent advances in electrospinning for bone tissue regeneration. J Tissue Eng 2012; 3: 2041731412443530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt MB, Mow VC, Chun LE, et al. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res 1990; 8: 353–363. [DOI] [PubMed] [Google Scholar]
- 9. Muir H, Bullough P, Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br 1970; 52: 554–563. [PubMed] [Google Scholar]
- 10. Flannery CR, Hughes CE, Schumacher BL, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun 1999; 254: 535–541. [DOI] [PubMed] [Google Scholar]
- 11. Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv 2013; 31: 706–721. [DOI] [PubMed] [Google Scholar]
- 12. Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med 2005; 24: 1–12. [DOI] [PubMed] [Google Scholar]
- 13. Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev 2009; 15: 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khanarian NT, Jiang J, Wan LQ, et al. A hydrogel-mineral composite scaffold for osteochondral interface tissue engineering. Tissue Eng Part A 2011; 18: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Shea TM, Miao X. Bilayered scaffolds for osteochondral tissue engineering. Tissue Eng Part B Rev 2008; 14: 447–464. [DOI] [PubMed] [Google Scholar]
- 16. Gudas R, Kalesinskas RJ, Kimtys V, et al. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy 2005; 21: 1066–1075. [DOI] [PubMed] [Google Scholar]
- 17. Xing L, Jiang Y, Gui J, et al. Microfracture combined with osteochondral paste implantation was more effective than microfracture alone for full-thickness cartilage repair. Knee Surg Sports Traumatol Arthrosc 2013; 21: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 18. Kok AC, Tuijthof GJ, den Dunnen S, et al. No effect of hole geometry in microfracture for talar osteochondral defects. Clin Orthop 2013; 471: 3653–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leng P, Wang Y-z, Zhang H-n. Repair of large osteochondral defects with mix-mosaicplasty in a goat model. Orthopedics 2013; 36: e331–e336. [DOI] [PubMed] [Google Scholar]
- 20. Solheim E, Hegna J, Øyen J, et al. Osteochondral autografting (mosaicplasty) in articular cartilage defects in the knee: results at 5 to 9 years. Knee 2010; 17: 84–87. [DOI] [PubMed] [Google Scholar]
- 21. Niemeyer P, Pestka JM, Kreuz PC, et al. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med 2008; 36: 2091–2099. [DOI] [PubMed] [Google Scholar]
- 22. Ossendorf C, Steinwachs MR, Kreuz PC, et al. Autologous chondrocyte implantation (ACI) for the treatment of large and complex cartilage lesions of the knee. BMC Sport Sci Med Rehab 2011; 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basad E, Ishaque B, Bachmann G, et al. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc 2010; 18: 519–527. [DOI] [PubMed] [Google Scholar]
- 24. Hangody L, Kish G, Karpati Z, et al. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects: a preliminary report. Knee Surg Sports Traumatol Arthrosc 1997; 5: 262–267. [DOI] [PubMed] [Google Scholar]
- 25. Evans EM, Freeman M, Miller A, et al. Metal sensitivity as a cause of bone necrosis and loosening of the prosthesis in total joint replacement. J Bone Joint Surg Br 1974; 56: 626–642. [DOI] [PubMed] [Google Scholar]
- 26. Fitzpatrick CK, Rullkoetter PJ. Influence of patellofemoral articular geometry and material on mechanics of the unresurfaced patella. J Biomech 2012; 45: 1909–1915. [DOI] [PubMed] [Google Scholar]
- 27. Imhof H, Sulzbacher I, Grampp S, et al. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest Radiol 2000; 35: 581–588. [DOI] [PubMed] [Google Scholar]
- 28. Fansa AM, Murawski CD, Imhauser CW, et al. Autologous osteochondral transplantation of the talus partially restores contact mechanics of the ankle joint. Am J Sports Med 2011; 39: 2457–2465. [DOI] [PubMed] [Google Scholar]
- 29. Lintz F, Pujol N, Pandeirada C, et al. Hybrid fixation: evaluation of a novel technique in adult osteochondritis dissecans of the knee. Knee Surg Sports Traumatol Arthrosc 2011; 19: 568–571. [DOI] [PubMed] [Google Scholar]
- 30. Reverte-Vinaixa MM, Joshi N, Diaz-Ferreiro EW, et al. Medium-term outcome of mosaicplasty for grade III-IV cartilage defects of the knee. J Orthop Surg (Hong Kong) 2013; 21: 4–9. [DOI] [PubMed] [Google Scholar]
- 31. Hoshiba T, Yamada T, Lu H, et al. Maintenance of cartilaginous gene expression on extracellular matrix derived from serially passaged chondrocytes during in vitro chondrocyte expansion. J Biomed Mater Res A 2012; 100: 694–702. [DOI] [PubMed] [Google Scholar]
- 32. Lu Y, Dhanaraj S, Wang Z, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res 2006; 24: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 33. Vijayan S, Bartlett W, Bentley G, et al. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: a two- to eight-year follow-up study. J Bone Joint Surg Br 2012; 94: 488–492. [DOI] [PubMed] [Google Scholar]
- 34. Hughes P, Eaves C, Hogge D, et al. High-efficiency gene transfer to human hematopoietic cells maintained in long-term marrow culture. Blood 1989; 74: 1915–1922. [PubMed] [Google Scholar]
- 35. Wang L, Zhao L, Detamore MS. Human umbilical cord mesenchymal stromal cells in a sandwich approach for osteochondral tissue engineering. J Tissue Eng Regen Med 2011; 5: 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodrigues MT, Lee SJ, Gomes ME, et al. Bilayered constructs aimed at osteochondral strategies: the influence of medium supplements in the osteogenic and chondrogenic differentiation of amniotic fluid-derived stem cells. Acta Biomater 2012; 8: 2795–2806. [DOI] [PubMed] [Google Scholar]
- 37. Koga H, Muneta T, Ju YJ, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells 2007; 25: 689–696. [DOI] [PubMed] [Google Scholar]
- 38. Betsch M, Schneppendahl J, Thuns S, et al. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One 2013; 8: e71602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horas U, Pelinkovic D, Herr G, et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. J Bone Joint Surg Am 2003; 85-A: 185–192. [DOI] [PubMed] [Google Scholar]
- 40. Innes J, Gordon C, Vaughan-Thomas A, et al. Evaluation of cartilage, synovium and adipose tissue as cellular sources for osteochondral repair. Vet J 2013; 197: 619–624. [DOI] [PubMed] [Google Scholar]
- 41. Ko J-Y, Kim K-I, Park S, et al. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 2014; 35: 3571–3581. [DOI] [PubMed] [Google Scholar]
- 42. Petrenko YA, Ivanov R, Petrenko AY, et al. Coupling of gelatin to inner surfaces of pore walls in spongy alginate-based scaffolds facilitates the adhesion, growth and differentiation of human bone marrow mesenchymal stromal cells. J Mater Sci Mater Med 2011; 22: 1529–1540. [DOI] [PubMed] [Google Scholar]
- 43. Shafiee A, Soleimani M, Chamheidari GA, et al. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J Biomed Mater Res A 2011; 99: 467–478. [DOI] [PubMed] [Google Scholar]
- 44. Bal BS, Rahaman MN, Jayabalan P, et al. In vivo outcomes of tissue-engineered osteochondral grafts. J Biomed Mater Res B Appl Biomater 2010; 93: 164–174. [DOI] [PubMed] [Google Scholar]
- 45. Frosch K-H, Stürmer KM. Metallic biomaterials in skeletal repair. Eur J Trauma 2006; 32: 149–159. [Google Scholar]
- 46. Jiang J, Tang A, Ateshian GA, et al. Bioactive stratified polymer ceramic-hydrogel scaffold for integrative osteochondral repair. Ann Biomed Eng 2010; 38: 2183–2196. [DOI] [PubMed] [Google Scholar]
- 47. Athanasiou KA, Darling EM, Hu JC. Articular cartilage tissue engineering. Synthesis Lect Tissue Eng 2009; 1: 1–182. [Google Scholar]
- 48. Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A 2009; 15: 3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One 2008; 3: e2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McNary SM, Athanasiou KA, Reddi AH. Engineering lubrication in articular cartilage. Tissue Eng Part B Rev 2012; 18: 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi Y, Xiong D. Microstructure and friction properties of PVA/PVP hydrogels for articular cartilage repair as function of polymerization degree and polymer concentration. Wear 2013; 305: 280–285. [Google Scholar]
- 52. Mikos AG, Temenoff JS. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron J Biotechn 2000; 3: 23–24. [Google Scholar]
- 53. Kaplan FS, Hayes WC, Keaveny TM, et al. Biomaterials. In: Simon SP. (ed.) Orthopedic Basic Science. Columbus, OH: American Academy of Orthopedic Surgeons; 1994. pp. 127–185. [Google Scholar]
- 54. Keaveny TM, Morgan EF, Niebur GL, et al. Biomechanics of trabecular bone. Annu Rev Biomed Eng 2001; 3: 307–333. [DOI] [PubMed] [Google Scholar]
- 55. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005; 26: 5474–5491. [DOI] [PubMed] [Google Scholar]
- 56. Kim K, Yeatts A, Dean D, et al. Stereolithographic bone scaffold design parameters: osteogenic differentiation and signal expression. Tissue Eng Part B Rev 2010; 16: 523–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simonet M, Schneider OD, Neuenschwander P, et al. Ultraporous 3D polymer meshes by low-temperature electrospinning: use of ice crystals as a removable void template. Polym Eng Sci 2007; 47: 2020–2026. [Google Scholar]
- 58. Filová E, Rampichová M, Litvinec A, et al. A cell-free nanofiber composite scaffold regenerated osteochondral defects in miniature pigs. Int J Pharm 2013; 447: 139–149. [DOI] [PubMed] [Google Scholar]
- 59. Shanmugasundaram S, Chaudhry H, Arinzeh TL. Microscale versus nanoscale scaffold architecture for mesenchymal stem cell chondrogenesis. Tissue Eng Part A 2010; 17: 831–840. [DOI] [PubMed] [Google Scholar]
- 60. Pham QP, Sharma U, Mikos AG. Electrospun poly(ϵ-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006; 7: 2796–2805. [DOI] [PubMed] [Google Scholar]
- 61. Zhang S, Chen L, Jiang Y, et al. Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration. Acta Biomater 2013; 9: 7236–7247. [DOI] [PubMed] [Google Scholar]
- 62. Rebollar E, Cordero D, Martins A, et al. Improvement of electrospun polymer fiber meshes pore size by femtosecond laser irradiation. Appl Surf Sci 2011; 257: 4091–4095. [Google Scholar]
- 63. Zhu N, Li M, Cooper D, et al. Development of novel hybrid poly(L-lactide)/chitosan scaffolds using the rapid freeze prototyping technique. Biofabrication 2011; 3: 034105. [DOI] [PubMed] [Google Scholar]
- 64. Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000; 21: 2529–2543. [DOI] [PubMed] [Google Scholar]
- 65. Woodfield T, Guggenheim M, Von Rechenberg B, et al. Rapid prototyping of anatomically shaped, tissue-engineered implants for restoring congruent articulating surfaces in small joints. Cell Prolif 2009; 42: 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Moroni L, De Wijn J, Van Blitterswijk C. 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials 2006; 27: 974–985. [DOI] [PubMed] [Google Scholar]
- 67. Woodfield TB, Malda J, De Wijn J, et al. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004; 25: 4149–4161. [DOI] [PubMed] [Google Scholar]
- 68. Bian W, Li D, Lian Q, et al. Fabrication of a bio-inspired beta-Tricalcium phosphate/collagen scaffold based on ceramic stereolithography and gel casting for osteochondral tissue engineering. Rapid Prototyping J 2012; 18: 68–80. [Google Scholar]
- 69. Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003; 24: 4337–4351. [DOI] [PubMed] [Google Scholar]
- 70. Guarino V, Gloria A, Raucci MG, et al. Hydrogel-based platforms for the regeneration of osteochondral tissue and intervertebral disc. Polymers 2012; 4: 1590–1612. [Google Scholar]
- 71. Kim K, Lam J, Lu S, et al. Osteochondral tissue regeneration using a bilayered composite hydrogel with modulating dual growth factor release kinetics in a rabbit model. J Control Release 2013; 168: 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Munoz-Pinto DJ, McMahon RE, Kanzelberger MA, et al. Inorganic-organic hybrid scaffolds for osteochondral regeneration. J Biomed Mater Res A 2010; 94: 112–121. [DOI] [PubMed] [Google Scholar]
- 73. Wendt D, Jakob M, Martin I. Bioreactor-based engineering of osteochondral grafts: from model systems to tissue manufacturing. J Biosci Bioeng 2005; 100: 489–494. [DOI] [PubMed] [Google Scholar]
- 74. Yunos D, Ahmad Z, Salih V, et al. Stratified scaffolds for osteochondral tissue engineering applications: electrospun PDLLA nanofibre coated Bioglass®-derived foams. J Biomater Appl 2013; 27: 537–551. [DOI] [PubMed] [Google Scholar]
- 75. Grayson WL, Bhumiratana S, Grace Chao P, et al. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthritis Cartilage 2010; 18: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Correia C, Pereira AL, Duarte AR, et al. Dynamic culturing of cartilage tissue: the significance of hydrostatic pressure. Tissue Eng Part A 2012; 18: 1979–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen H-C, Chang Y-H, Chuang C-K, et al. The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials 2009; 30: 674–681. [DOI] [PubMed] [Google Scholar]
- 78. Hosseinkhani H, Azzam T, Kobayashi H, et al. Combination of 3D tissue engineered scaffold and non-viral gene carrier enhance in vitro DNA expression of mesenchymal stem cells. Biomaterials 2006; 27: 4269–4278. [DOI] [PubMed] [Google Scholar]
- 79. Lin C-Y, Chang Y-H, Lin K-J, et al. The healing of critical-sized femoral segmental bone defects in rabbits using baculovirus-engineered mesenchymal stem cells. Biomaterials 2010; 31: 3222–3230. [DOI] [PubMed] [Google Scholar]
- 80. Leng P, Ding C-r, Zhang H-n, et al. Reconstruct large osteochondral defects of the knee with hIGF-1 gene enhanced Mosaicplasty. Knee 2012; 19: 804–811. [DOI] [PubMed] [Google Scholar]
- 81. Reyes R, Delgado A, Sanchez E, et al. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGFβ1 from a bilayered alginate-PLGA scaffold. J Tissue Eng Regen Med. Epub ahead of print 26 June 2012. DOI: 10.1002/term.1549. [DOI] [PubMed] [Google Scholar]
- 82. Yi O, Kwon H-J, Kim H, et al. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res 2012; 113: 40–45. [DOI] [PubMed] [Google Scholar]
- 83. Aulin C, Jensen-Waern M, Ekman S, et al. Cartilage repair of experimentally induced osteochondral defects in New Zealand White rabbits. Lab Anim 2013; 47: 58–65. [DOI] [PubMed] [Google Scholar]
- 84. Scherer F, Anton M, Schillinger U, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther 2002; 9: 102–109. [DOI] [PubMed] [Google Scholar]
- 85. Reyes R, Delgado A, Solis R, et al. Cartilage repair by local delivery of TGF-β1 or BMP-2 from a novel, segmented polyurethane/polylactic-co-glycolic bilayered scaffold. J Biomed Mater Res A 2014; 102: 1110–1120. [DOI] [PubMed] [Google Scholar]
- 86. Maehara H, Sotome S, Yoshii T, et al. Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2). J Orthop Res 2010; 28: 677–686. [DOI] [PubMed] [Google Scholar]
- 87. Nakayama J-i, Fujioka H, Nagura I, et al. The effect of fibroblast growth factor-2 on autologous osteochondral transplantation. Int Orthop 2009; 33: 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Noel D, Gazit D, Bouquet C, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells 2004; 22: 74–85. [DOI] [PubMed] [Google Scholar]
- 89. Sakata R, Kokubu T, Nagura I, et al. Localization of vascular endothelial growth factor during the early stages of osteochondral regeneration using a bioabsorbable synthetic polymer scaffold. J Orthop Res 2012; 30: 252–259. [DOI] [PubMed] [Google Scholar]
- 90. Jung MR, Shim IK, Chung HJ, et al. Local BMP-7 release from a PLGA scaffolding-matrix for the repair of osteochondral defects in rabbits. J Control Release 2012; 162: 485–491. [DOI] [PubMed] [Google Scholar]
- 91. Dormer NH, Singh M, Zhao L, et al. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A 2012; 100: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tiwary R, Aithal HP, Kinjavdekar P, et al. Effect of IGF-1 and uncultured autologous bone-marrow-derived mononuclear cells on repair of osteochondral defect in rabbits. Cartilage 2014; 5: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang X, Wenk E, Zhang X, et al. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release 2009; 134: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Teng MS, Yuen AS, Kim HT. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res 2008; 466: 1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Fransès R, McWilliams D, Mapp P, et al. Osteochondral angiogenesis and increased protease inhibitor expression in OA. Osteoarthritis Cartilage 2010; 18: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Diederichs S, Baral K, Tanner M, et al. Interplay between local versus soluble transforming growth factor-beta and fibrin scaffolds: role of cells and impact on human mesenchymal stem cell chondrogenesis. Tissue Eng Part A 2012; 18: 1140–1150. [DOI] [PubMed] [Google Scholar]
- 97. Pérez RA, Won J-E, Knowles JC, et al. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv Drug Deliv Rev 2013; 65: 471–496. [DOI] [PubMed] [Google Scholar]
- 98. Chen F-M, Zhang M, Wu Z-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials 2010; 31: 6279–6308. [DOI] [PubMed] [Google Scholar]
- 99. Mehta M, Schmidt-Bleek K, Duda GN, et al. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv Drug Deliv Rev 2012; 64: 1257–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sohier J, Vlugt T, Cabrol N, et al. Dual release of proteins from porous polymeric scaffolds. J Control Release 2006; 111: 95–106. [DOI] [PubMed] [Google Scholar]
- 101. Orth P, Kaul G, Cucchiarini M, et al. Transplanted articular chondrocytes co-overexpressing IGF-I and FGF-2 stimulate cartilage repair in vivo. Knee Surg Sports Traumatol Arthrosc 2011; 19: 2119–2130. [DOI] [PubMed] [Google Scholar]
- 102. Trippel S, Corvol M, Dumontier M, et al. Effect of somatomedin-C/insulin-like growth factor I and growth hormone on cultured growth plate and articular chondrocytes. Pediatr Res 1989; 25: 76–82. [DOI] [PubMed] [Google Scholar]
- 103. Van der Kraan P, Buma P, Van Kuppevelt T, et al. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthritis Cartilage 2002; 10: 631–637. [DOI] [PubMed] [Google Scholar]
- 104. Luyten FP, Hascall VC, Nissley SP, et al. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys 1988; 267: 416–425. [DOI] [PubMed] [Google Scholar]
- 105. Madry H, Kaul G, Cucchiarini M, et al. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I). Gene Ther 2005; 12: 1171–1179. [DOI] [PubMed] [Google Scholar]
- 106. Chen J, Chen H, Li P, et al. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 2011; 32: 4793–4805. [DOI] [PubMed] [Google Scholar]