Abstract
Background
Correct specification of cell lineages and establishing angiogenic privilege within the developing cornea are essential for normal vision but the mechanisms controlling these processes are poorly understood.
Results
We show that the homeodomain transcription factor PItX2 is expressed in mesenchymal cells of the developing and mature cornea and use a temporal gene knockout approach to demonstrate that PITX2 is required for corneal morphogenesis and the specification of cell fates within the surface ectoderm and mesenchymal primordia. PITX2 is also required to establish angiogenic privilege in the developing cornea. Further, the expression of Dkk2 and suppression of canonical Wnt signaling activity levels are key mechanisms by which PITX2 specifies ocular surface ectoderm as cornea. In contrast, specifying the underlying mesenchyme to corneal fates and establishing angiogenic privilege in the cornea are less sensitive to DKK2 activity. Finally, the cellular expression patterns of FOXC2, PITX1, and BARX2 in Pitx2 and Dkk2 mutants suggest that these transcription factors may be involved in specifying cell fate and establishing angiogenic privilege within the corneal mesenchyme. However, they are unlikely to play a role in specifying cell fate within the corneal ectoderm.
Conclusions
Together, these data provide important insights into the mechanisms regulating cornea development.
Keywords: Temporal gene knockout, ocular anterior segment, glaucoma, canonical Wnt signaling, Dkk2
INTRODUCTION
The cornea provides important properties that are required for normal visual acuity, including transparency, refraction, and protection of inner components of the eye from external environmental factors. The mature cornea consists of three cellular layers, a 6–7 cell thick corneal epithelium that comprises the outer surface, a monolayer f endothelium that forms the inner surface, and a stroma layer that lies between the epithelium and the endothelium and consists of a highly ordered lamellar arrangement keratocytes and specialized collagen bundles (Chow and Lang, 2001, Cvekl and Tamm, 2004). The corneal epithelium arises from surface ectoderm during development, where as the endothelium and stroma are formed from mesenchyme that, depending upon the species, largely or totally derives from neural crest (Gage et al., 2005, Le Douarin, 1980, Le Lievre and Le Douarin, 1975). Understanding how intrinsic and extrinsic factors mesh to control cell fate decisions and gene expression in each cellular layer during development is likely to enhance the development of new and improved strategies for treating corneal diseases.
Normal vision also requires the complete absence of blood and lymphatic vessels from the cornea, a property referred to as angiogenic privilege. Loss of angiogenic privilege as a consequence of developmental abnormalities or postnatal insult results in corneal neovascularization, a sight threatening condition. Therefore, a detailed understanding of how angiogenic privilege is initially established during development and subsequently preserved in the mature cornea is important for expanding our knowledge of corneal development and identifying new, more effective therapies.
The onset of corneal development is morphologically apparent when neural crest mesenchyme begins to migrate between the newly formed lens vesicle and the overlying surface ectoderm (Cvekl and Tamm, 2004). The endothelium is the first differentiated corneal layer to differentiate, and it arises from mesenchymal cells that contact with the anterior lens epithelia. Subsequently, mesenchymal cells between the newly formed endothelium and the overlying ocular surface ectoderm become compact and adopt an ordered and lamellar arrangement to form the corneal stroma. Blood vessels never appear in the developing cornea, indicating that angiogenic privilege is established early during corneal morphogenesis. Cell signaling from cells of the anterior lens vesicle and the distal rim of the optic cup acts directly upon the mesenchyme early in corneal development to induce expression of transcription factor genes that are broadly required for normal anterior segment development, including Foxc1 and Pitx2 (Matt et al., 2005, Molotkov et al., 2006). Of these, only Foxc1 has been established as a gene that specifies cell fates and angiogenic privilege in the developing cornea (Kidson et al., 1999, Seo et al., 2012). Concurrent with development of the endothelium and stroma, the overlying ocular surface ectoderm is specified as cornea and signaling from the underlying mesenchymal cells is for this process (Pearton et al., 2005).
The ocular surface ectoderm and underlying mesenchyme also give rise to the conjunctiva, which lies between the cornea and the posterior margin of the eyelid. The cellular components of the conjunctiva are highly distinct from those of the cornea, and the conjunctival mesenchyme is highly vascularized. The mechanisms underlying segregation of the multipotent ocular surface ectoderm and mesenchyme into corneal versus conjunctival fates during development are unknown.
Given its fundamental role in governing development of early anterior segment tissues (Evans and Gage, 2005a, Gage et al., 1999), we hypothesize that Pitx2 is also required for specifying cell lineages and establishing angiogenic privilege within the developing cornea. Pitx2 encodes a paired-like homeodomain transcription factor that is important in both development and disease. Heterozygous mutations in human PITX2 are one genetic cause of Axenfeld-Rieger Syndrome (ARS), which features dysgenesis of anterior segment structures within the eye and a high risk for glaucoma (Semina et al., 1996). ARS patients with PITX2 mutations, as well as Pitx2 heterozygous mice, exhibit decreased central corneal thickness (CCT), consistent with a potential role for PITX2 in corneal development (Asai-Coakwell et al., 2006). Global or neural crest-specific ablation of Pitx2 results in the arrest of anterior segment development at a stage prior to the initiation of corneal development, thereby making these models unsuitable for assessing gene function in the latter process (Evans and Gage, 2005a, Gage et al., 1999). To circumvent this blockade, we employed a temporal gene knockout approach in the current study. We demonstrate that PITX2 is required for specifying lineages and establishing angiogenic privilege in the developing cornea. In the absence of PITX2, the surface ectoderm and mesenchyme acquire gene expression patterns more consistent with conjunctival fates, Dkk2 expression is not maintained within the corneal mesenchyme, and canonical Wnt signaling activity becomes elevated within the developing cornea. Maintenance of normal canonical Wnt signaling through its downstream target Dkk2 appears to be an essential mechanism by which PITX2 controls normal specification of the corneal epithelium.
RESULTS
Pitx2 is required for normal histogenesis and establishing angiogenic privilege in the developing cornea
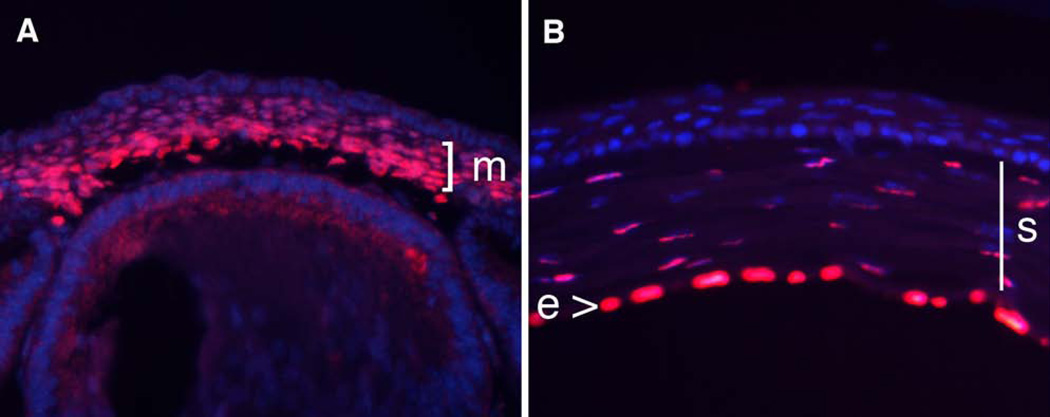
As shown previously, central corneal thickness is reduced in mice and humans harboring a 50% reduction in PITX2, suggesting a potential direct role in cornea development (Asai-Coakwell et al., 2006). However, this result could also arise from an indirect effect such as elevated intraocular pressure leading to corneal thinning. Therefore, we analyzed protein expression in developing and mature corneas in the mouse to determine whether there was the potential for PITX2 to have a direct effect on corneal development. We found that PITX2 protein is uniformly expressed in the mesenchymal layer of the developing cornea, as well as in the stroma and endothelium layers in the mature cornea, consistent with a potential role for PITX2 in regulating corneal development (Figure 1).
Figure 1. PITX2 expression in developing and mature corneas.
PITX2 immunostaining on e14.5 (A) and 6-week old (B) cornea. E, corneal endothelium; M, corneal mesenchyme; S, corneal stroma.
Anterior segment development is arrested in global and neural crest-specific Pitx2 knockout mice prior to initiation of corneal development (Evans and Gage, 2005b, Gage et al., 1999). Therefore, we employed a temporal knockout strategy that allowed us to rescue the initial arrest in anterior segment development but still ablate Pitx2 at the beginning of corneal development (Zacharias et al., 2011). Briefly, we crossed mice carrying our conditional Pitx2flox allele together with mice carrying the UBC-CreERt2 transgene, which ubiquitously expresses a Cre fusion protein that is inactive in the absence of tamoxifen (Gruber et al., 2007, Ruzankina et al., 2007). Timed pregnant females carrying prospective mutants (UBC-CreERt2:Pitx2flox/null) and controls (Pitx2flox/null) were injected with tamoxifen at e10.5 to activate the CreERT2 fusion protein and ablate Pitx2 in the prospective mutant (Pitx2-tko) mice at the beginning of corneal morphogenesis. The Pitx2 gene was deleted and PITX2 protein is degraded in Pitx2-tko embryos within 24 hours, making this system highly efficient (Figure 2B). PITX2-positive cells do not reemerge in older embryos (Figure 2C–H).
Figure 2. Tamoxifen induced ablation of PITX2 is highly efficient.
Immunohistochemistry was used on representative control and Pitx2-tko mutant eyes to detect PITX2 expression at the indicated ages. All timed pregnant females were injected with tamoxifen at e10.5 to ablate the Pitx2 gene in prospective mutants. Only rare PITX2+ cells were present in mutant mice. C, cornea; Cn, conjunctiva.
We first used a histological approach to investigate the consequences of ablating Pitx2 (Figure 3). Mesenchymal cells in control littermates begin to populate the space between the developing lens and overlying surface ectoderm by e11.5, and their number is markedly increased by e12.5 (Figure 3A,B). By e14.5, a monolayer of endothelium is present on the inner surface of the mesenchymal layer and an obvious anterior chamber has formed (Figure 3C). Beginning at e14.5 and continuing through e16.5, cells within the emerging stroma cells adopt an increasingly organized and laminar appearance within the mesenchyme located between the endothelium and the overlying surface ectoderm. By e16.5, all three layers of the cornea are well delineated (Figure 3D). By e11.5, corneas in Pitx2-tko are indistinguishable from controls. However, by e12.5 the mesenchymal component in mutant embryos is thickened relative to corneas of control littermates (Figure 3E,F). At 14.5, the endothelium is absent from Pitx2-tko corneas, and the putative stromal cell layer is significantly thicker and less well organized than in controls. In addition, multiple abnormal histological layers are often visible within the putative stroma (Figure 3G). The disorganization of the corneal tissues persists through e16.5 and the lack of an anterior chamber confirms the absence of a functional endothelium (Figure 3H and data not shown). The ectodermal layer of Pitx2-tko corneas is modestly thickened relative to control corneas. Collectively, these data establish that PITX2 is required for correct morphogenesis within each of three layers of the developing cornea.
Figure 3. Altered corneal morphogenesis in Pitx2-tko eyes following ablation of Pitx2 by injection of tamoxifen at e10.5.
H&E-stained coronal sections of control and mutant anterior segments at e11.5 (A,E), e12.5 (B,F), e14.5 (C,G), and e16.5 (D,H). Note that in the corneas of Pitx2-tko mutants there is an overabundance of mesenchyme beginning at 12.5, the endothelium and stroma layers do not form, the surface ectoderm is modestly thickened, and the anterior chamber is absent. Ec, corneal ectoderm; M, corneal mesenchyme; L, lens; Ac, anterior chamber; En, corneal endothelium.
We also noted the presence of blood vessels in corneas of Pitx2-tko mice. To more readily visualize this, Pitx2-tko and control eyes were immunostained for presence of the blood vessel endothelial cell marker, CD31 (REFERENCE). As expected, the avascular control corneas are devoid of CD31 staining (Figure 4A). In contrast, by late gestation Pitx2-tko corneas are heavily invested with CD31+ blood vessels by late gestation (Figure 4C). Because lymphangiogenesis frequently parallels angiogenesis, we also tested control and Pitx2-tko eyes for the presence of lymphatic vessels. By late in gestation, LYVE1+ lymphatic vessels are absent form control corneas but readily detectable in Pitx2-tko corneas by late in gestation (Figure 4B,D). These data confirm that PITX2 function is required to establish angiogenic privilege.
Figure 4. PITX2 is required to establish angiogenic privilege in the developing cornea.
Late gestation control and Pitx2-tko mutant corneas immunostained to detect the presence of CD31 (A,C) and LYVE1 (B,D) markers of the endothelium of blood vessels and lymphatic vessels, respectively. C, corneal stroma; L, lens.
Gene expression in Pitx2-tko corneal mesenchyme resembles that of conjunctival mesenchyme
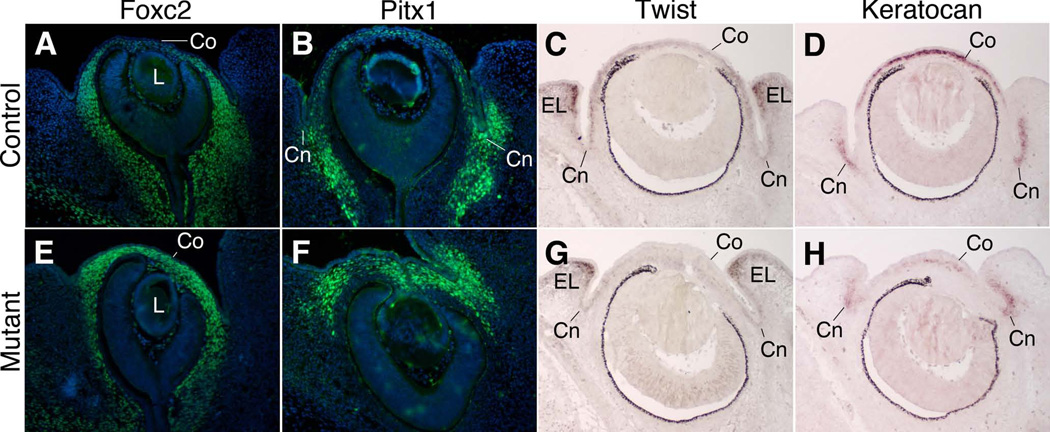
We next characterized whether the morphological changes observed in mutant corneas during the first several days following Pitx2 ablation were accompanied by alterations in the spatial or temporal patterns of gene expression. We observed that in control embryos at e12.5, immunostaining to detect the forkhead transcription factor FOXC2 distinguishes the corneal mesenchyme, where it is not expressed, and the periocular mesenchyme of the future conjunctiva and posterior of the optic cup, where it is expressed (Figure 5A). In contrast to control embryos, in Pitx2-tko littermates, FOXC2 is uniformly high throughout the periocular mesenchyme, including the presumptive corneal mesenchyme (Figure 5E). Similarly, we immunostained sections for the homeodomain transcription factor PITX1. In control mice at e13.5, PITX1 is highly expressed in the conjunctival mesenchyme but only minimally in the corneal mesenchyme of control mice at e13.5 (Figure 5B). In Pitx2-tko mice, PITX1 expression is uniformly high in both the conjunctival and corneal mesenchyme (Figure 5F). In situ hybridization was then used to detect expression of the gene for the bHLH transcription factor Twist1. In control embryos at e14.5, Twist1 is expressed in the corneal mesenchyme but not in the conjunctival mesenchyme (Figure 5C, (Thut et al., 2001)). In Pitx2-tko siblings, Twist1 expression is not detectable in either the corneal or conjunctival mesenchyme (Figure 5G). Finally, in situ hybridization was used to examine the expression of Keratocan, which has been identified as a gene whose expression pattern is specific to cells in the corneal stroma during late development and continuing in adults (Liu et al., 1998). Here, however, we found a more complex expression pattern. In control embryos at e14.5, high levels of Keratocan are expressed in the developing corneal mesenchyme, whereas Keratocan was also expressed at lower levels in the conjunctival mesenchyme (Figure 5D). In Pitx2-tko littermates, the expression level in the corneal mesenchyme of was consistently lower than in control corneas and the pattern was more diffuse (Figure 5H). In this regard, the level and pattern more closely resembled that of the conjunctival mesenchyme in controls. To assess whether differentiation of the corneal mesenchyme was simply delayed, we assessed Keratocan expression in older embryos. However, we observed the same complex expression pattern as in younger eyes with Keratocan expression present in the mesenchyme of the cornea and conjunctiva (Figure 6). As in the younger embryos, the expression pattern in the mesenchyme of mutant corneas more closely resembled that found in the conjunctival mesenchyme. Together, these data show that following ablation of Pitx2, gene expression in the presumptive corneal mesenchyme is altered at the developmental time point (e12.5) that coincides with the first morphological evidence of altered corneal development, and the protein and gene expression patterns in the corneal mesenchyme of Pitx2-tko mutants resemble that of the conjunctival mesenchyme.
Figure 5. Gene expression in the corneal mesenchyme is altered following ablation of Pitx2.
Immunohistochemistry was used on control and Pitx2-tko mutant corneas to detect expression of the FOXC2 (A,E) and PITX1 (B,F) transcription factors at e12.5 and e13.5, respectively. FOXC2 and PITX1 protein expression expands from the conjunctiva into the cornea in mutant eyes. In situ hybridization was used to detect expression of the Twist1 (C,G) and Keratocan (D,H) mRNA at e14.5. Twist1 mRNA is expressed in the mesenchyme of control but not Pitx2-tko mutant corneas. Expression of Keratocan in mesenchyme of Pitx2tko corneas is reduced and more diffuse when compared to expression in mesenchyme of control corneas. CO, corneal mesenchyme; L, lens; Cn, conjunctival mesenchyme; EL, eyelid mesenchyme.
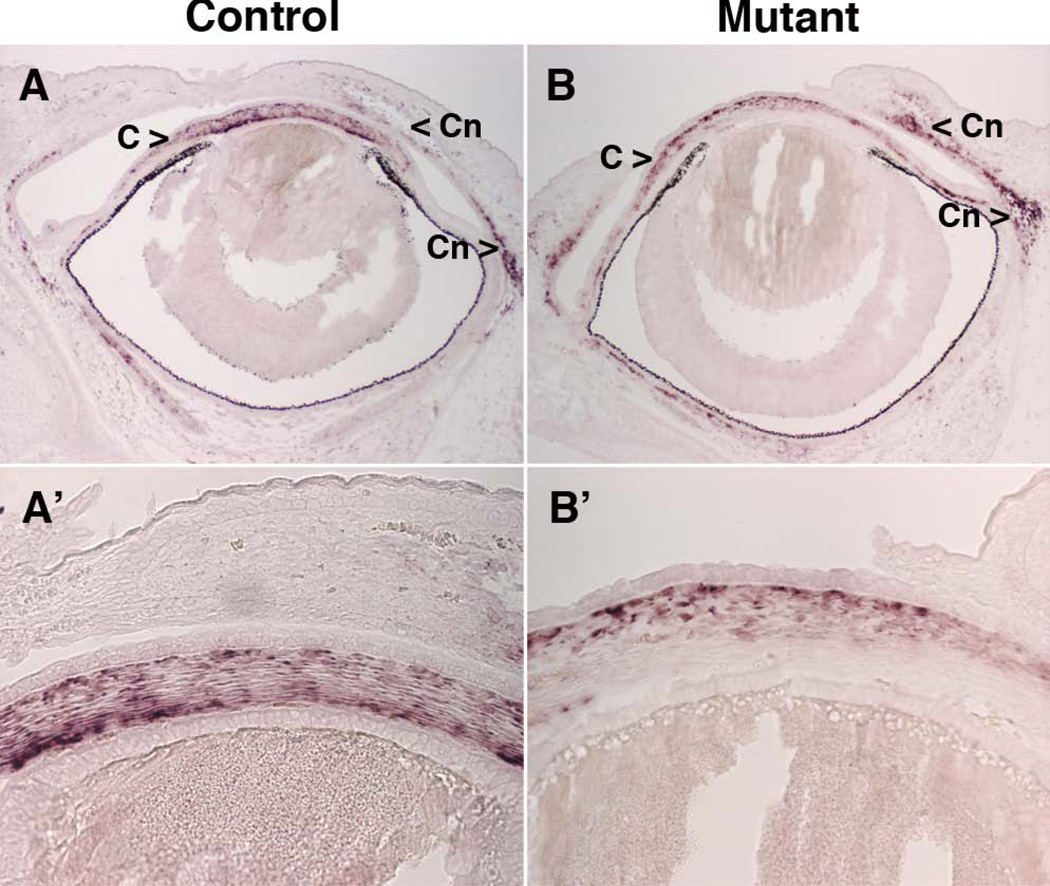
Figure 6. Keratocan expression in e16.5 control and Pitx2-tko mutant eyes.
In situ hybridization was used to detect Keratocan mRNA in e16.5 embryos. Panels A' and B' are magnified views of the central corneas from the eyes shown in Panels A and B, respectively. C, corneal mesenchyme; Cn, conjunctival mesenchyme.
Gene expression in Pitx2-tko corneal ectoderm resembles that of conjunctival ectoderm
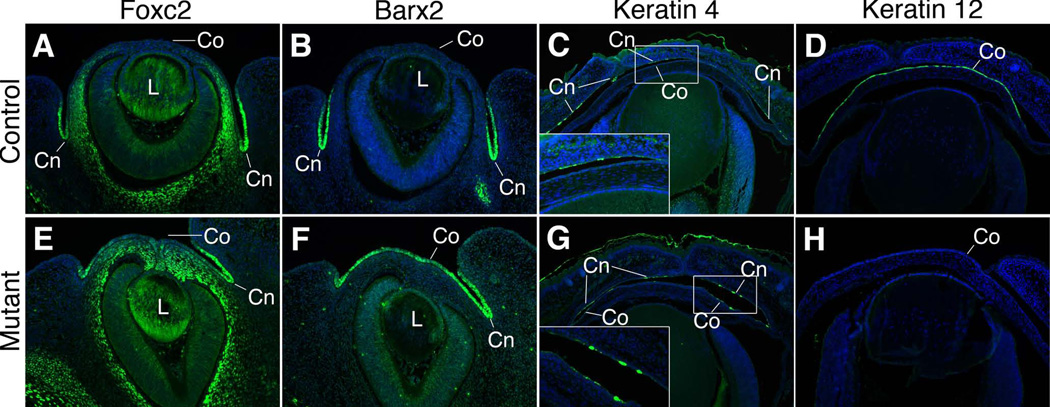
Signaling from the underlying mesenchyme dictates the fate of the overlying surface ectoderm, including in the cornea (Pearton et al., 2005). Therefore, based the apparent fate switch in the underlying mesenchyme from a corneal to a conjunctival character, we examined markers whose expression patterns differentiate between corneal and conjunctival ectoderm. However, at e13.5, we found that in control embryos the transcription factors FOXC2 and BARX2 are specifically expressed in the conjunctival ectoderm but not in corneal ectoderm of control embryos (Figure 7A,B). In Pitx2-tko littermates, FOXC2 and BARX2 are both expressed throughout both the conjunctival and corneal ectoderm, which is consistent with at least a partial fate switch at these this timepoint (Figure 7E,F). Further evidence for a fate switch within the ectoderm was revealed by assessing the expression of the intermediate filament proteins cytokeratin 4 (cK4), a specific marker of conjunctival ectoderm and cytokeratin 12 (cK12), a specific marker of corneal ectoderm at e16.5 (Figure 7C,D). In Pitx2-tko eyes, expression of the cK4 expands to include cells within both the conjunctival and corneal ectoderm (Figure 7G), while expression of the corneal marker cK12 is absent (Figure 7H). Therefore, the results of examining multiple markers expressed in each embryonic tissue support the conclusion that following the loss of Pitx2, corneal cell lineages are not specified correctly and that both the mesenchyme and the ectoderm partially adopt conjunctival fates.
Figure 7. Gene expression in the corneal ectoderm is altered following ablation of Pitx2.
Control and Pitx2-tko eyes were immunostained to detect the expression of FOXC2 at e13.5 (A,E), BARX2 at e13.5 (B,F), and keratin 4 (C,G) and keratin 12 (D,H) at e16.5. FOXC2, BARX2 and keratocan 4 are limited to the conjunctival ectoderm in control eyes but also expressed in the corneal ectoderm in Pitx2tko mutant eyes. Keratocan 12 is expressed in the corneal ectoderm of control eyes but is absent from Pitx2-tko mutant eyes. Note: the FOXC2 signal in the lens is background artifact due to the inherent stickiness of crystalline proteins. Co, corneal ectoderm; Cn, conjunctival ectoderm.
PITX2 is required to maintain expression of Dkk2 in ocular neural crest
We have previously demonstrated that in neural crest during early anterior segment morphogenesis PITX2 is required for activation of Dkk2, which encodes an extracellular antagonist of canonical Wnt signaling, (Gage et al., 2008). Dkk2 expression is activated in Pitx2-tko embryos prior to tamoxifen injection due to the initial presence of PITX2 protein in these embryos (data not shown). Since the previous experiments did not determine whether PITX2 is also required for maintenance of Dkk2 expression, we examined Dkk2 expression in Pitx-tko eyes following tamoxifen injection. We found that in mutant eyes Dkk2 mRNA is undetectable by e14.5 (Figure 8C). As a consequence, canonical Wnt signaling activity levels as measured by Axin2 expression become elevated in Pitx2-tko eyes (Figure 8D). Collectively, we conclude that functional PITX2 is required to both activate and maintain Dkk2 expression. The absence of DKK2 results in elevated canonical Wnt signaling activity levels, providing a potential mechanism contributing to the observed morphological and gene expression changes in the developing corneas of Pitx2-tko eyes.
Figure 8. Expression of canonical Wnt pathway genes is altered during corneal development in Pitx2-tko mice.
In situ hybridization was used to detect expression of the Dkk2 (A,C) and Axin2 (B,D) mRNA at e14.5. Dkk2 expression is not maintained in the corneal mesenchyme while Axin2 expression is elevated in both the corneal surface ectoderm and underlying mesenchyme of Pitx2-tko eyes. E, corneal ectoderm; M, corneal mesenchyme.
Loss of Dkk2 expression accounts for a subset of corneal phenotypes in PItx2-tko mice
We examined developing corneas of Dkk2-null mice and found they exhibit morphological changes that are similar to those present in Pitx-tko mutant corneas, including disruption of normal organization within the stromal and endothelium layers of the mesenchyme, and modest thickening of the overlying ectoderm (Figure 9A,E) (Gage et al., 2008). An important difference between Pitx2-tko and Dkk2−/− mice, however, is the degree of corneal angiogenesis. While ectopic CD31+ blood vessels are present in the peripheral cornea of Dkk2-null eyes, in a position overlying the ciliary body and where blood vessels are not found in wild type controls (Figure 9C,F), unlike what is observed in Pitx2-tko eyes, these blood vessels do not extend into the central cornea (Figure 9B,F). Further, in the Dkk2−/− eyes, there is no evidence of LYVE1+ lymphatic vessels in the cornea (Figure 9D,H). Collectively, these results suggest that corneal morphogenesis is disrupted but the loss of angiogenic privilege in Pitx2-tko eyes is only partly caused by decreased Dkk2 expression.
Figure 9. Altered morphogenesis and relaxed angiogenic privilege in Dkk2 mutant mice.
Control and Dkk2 mutant sections taken from e16.5 embryos stained with H&E. Comparable sections were immunostained to detect CD31 or LYVE1. CD31+ blood vessels were ectopically located in the peripheral cornea adjacent to the ciliary body but did not extend towards the central cornea. Panels C and F are magnified images of the boxed areas in panels B and F, respectively. C, cornea; CB, ciliary body.
Next, we examined the corneal and conjunctival markers that were altered in Pitx2-tko mice to determine whether they were sensitive to loss of DKK2 and the associated changes in canonical Wnt signaling activity levels. We began with the markers whose expression patterns distinguish between differentiating cornea and conjunctival mesenchyme. Interestingly, the expression patterns of FOXC2, PITX1, Twist, and Keratocan were unaltered in Dkk2 mutant eyes relative to wild type controls (Figure 10). From these results, we infer that loss of DKK2 and the associated increase in canonical Wnt signaling activity is unlikely to account for the changes in expression of these genes present in PItx2-tko eyes. We also examined the early conjunctival ectoderm markers, FOXC2 and BARX2 in control and mutant embryos at e14.5 (Figure 11A,B). In contrast to Pitx2-tko embryos, neither protein is expressed in the corneal ectoderm of Dkk2−/− embryos at this same stage (Figure 11E,F). Despite the lack of an effect on the early ectodermal markers in the Dkk2−/− embryos, at later timepoints, expression of the corneal marker keratin 12 is lost while expression of the conjunctival marker keratin 4 is activated in the anatomical corneal ectoderm of Dkk2−/− embryos. Collectively, these data suggest that loss of DKK2 expression in the mesenchyme biases cells within the overlying central ocular surface ectoderm toward conjunctival rather than corneal fates. However, the expanded expression of FOXC2 and BARX2 observed in Pitx2-tko embryos is unlikely to contribute this fate switch since expression of these transcription factors is unchanged in Dkk2 mice.
Figure 10. Analysis of mesenchymal marker expression in control and Dkk2 mutant eyes.
Immunohistochemistry was used on control and Dkk2 mutant eyes at e12.5 to detect FOXC2 and PITX1 protein. In situ hybridization was used on embryos of the same genotypes at e14.5 to detect expression of Twist1 and Keratocan mRNA. All markers were unchanged in Dkk2 mutant eyes relative to controls. Note: the FOXC2 signal in the lens is background artifact due to the inherent stickiness of crystalline proteins. Co, corneal mesenchyme; Cn, conjunctival mesenchyme; L, lens.
Figure 11. Analysis of surface ectoderm marker expression in control and Dkk2 mutant eyes.
Immunohistochemistry was used on control and Dkk2 mutant eyes to detect FOXC2 and BARX2 at e14.5, and cK12 and cK4 at e18.5. FOXC2 and PITX2 expression was unchanged in mutant eyes while cK12 and cK4 phenocopied the expression changes identified in Pitx2-tko eyes. Cn, conjunctiva ectoderm; Co, corneal ectoderm; L, lens.
DISCUSSION
We have used a temporal knockout approach to reveal a requirement for the homeodomain transcription factor PITX2 in multiple steps of corneal development. In the absence of PITX2, corneal morphogenesis and the specification of cell fates within both the surface ectoderm and mesenchymal primordia are disrupted. PITX2 is also required to establish angiogenic privilege in the developing cornea. Control of canonical Wnt signaling activity levels as a result activation of the downstream target Dkk2 in the underlying mesenchyme appears to be a key mechanism by which PITX2 governs the correct specification of central ocular surface ectoderm to a corneal epithelial fate. In contrast, the correct specification of the underlying mesenchymal cells to corneal fates and establishing angiogenic privilege appear to be less sensitive to loss of DKK2 activity. We identify several transcription factors whose patterns in Pitx2 and Dkk2 mutants suggest that they may be involved in cell fate specification and establishing angiogenic privilege within the presumptive corneal mesenchyme but are unlikely to play a role in cell fate specification within the corneal ectoderm.
Normal corneal development requires a set of transcription factors in the neural crest mesenchyme
The current work extends our understanding of the transcriptional code that is required for normal corneal development. LMX1b encodes a homeodomain transcription factor that is expressed in the ocular neural crest beginning early in eye development and is associated with Nail-Patella Syndrome (McIntosh et al., 1998, Pressman et al., 2000, Vollrath et al., 1998). Although human patients are not known to exhibit corneal defects, Lmx1b knockout mice have defects in development of the corneal stroma (Pressman et al., 2000). Heterozygous mutations in FOXC1, encoding a forkhead transcription factor are a second cause of Axenfeld-Rieger Syndrome (Lehmann et al., 2000). Like Pitx2, expression of Foxc1 in ocular neural crest mesenchyme depends on retinoic acid signaling. Development of the corneal stroma and endothelium is impaired in Foxc1 knockout mice (Kidson et al., 1999). In addition, Foxc1 is required both to establish and maintain angiogenic privilege within the cornea (Seo et al., 2012).
A striking feature of these three transcription factors is that none are specifically limited to the corneal mesenchyme during eye development. Indeed, all three are also co-expressed within neural crest fated to form additional tissues such as the conjunctival mesenchyme, the schlera, and structures within the iridocorneal angle. Therefore, although each is required, the ability to specify corneal fates is not uniquely encoded by any one of these three factors or in combination. This raises the possibility that they cooperate together with one or more additional transcription factors that are themselves specific to the corneal mesenchyme. Alternatively, there may be other transcription factors whose expression patterns encompass more than the cornea but the corneal mesenchyme is the unique site where they are expressed together with the FOXC1, LMX1B, and PITX2.
Surface ectoderm is more sensitive to increases in canonical Wnt signaling activity levels
Our data provide new insights in the relative sensitivities of the surface ectoderm and underlying mesenchyme within the presumptive cornea to Dkk2 expression and the resulting suppression of canonical Wnt signaling activity levels. Ablation of Dkk2 is sufficient to cause an apparent switch from a corneal fate to a conjunctival fate within the surface ectoderm, as evidenced by the absence of the mature corneal marker, keratin 12 and the presence of the mature conjunctival marker, keratin 4. In contrast, none of the mesenchymal markers examined were altered in presumptive corneas of Dkk2 mutant mice and angiogenic privilege was only partially affected as compared to Pitx2 mutant corneas.
The difference in sensitivities to changes in canonical Wnt signaling activity levels between the surface ectoderm and mesenchyme within the presumptive cornea mirrors the expression patterns of both Wnt ligands and receptors. A minimum of four genes encoding Frizzled receptor proteins are expressed within the surface ectoderm of the developing cornea, meaning that this layer is likely to be highly competent to respond to available Wnt ligands (Liu et al., 2003). In contrast, only Frizzled 7 appears to be expressed within the underlying mesenchyme during corneal development, suggesting that these cells may be less responsive to Wnt ligand (Liu et al., 2003). The surface ectoderm is also the major source of available Wnt ligand within the region (Liu et al., 2003). In contrast, the underlying neural crest mesenchyme is the source of DKK2 (Gage et al., 2008). Collectively, these observations indicate that, during normal corneal development, paracrine inhibition by DKK2 produced within the neural crest is required to suppress the autocrine effect of Wnt ligand on the surface ectoderm.
The mechanism(s) underlying the broader phenotype in the absence of PITX2 relative to DKK2 are currently unclear and will require more investigation. Recently, we have developed preliminary data suggesting that Dkk1 expression is also dramatically reduced in the absence of PITX2 (Chen et al, preliminary results). This suggests the possibility that canonical Wnt signaling activity levels are more elevated in the Pitx2 mutants since the response would be the sum of losing both DKK1 and DKK2. If true, the higher activity levels may be sufficient to change fate choices by the mesenchyme and alter establishment of angiogenic privilege in a way that the presumably lesser activity levels Dkk2 mutant corneas do not. An additional possibility that is not mutually exclusive is that fate determination within the mesenchyme and establishing angiogenic privilege may be dependent on input from other signaling pathways such as Nodal, FGF, TGFβ, and BMP, all of which are active within the developing anterior segment (Govindarajan et al., 2000, Saika et al., 2001, Zhao et al., 2002).
Early transcription factor markers could play role in specification of corneal mesenchyme or establishing angiogenic privilege
We have identified several markers whose expression patterns within surface ectoderm and mesenchyme distinguish between corneal and conjunctival fates early during the partitioning of these two tissues. These markers represent important new tools for analysis of fates, particularly at early stages of the process when these patterns proteins are differentially expressed. As each of these genes encodes a transcription factor, it raises the question whether their regionalized expression patterns may be functionally important for partitioning the surface ectoderm or mesenchyme into corneal versus conjunctival fates. For example, is exclusion of the normally conjunctival-specific expression of FOXC2, PITX1, and BARX2 required for normal development of the corneal ectoderm and mesenchyme, and for establishing angiogenic privilege? The expansion of FOXC2 expression into the cornea is particularly interesting with respect to establishing angiogenic privilege since this transcription factor is well known to promote angiogenesis under other normal and pathological conditions, including cancer. Interestingly, alterations in functional levels of the highly related family member, FOXC1 have recently been shown to promote loss of angiogenic privilege in the cornea (Seo et al., 2012).
Pitx2 function in later developing tissues and mature structures
Schlemm’s canal and the trabecular meshwork within the iridocorneal angle begin to differentiate towards the end of corneal development and congenital defects in Pitx2-attribultable Axenfeld-Rieger patients suggest the likelihood that PITX2 is required for development of these structures, in addition to the cornea. Pitx2 is highly expressed within these structures during eye development, lending support for functional significance (data not shown). Long-term expression within the mature corneal stroma and endothelium, Schlemm’s canal, and trabecular meshwork also raises the possibility that PITX2 may play essential roles in maintenance of cell fate and renewal within these structures. Application of the temporal knockout strategy employed here at later stages of eye development and in mature animals will now allow these important questions that were not previously experimentally accessible to be addressed.
Experimental Procedures
Mouse strains and animal husbandry
All experiments were conducted in accordance with the NIH Guidelines for the Care and Use of Experimental Animals and all procedures involving mice were approved by the University of Michigan Committee on Use and Care of Animals. Generation of the Pitx2null+, Pitx2flox, UBC-CreERT2, and Dkk2null mouse strains has been described previously (Gage et al., 1999, Gruber et al., 2007, Li et al., 2005, Ruzankina et al., 2007). Mice were mated to generate timed pregnancies. The relevant crosses are UBC-CreERT2; Pitx2+/null × Pitx2flox/flox; R26R/R26R and Dkk2+/null × Dkk2+/null. If indicated, a single intraperitoneal injection of tamoxifen (Sigma) suspended in corn oil at a dose of 100 mg per gram body weight was administered to the pregnant dam at noon on the day noted. The resulting embryos were genotyped for Cre or Pitx2 using PCR-based methods (Evans and Gage, 2005a, Suh et al., 2002), and processed for histology as previously described (Evans and Gage, 2005a).
Embryo processing and histology
All embryos were fixed in 4% paraformaldehyde diluted in PBS, washed in PBS, dehydrated though graded alcohols, and processed into Paraplast Plus (McCormick Scientific; St. Louis, MO) for paraffin sectioning. Mounted paraffin sections for morphological analysis were dewaxed, rehydrated, and stained with hematoxylin and eosin.
Immunostaining & RNA In Situ Hybridization
Paraffin sections were immunostained as previously described (Evans and Gage, 2005a). Primary antibodies against PITX2 (gift from T. Hjalt, Lund University), CD31 (BD Pharminogen), LYVE-1 (Abcam), FOXC2 (Abcam), PITX1 gift from Jacques Drouin, International Cancer Research Center of Montreal), BARX2 (Santa Cruz Biotechnology), keratin 4 (Abcam), and keratin 12 (gift from Winston Kao, University of Cincinnati) were used. Digoxigenin-labeled riboprobes against Pitx2, Dkk2 (gift from Gary Hammer, University of Michigan), Axin2 (gift from Sally Camper, University of Michigan), Twist1, and Keratocan (gift from Winston Kao, University of Cincinnati) were generated and used to stain paraffin sections as previously described (Cushman et al., 2001, Martin et al., 2002).
ACKNOWLEDGEMENTS
The authors thank Millie Mo and Mie Kasanuki for technical assistance. The authors also thank Lisheng Chen and Peter Hitchcock for helpful discussions and for comments on the manuscript. This work was supported by the National Eye Institute of the National Institutes of Health (EY007003, EY014126) (PJG).
REFERENCES
- Asai-Coakwell M, Backhouse C, Casey RJ, Gage PJ, Lehmann OJ. Reduced human and murine corneal thickness in an Axenfeld-Rieger syndrome subtype. Invest Ophthalmol Vis Sci. 2006;47:4905–4909. doi: 10.1167/iovs.06-0457. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Watkins-Chow DE, Brinkmeier ML, Raetzman LT, Radak AL, Lloyd RV, Camper SA. Persistent Prop1 expression delays gonadotrope differentiation and enhances pituitary tumor susceptibility. Hum Mol Genet. 2001;10:1141–1153. doi: 10.1093/hmg/10.11.1141. [DOI] [PubMed] [Google Scholar]
- Cvekl A, Tamm ER. Anterior eye development and ocular mesenchyme: new insights from mouse models and human diseases. Bioessays. 2004;26:374–386. doi: 10.1002/bies.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005a;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Human molecular genetics. 2005b;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Qian M, Wu D, Rosenberg KI. The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol. 2008;317:310–324. doi: 10.1016/j.ydbio.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt TA. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, Ito M, Makarenkova HP, Lang RA, Overbeek PA. Endogenous and ectopic gland induction by FGF-10. Dev Biol. 2000;225:188–200. doi: 10.1006/dbio.2000.9812. [DOI] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidson SH, Kume T, Deng K, Winfrey V, Hogan BL. The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev Biol. 1999;211:306–322. doi: 10.1006/dbio.1999.9314. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM. Migration and differentiation of neural crest cells. In: Moscona A, Monroy A, editors. Current topics in development, part II. New York: Academic Press; 1980. [DOI] [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Lehmann OJ, Ebenezer ND, Jordan T, Fox M, Ocaka L, Payne A, Leroy BP, Clark BJ, Hitchings RA, Povey S, Khaw PT, Bhattacharya SS. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet. 2000;67:1129–1135. doi: 10.1016/s0002-9297(07)62943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, Hurley M, Guo C, Boskey A, Sun L, Harris SE, Rowe DW, Ke HZ, Wu D. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet. 2005;37:945–952. doi: 10.1038/ng1614. [DOI] [PubMed] [Google Scholar]
- Liu CY, Shiraishi A, Kao CW, Converse RL, Funderburgh JL, Corpuz LM, Conrad GW, Kao WW. The cloning of mouse keratocan cDNA and genomic DNA and the characterization of its expression during eye development. J Biol Chem. 1998;273:22584–22588. doi: 10.1074/jbc.273.35.22584. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Martin DM, Skidmore JM, Fox SE, Gage PJ, Camper SA. Pitx2 distinguishes subtypes of terminally differentiated neurons in the developing mouse neuroepithelium. Dev Biol. 2002;252:84–99. doi: 10.1006/dbio.2002.0835. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Mcintosh I, Dreyer SD, Clough MV, Dunston JA, Eyaid W, ROIG CM, Montgomery T, Ala-Mello S, Kaitila I, Winterpacht A, Zabel B, Frydman M, Cole WG, Francomano CA, Lee B. Mutation analysis of LMX1B gene in nail-patella syndrome patients. Am J Hum Genet. 1998;63:1651–1658. doi: 10.1086/302165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearton DJ, Yang Y, Dhouailly D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc Natl Acad Sci U S A. 2005;102:3714–3719. doi: 10.1073/pnas.0500344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman CL, Chen H, Johnson RL. LMX1B, a LIM homeodomain class transcription factor, is necessary for normal development of multiple tissues in the anterior segment of the murine eye. Genesis. 2000;26:15–25. [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGFbeta2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, Siegel-Bartelt J, Bierke-Nelson D, Bitoun P, Zabel BU, Carey JC, Murray JC. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996;14:392–399. doi: 10.1038/ng1296-392. [DOI] [PubMed] [Google Scholar]
- Seo S, Singh HP, Lacal PM, Sasman A, Fatima A, Liu T, Schultz KM, Losordo DW, Lehmann OJ, Kume T. Forkhead box transcription factor FoxC1 preserves corneal transparency by regulating vascular growth. Proc Natl Acad Sci U S A. 2012;109:2015–2020. doi: 10.1073/pnas.1109540109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H, Gage PJ, Drouin J, Camper SA. Pitx2 is required at multiple stages of pituitary organogenesis: pituitary primordium formation and cell specification. Development. 2002;129:329–337. doi: 10.1242/dev.129.2.329. [DOI] [PubMed] [Google Scholar]
- Thut CJ, Rountree RB, Hwa M, Kingsley DM. A large-scale in situ screen provides molecular evidence for the induction of eye anterior segment structures by the developing lens. Dev Biol. 2001;231:63–76. doi: 10.1006/dbio.2000.0140. [DOI] [PubMed] [Google Scholar]
- Vollrath D, Jaramillo-Babb VL, Clough MV, Mcintosh I, Scott KM, Lichter PR, Richards JE. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091–1098. doi: 10.1093/hmg/7.7.1091. [DOI] [PubMed] [Google Scholar]
- Zacharias AL, Lewandoski M, Rudnicki MA, Gage PJ. Pitx2 is an upstream activator of extraocular myogenesis and survival. Developmental biology. 2011;349:395–405. doi: 10.1016/j.ydbio.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Chen Q, Hung FC, Overbeek PA. BMP signaling is required for development of the ciliary body. Development. 2002;129:4435–4442. doi: 10.1242/dev.129.19.4435. [DOI] [PubMed] [Google Scholar]