Abstract
Plant sterols have shown potent anti-proliferative effects and apoptosis induction against breast and prostate cancers. However, the effect of sterols against hepatic cancer has not been investigated. In the present study, we assessed whether the stigmasterol isolated from Navicula incerta possesses apoptosis inductive effect in hepatocarcimona (HepG2) cells. According to the results, Stigmasterol has up-regulated the expression of pro-apoptotic gene expressions (Bax, p53) while down-regulating the anti-apoptotic genes (Bcl-2). Probably via mitochondrial apoptosis signaling pathway. With the induction of apoptosis caspase-8, 9 were activated. The DNA damage and increase in apoptotic cell numbers were observed through Hoechst staining, annexin V staining and cell cycle analysis. According to these results, we can suggest that the stigmasterol shows potent apoptosis inductive effects and has the potential to be tested as an anti-cancer therapeutic against liver cancer. [BMB Reports 2014; 47(8): 433-438]
Keywords: Apoptosis, Bcl-2 family, Caspase8, 9, Marine microalgae, Navicula incerta, Stigmasterol
INTRODUCTION
As photoautotrophs, the simple growth requirements of microalgae make them attractive source for bioprocessing, especially aimed at sustainable production of high value-added compounds which could attract large demand by the pharmaceutical industry (1, 2). Moreover, due to their culturability, continuous supply of these compounds could be ensured. Benthic diatom Navicula incerta is a phytoplankton mainly used in aquaculture. In a previous study conducted in our laboratory, we have characterized its protein fraction for the anti-oxidant activity and alcohol-induced liver protection on the HepG2 cells (3). The current study was aimed to isolate bioactive metabolites from Navicula incerta organic extracts.
The plant-derived phytosterols are structurally similar to cholesterol with a double bond at the C5-6 position (4). Several previous studies reported that phytosterol has apoptosis inductive effects in some kinds of carcinoma cell lines such as human colon cancer cell (HT-29), human prostate cancer cell (LNCaP), human breast cancer cell (MDA-MB-231) (5-8). Stigmasterol is also belongs to the group of phytosterol. In this experiment, we investigated the ability of stigmasterol to induce apoptosis in human hepatocarcinoma cell model.
The incidents of liver cancer are increasing due to food habits, stress and insufficient exercise. Liver cancer is very difficult to cure as currently existing treatment methods include surgery, chemotherapy, radiation, immunotherapy and monoclonal antibody therapy. Except surgery, most of the cancer therapy methods involves in inhibition of cancer cells growing. Induction of apoptosis in cancer cells would play an active role in elimination of cancer cells (9). Apoptosis is a normal physiological process of cell death which maintains the homeostasis of healthy tissue by removing unwanted cells. However cancer cells escape the process of apoptosis and here by increase the cell proliferation rate ultimately leading to tumor formation. However, the main aim of cancer therapy is to control the cancer cell growth without causing damage to normal cells (9, 10). Therefore, induction of apoptosis selectively in cancer cells is considered as one of the strong cancer preventive strategy.
The mechanisms of apoptosis are highly complex and sophisticated, involving an energy-dependent cascade of molecular events. There are two main apoptotic pathways: the extrinsic and intrinsic pathway (11-13). In the intrinsic pathway, is regulated by activation or deactivation of Bcl-2 family proteins. In response to signals generated by variety of genotoxic stress such as DNA damage, p53, a tumor suppressor gene is activated. This activation induces the expression of Bax and sub sequent proteins which leads to activation of caspase cascade. Bcl-2 of Bcl-2 family is an anti-apoptotic gene which functions to inhibit apoptotic process and hence highly expressed in cancer cells. Apoptotic inducing therapeutics mainly targets to suppress the Bcl-2 levels and induce Bax expression levels.
In this study efforts have been taken to characterize stigmasterol isolated from Navicula incerta organic extract for its apoptotic inducing effects in hepatocarcinoma cell model. Moreover, experiments have been conducted to elucidate the underlying mechanism of stigmasterol induced apoptosis.
RESULTS AND DISCUSSION
Purification and structure elucidation of stigmasterol
Several peptides isolate from N. incerta has shown potent anti-oxidant and alcohol-induced liver protection in HepG2 cell line (3). However, the presence of biologically active compounds in N. incerta has not been analyzed before. Therefore in this study, focus was given to isolated and characterizes bioactive compounds from N. incerta. As depicted in Fig. 1A, fraction 2 (20.313 g), extracted from N. incerta (50 g) by MeOH : CH2CH2 1:1 solvent was gain fractionated by silica gel open column chromatography (into 11 fractions (Fr.2-1 – Fr.2.11)). Among them, Fr. 2.3 and 2.4 were mixed and further purified to isolate stigmastrol.
Fig. 1. (A) Isolation profiles of stigmastsrol from Navicula incerta extract, (B) Structure of stigmasterol isolated from Navicula incerta. (C) Cell viability of HepG2 cells treated with stigmasterol for 24 h. Cells were cultured in serum-free media. After that HepG2 cells were treated with different concentrations (5, 10, 20 uM) of stigmasterol for 24 h and cell viability was assessed by the MTT assay. a-cDifferent letters on each bar indicates significant difference (P < 0.05) according to Duncan’s multiple range test.
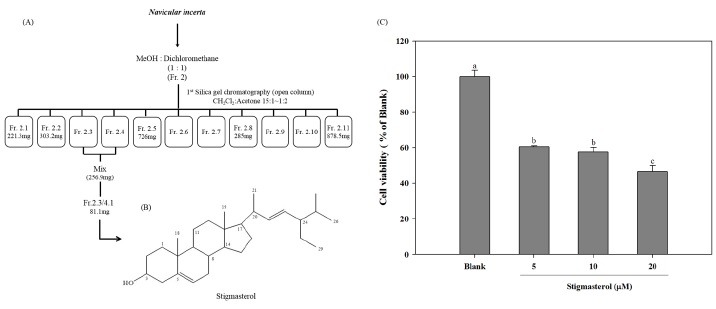
Pooled Fr. 2.3 and 2.4 (256.9 mg) were separated on silica gel, eluting with hexane : ethyl acetate (10 : 1) to obtain 5 fractions; Fr. 2.3/4.1 (81.1 mg), Fr. 2.3/4.2 (64.2 mg), Fr. 2.3/4.3 (19.8 mg), Fr. 2.3/4.4 (10.3 mg), Fr. 2.3/4.5 (15.7 mg). From these fractions, Fraction 2.3/4.1 was further purified with preparative thin layer chromatography (PTLC) eluted with chloroform. Recrystallization of Fr. 2.3/4.1 from chloroform gave white solid (17.2 mg) of stigmasterol (Stigmasta-5,22- dien-3-ol): white solid; 1H NMR (CDCl3, 400 MHz) δ 0.67-2.32 [44H, m, containing 1.01 (3H, s, CH3), 0.81 (3H, s, CH3)], 5.36 (1H, d, J = 5.2 Hz, H-6), 5.15 (1H, m, one of 22-H or 23-H), 5.02 (1H, m, one of 22-H or 23-H), 3.53 (1H, m, 3-H); 13C NMR (CDCl3, 100 MHz) δ 140.7 (C-5), 138.3 (C-23), 129.3 (C-22), 121.7 (C-6), 71.8 (C-3), 56.8 (C-14), 55.9 (C-17), 51.2 (C-24), 50.1 (C-9), 42.3 (C-13), 42.2 (C-20), 40.5 (C-4), 39.7 (C-12), 37.2 (C-10), 36.5 (C-2), 31.9 (C-8), 31.9 (C-25), 31.9 (C-1), 31.6 (C-7), 28.9 (C-15), 25.4 (C-16), 24.4 (C-28), 21.2 (C-11), 21.2 (C-18), 21.1 (C-27), 19.4 (C-19), 19.0 (C-21), 12.2 (C-26), 12.0 (C-29); LREIMS m/z 412 [M]+ (2). C29H48O (M.W: 412.00).
Stigmasterol isolated from marine microalgae, Navicula incerta belongs to the family of phytosterols. It has a double bond on the side chain and consists of three cyclohexane rings and one cyclopentane ring (Fig. 1B). According to the chemical formula, the C29H48O is evolved from the family of cholesterol. These kinds of phtytosterols are known to have an inductive effect of apoptosis in breast cancer, prostate cancer and human colon cancer cells (5,6,8,9).
Effect of stigmasterol on HepG2 cell viability
In order to evaluate the effect of stigamsterol isolated from N. incerta on HepG2 cells, the HepG2 cells were treated with different concentrations of stigmasterol. Stigmasterol has shown a significant toxicity on HepG2 cells in a dose-dependent manner, approximately 40%, 43% and 54% toxicity at concentrations of 5, 10 and 20 μM, respectively (Fig. 2). According to the results of cell viability test, the isolated compound, stigmasterol has shown potent cytotoxicity against in HepG2 cells. Previous studies have also shown anti-proliferative effect of β-sitosterols belonging to the group of phytosterol in several human cancer cells at similar concentrations (16 μM) (6,7,17,18).
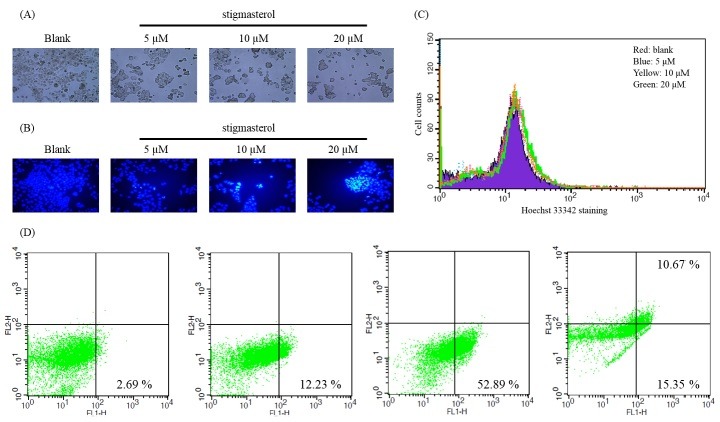
Fig. 2. (A) Morphological changes of stigmasterol treated HepG2 cells. For observation of morphological changes, cultured HepG2 cells were treated with stigmasterol for 24 h and morphological changes were detected under a light microscope (viewed at magnification of 100×). (B) Fluorescence micrographs showing the stigmasterol induced DNA damage. Stigmasterol treated cells were stained with Hoechst 33342 dye and detected under fluorescence microscope (viewed at magnification of 400×). The blue fluorescence in the nucleus indicates DNA fragmentation. (C) Cell numbers were counted using FACS after Hoechst 33342 staining. (Red; blank, Blue; 5 μM, Yellow; 10 μM, Green; 20 μM) (D) Flow cytometric analysis of the effect of stigmasterol in HepG2 cells experimented using Annexin V-PI staining assay.
It has been reported that the double bonds in phytosterols are responsible for apoptosis induction effect (16). Moreover, it is considered that the interaction occurs with mRNA and protein of the cells with double bonds of phytosterols in C-5, C-22 positions. We can suggest that stigmasterol may also show apoptotic induction effect due to the presence of these structural features.
Stigmasterol induces morphological changes and DNA damage in HepG2 cells
In the process of apoptosis, cells undergo morphological transformations including condensation of cytoplasm and nucleus, blebbing of cell membrane and DNA fragmentation. These fundamental changes in apoptotic cells can be observed through microscopic techniques.
As depicted in Fig. 3A, stigmasterol induced morphological changes after 24 h exposure to various concentrations (5, 10, 20 μM). According to the light microscopic results, the number of attached cells was remarkably reduced with increasing doses of stigmasterol and also changes in the cell membrane were observed. These results indicated that stigmasterol has toxic effects on HepG2 cells compared to non-treated blank group.
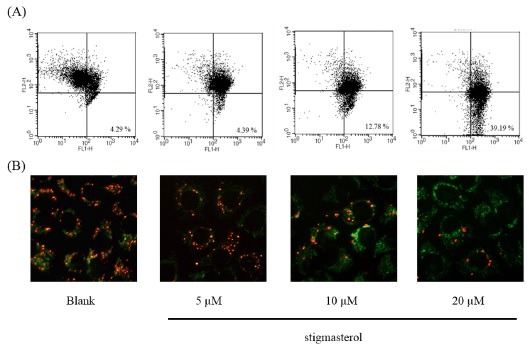
Fig. 3. Effect of stigmasterol on the mitochondrial membrane potential of HepG2 cells. (A) Fluorescence microscopic image of treated HepG2 cells stained with the MitoCaptureTM mitochondrial dye (Biovision). (B) Flow cytometric analysis of mitochondrial membrane potential.
In order to determine the effect of stigmasterol on the DNA damage, HepG2 cells were stained with Hoechst 33342 dye after 24 h of sample treatment and the DNA damages were observed under fluorescence microscope (Fig. 3B). The nuclear degradation of HepG2 cells was clearly observed under fluorescence light. The nuclei with chromatin concentration and apoptotic bodies were observed in the cells exposed to stigmasterol at higher concentrations. These observations suggest that stigmasterol may induce cell death in HepG2 cells through typical apoptotic pathway.
To evaluate Hoechst 33342 staining quantitatively, FACS analysis was performed. HepG2 cells were treated with stigmasterol for 24 h. then the cells were stained with Hoechst 33342 dye and were detected by fluorescence. As shown in graph (Fig. 2C), cell early apoptotic number is increasing in stigmasterol treated cell groups dose dependently. Upon these data, it is clear that stigmasterol may has induction activity of apoptosis on HepG2 cells.
Induction of apoptosis by stigmasterol in HepG2 cells analyzed by Annexin V-PI staining
To investigate the apoptotic effects of stigmasterol. HepG2 cells were treated with different concentrations of stigmasterol for 24 h and staining with Annexin V-PI dye. Phosphatidylserine-specific fluoprescene isothiocyanate (FITC)-Annexin V staining assay offers the possible of detecting early stage apoptosis before the loss of apoptosis cell death (19). Propidium iodide (PI) staining can be visible in late stage of apoptosis because the damaged or dead cells are permeable to PI. Early apoptotic cells are Annexin V positive and PI negative. Fig. 4A shows the contour diagrams of Annexin V and PI stained HepG2 cells by FACS after 24 h of incubation with different concentrations of stigmasterol. As you can see FACS analysis data, in lower right quadrants the cell population increases dose dependent manner. These results evaluate that the stigmasterol induce to apoptosis in HepG2 cells after 24 h treatment.
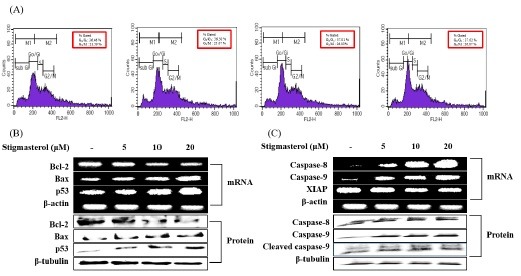
Fig. 4. Apoptotic inducing effect of stigmasterol in HepG2cells. Cells were treated with different concentrations of stigmasterol for 24 h. (A) Cell cycle progression patterns of HepG2 cells treated with stigmasterol. Stigmasterol treated cells were stained with PI and detected using FACS (FACS Calibur, BD Sciences, Heidelberg, Germany). (B) The expression levels of Bcl-2, Bax and p53, were detected using RT-PCR and western blot analysis, respectively. β-actin and β-tubulin were used as an internal standard. (C) The expression levels of caspase-8, -9 and XIAP were detected using RT-PCR and western blot analysis. β-actin and β-tubulin were used as an internal standard.
Induce apoptotic effect of stigmasterol through mitochondrial membrane potential in HepG2 cells
Mitochondrial membrane potential (MMP) has an important role in apoptosis process. MMP is a component of inducing force of mitochondrial electron transporter chain. Reducing of MMP is occurring by oxidative stress and secretion of cytochrome c which activates mitochondrial pathway of apoptosis. To investigate the inducing effect of stigmasterol, HepG2 cells were treated with stigmasterol in difference concentration (5, 10, 20 μM) for 24 h. As shown in Fig. 3, FACS anaylsis suggested that stigmasterol treatment increased the lower right quadratic cell population in FITC channel (FL1-H), compared to the blank group. This high green fluorescence is detected due to cytosolic monomers of the mitochondrial dye. Stigmasterol blocked the mitochondrial electron transtporter chain and reduces MMP. Due to altered MMP the dye could not penetrate into the mitochondria and remained in the cytosol as monomers. The monomers of MitoCaptureTM dye give high green fluorescence which is detected in the lower right quadrant of the FACS dot plot. The mitochondria-sensitive dye penetrates, aggregates in mitochondria and gives bright red fluorescence when mitochondria are healthy. Commonly the red fluorescence is detected in the PI (FL2-H) channel of FACS. The FACS analysis and the fluorescence figures clearly explain that with the stigmasterol treatment the number of green fluorescent cells were increased indicating the breakdown of MMP.
Effect of stigmasterol on the expression of apoptosis signaling molecules
To confirm expression levels of genes and proteins which are related with apoptosis pathway such as Bcl-2, Bax, caspase-8, 9, XIAP and p53 were examined. Activation of caspase is affected by Bcl-2 family proteins, which play an important role in intrinsic pathway (20, 21). The caspase-8, 9 members of caspase family is activated in apoptotic conditions and carries out the execution of the cell. The Bcl-2 family consists of both anti-apoptotic molecules, such as Bcl-2 and pro-apoptotic molecules, such as Bax, which are the most important effectors for characterization of apoptosis (22, 23). Tumor suppressor factor p53 regulate the Bcl-2 family proteins and leads to the progression of apoptosis. X-linked inhibitor of apoptosis protein (XIAP) stops apoptotic cell death that is induced either by viral infection or by overproduction of caspases. Negative regulation of XIAP is also one of the most effective ways to induce apoptosis for anticancer therapy. Therefore, we investigated the changing levels of these factors as an important regulators involved in apoptosis to examine the apoptotic effects of stigmasterol using RT-PCR and Western blot analysis.
According to the results of RT-PCR, stigmasterol induced the up-regulation of caspase-8, 9, Bax, and p53 expression levels in a dose-dependent manner. These results comply with the results of Western blotting. In Western blot results, caspase-8, 9, Bax and p53 levels are increasing in a dose-dependent manner. Bcl-2 and XIAP genes which are anti-apoptotic molecules are down-regulated in a dose-dependent manner by stigmasterol (Fig. 4A, B). According to the results, stigmasterol significantly activates the caspase cascade via intrinsic apoptotic pathway and thereby leading to cell death.
This study was involved in purification and characterization of stigmasterol from marine microalgae, Navicula incerta. The apoptotic inductive effect of stigmasterol was explored detail using hepatocarcinoma cell line HepG2. The cell death was resulted due to stigmasterol induced expression of pro-apoptotic gene expression while inhibiting the anti-apoptotic factors. The stigmasterol induced apoptosis was mediated through intrinsic mitochondrial pathway which leads to the activation of caspase cascade and subsequent cell death. Thus, it could be concluded that stigmasterol possess potential apoptosis inductive effects and with further studies it could be developed as a potential candidate for hepatic cancer treatment.
Flow cytometric measurement of stigmasterol induced cell cycle arrest
Cell cycle check points are one of the main parameters to determine apoptosis process in cancer cell lines. In the cell cycle, there are 4 main phases; S, G2/M, sub G1, G0/G1 phase. Among them, G0/G1 and G2/M phases are important check point in apoptotic cell. If there is any DNA damage the cell are not proceeding to the next generation and thus the life cycle of the cell is terminated (24, 25).
In order to check the effect of stigmasterol on cell cycle progression, HepG2 cells were cultured in 6 cm2 dishes treated with different concentrations (5, 10, 20 μM) of stigmasterol and cell cycle was analyzed using FACS. As shown in the results HepG2 cells were arrested at G2/M phase of the cell cycle due to stigmasterol treatment. We observed that the number of cells in G2/M phase was (26.87%) highest at the higher concentration (20 μM) of stigmasterol and also G0/G1 phase was (37.02%) highest at the same concentration (Fig. 3C). At key transitions during eukaryotic cell cycle progression, signaling pathways monitor the successful completion of upstream events prior to proceeding to the next phase. Defects in the cell components have resulted in the arrest of cell cycle at G0/G1 and G2/M check point which may allow the damaged cell to undergo apoptosis. These results confirm that the compound arrests the cell cycle and thereby leading to cell death.
MATERIALS AND METHODS
Materials and chemicals
For NMR spectroscopy (1H 400MHz, 13C 100MHz, JEOL JNMECP 400 NMR spectrometer), chloroform-D (Cambridge Isotope Laboratories, Inc., USA) was used as the solvent. Human liver hepatocellular carcinoma cell line (HepG2) was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cell culture media Dulbecco’s Modified Eagle Medium (DMEM), penicillin/streptomycin, fetal bovine serum (FBS) were purchased from Gibco BRL, Life Technology (NY, USA). 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All antibodies used for Western blot analysis were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Other chemicals and reagents used of analytical grade commercially available.
Preparation and isolation of stigmasterol from Navicula incerta
The bentic diatom, Navicula incerta (strain KMMCC B-001) used in this experiment was generously provided by Korea Marine Microalgae Culture Center. The sample was cultured using standard F/2 (Guillard’s) medium until the required biomass in obtained (2).
First, 50 g of N. incerta was extracted for 24 h in the room temperature with 3 L of methanol. After that, methanol extract was filtered and evaporated. (Fr. 1, 20 g). Then the microalgae was extracted again with 3 L of methanol : dichloromethane (1:1) (Fr. 2, 20 g). Final extraction was done with 3 L of dichloromethane (Fr. 3, 3 g). Methanol : dichloromethane (1:1) extract of (Fr. 2) N. incerta (20.313 g) was separated by silica gel column and eluted with Dichloromethane : acetone gradient (30:1 to 1:2) to obtain 11 fractions (Fig. 1A). Fr. 2.3/4 were selected for further purification. For this purification hexane : ethyl acetate solvent gradient was used.
Cell culture and cell viability assay
HepG2 cells were grown in DMEM media containing 10% FBS and 100 μg/mL streptomycin/penicillin, 5% CO2 at 37℃. To determine cell viability after exposure to different concentrations (5, 10 and 20 μM) of stigmasterol, MTT cell viability assay was performed. After 24 h of incubation with compound, 100 μL of MTT (1 mg/mL) was added and incubated again for 4 h. Then the formed formazan salt was dissoleved in 100 μL of DMSO and the absorbance was measured at 550 nm (GENiosⓇ microplate reader, Tecan Austria GmbH, Austria).
Morphological changes, Hoechst 33342 staining and cell counting by flow cytometry
HepG2 cells treated with stigmasterol were fixed in 4% paraformaldehyde (Sigma) in phosphate buffered saline (PBS) for 10 min at room temperature. The fixed cells were detected for morphological changes using a light microscope (CTR 6000; Leica, Wetzlar, Germany). After observation, the cells, they were stained with 1 μg/ml of the fluorescent DNA-binding dye, Bisbenzimide Hoechst 33342 (Sigma) and incubated for 1 h to reveal nuclear condensation/aggregation. The Hoechst- stained cells were visualized and photographed under fluorescence microscope (CTR 6000; Leica, Wetzlar, Germany).
For check cell numbers of Hoechst staining, HepG2 cells treated with stigmasterol were incubated 24 h in 6 cm2 dish at cell density of 1 × 106 cells/ml. After 24 h, cells were wash 2 times and were detached. Then cells were stained by Hoechst 33342 dye for 20 min. After that, cells were washed with PBS 2 times. The samples were then analyzed by a flow cytometry (FACS Calibur, BD Sciences, Heidelberg, Germany) within 1 h.
Annexin V-FITC and propidium iodide staining apoptosis tests
The HepG2 cells were cultured in 6 cm2 dish at a density of 5 × 105 and stigmasterol was treated at different concentrations. The cells were then incubated for 24 h. The subsequent procedures were carried out according to the manufacturer's manual provided with the Annexin V-FITC kit (BD Sciences). Briefly, the cells were washed 3 times with PBS and suspended in binding buffer at a concentration of 1 × 105 cells/mL followed by additions of FITC Annexin V and propidium iodide (PI) and left for 15 min in the dark. The samples were then analyzed by a flow cytometer (FACS Calibur, BD Sciences, Heidelberg, Germany) within 1 h.
Determination of mitochondrial membrane potential (MMP)
The MitoCaptureTM mitochondrial apoptosis kit (Biovision) was used to detect changes in mitochondrial membrane potential. Briefly, HepG2 cells were grown at a density of 1 × 106 cells/ml and apoptosis was induced by stigmasterol various concentrations with 24 h. A blank was incubated with the vehicle without induction, concurrently. The assay was conducted according to manufacturer’s guidelines.
RNA extraction and reverse-transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from HepG2 cells treated with and without stigmaterol for 24 h using TRIzolⓇ reagent (Invitrogen Co., CA, USA) as reported in manufacturer’s manual (14). The assay used for RT-PCR described by Lee et al. (26) was modified by increasing cycles and annealing time. The target cDNA was amplified using the following primers (refer to supplementary Table 1). The amplification was carried out at 95℃ for 45 s, 60℃ for 1 min and 72℃ for 45 s. after 30 cycles, the PCR products were electrophoresed on 1.5% agarose gel stained with ethidium bromide and visualized under UV light, using AlphaEaseⓇ gel image analysis software (Alpha innotech, CA, USA).
Western blot analysis
Western blotting was performed according to standard procedures (16). Cells with stigmasterol were lysed in RIPA buffer (Sigma-aldrich Corp., St. Louis, MO, USA). Proteins lysed from cells were separated using a 10% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane (Amersham Pharmacia Biotech., England, UK). The membrane was blocked for 2 h with 3% of BSA. The respective proteins were detected with a chemiluminescent ECL assay kit (Amersham Pharmacia Biosciences, NJ, USA). The Western blot bands were visualized using a LAS-3000 system and quantified by Multi Gauge V3.0 software (Fujifilm Life Science, Tokyo, Japan).
Flow cytometry analysis for measurement of cell cycle arrest
The cells were harvested and fixed in ice-cold 70% ethanol and stored at 4℃. Prior to analysis, the cells were suspended in 1 ml of cold propidium iodide solution containing 50 μg/ml RNase A, 250 μg/ml PI and further incubated on ice for 20 min in the dark. Flow cytometric analyzes were carried out using a flow cytometer (FACS Calibur, BD Sciences, Heidelberg, Germany) and Cell Quest software was used to distinguish phases of the cell cycle.
Statistical analysis
Each value was expressed as means ± S.E.M (n = 3). The statistical significance of differences was analyzed by ANOVA using SAS (Chicago, IL, USA).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported by a grant from Marine Bioprocess Research Center of the Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Republic of Korea
References
- 1.Spolaore P., Joannis-Cassan C., Duran E., Isambert A. Commercial applications of microalgae. J. Biosci. Bioeng. (2006);101:87–96. doi: 10.1263/jbb.101.87. [DOI] [PubMed] [Google Scholar]
- 2.Plaza M., Herrero M., Cifuentes A., Ibanez E. Inovative natural functional ingredients from Microalgae. J. Agric. Food Chem. (2009);57:7159–7170. doi: 10.1021/jf901070g. [DOI] [PubMed] [Google Scholar]
- 3.Kang K. H., Qian Z. J., Ryu B., Kim D., Kim S. K. Protective effects of protein hydrolysate from Marine Microalgae Navicula incerta on ethanol-induced toxicity in HepG2/CYP2E1 cells. Food Chem. (2011);132:677–685. doi: 10.1016/j.foodchem.2011.10.031. [DOI] [Google Scholar]
- 4.Ryan E., McCarthy F., Maguire A., O’Brien N. Phytosterol oxidation products: their formation, occurance and biological effects. Food Rev. Int. (2009);25:157–174. doi: 10.1080/87559120802682797. [DOI] [Google Scholar]
- 5.Awad A. B., Fink C. S. Phytosterols as anticancer dietary components: evidence and mechanism of action. J. Nutr. (2000);130:2127–2130. doi: 10.1093/jn/130.9.2127. [DOI] [PubMed] [Google Scholar]
- 6.Von Holtz R. L., Fink C. S., Hennessey T. β-Sitosterol sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr. Cancer. (1998);32:8–12. doi: 10.1080/01635589809514709. [DOI] [PubMed] [Google Scholar]
- 7.Downie A., Fink C. S., Awad A. B. Effect of phytosterols on MDA-MB-231 human breast cancer cell growth. FASEB J. (1999);13:A333. (abs.) [Google Scholar]
- 8.Yvonne C. O’Callaghan, David A. Foley, Niamh M. O’Connell, Florence O. McCarthy, Anita R. Maguire, Nora M. O’brien. Cytotoxicity and apoptosis effects of the oxidized derivatives of stigmasterol in the U937 human monocytic cell line. J. Agric. Food Chem. (2010);58:10793–10798. doi: 10.1021/jf1023017. [DOI] [PubMed] [Google Scholar]
- 9.Raicht R. F., Cohen L. I., Fazzini E. P., Sarwal A. N., Takahashi M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res. (1980);40:403–405. [PubMed] [Google Scholar]
- 10.Hetz C. A., Torres V., Quest A. F. Beyond apoptosis: non-apoptotic cell death in physiology and disease. Biochem. Cell Biol. (2005);83:579–588. doi: 10.1139/o05-065. [DOI] [PubMed] [Google Scholar]
- 11.Gerl R., Vaux D. L. Apoptosis in the development and treatment of cancer. Carcinogenesis. (2005);26:263–270. doi: 10.1093/carcin/bgh283. [DOI] [PubMed] [Google Scholar]
- 12.Haimovitz-Friedman A., Kan C. C., Enleiter D., Persaud R. S., McLoughlin M., Fuks Z., Kolesnick R. N. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. (1994);180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santana P., Pena L. A., Haimovitz-Friedman A. Acid sphingomyelinase deficient lymphoblasts and mice are defective in radiation induced apoptosis. Cell. (1996);86:189–199. doi: 10.1016/S0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 14.Narula J., Kharbanda S., Khaw B-A. Apoptosis and the heart. Chest. (1997);112:1358–1362. doi: 10.1378/chest.112.5.1358. [DOI] [PubMed] [Google Scholar]
- 15.Himaya S. W. A., Ryu B., Qian Z. J., Li Y., Kim S. K. 1-(5-bromo-2-hydroxy-4-methoxyphenyl)ethanone [SE1] suppresses pro-inflammatory responses by blocking NF-κB and MAPK signaling pathways in activated microglia. Eur. J. Pharmacol. (2011);670:608–616. doi: 10.1016/j.ejphar.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Ryu B., Li Y., Qian Z. J., Kim M. M., Kim S. K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. Biol. Interact. (2009);179:192–201. doi: 10.1016/j.cbi.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Awad A. B., Chen Y. C., Fink C. S., Hennessey T. Beta-sitosterols inhibits HT-29 human colon cancer cell growth and alters membrane lipids. Anticancer Res. (1996);16:2797–2804. [PubMed] [Google Scholar]
- 18.Trouillas P., Corbiere C., Liagre B., Duroux J. L., Beneytout J. L. Structure-function relationship for saponin effects on cell cycle arrest and apoptosis in the human 1547 osteosarcoma cells: a molecular modeling approach of natural molecules structurally close to diosgenin. Bioorg. Med. Chem. (2005);13:1141–1149. doi: 10.1016/j.bmc.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Vermes I., Haanen C., Steffens-Nakken H., Reutellingsperger C. A novelassay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 20.Antonsson B. Bax and other pro-apoptotic Bcl-2 family ‘‘killer-proteins” and their victim the mitochondrion. Cell Tissue Res. (2001);306:347–361. doi: 10.1007/s00441-001-0472-0. [DOI] [PubMed] [Google Scholar]
- 21.Zornig M., Hueber A., Baum W., Evan G. Apoptosis regulators and their role in tumorigenesis. Biochem. Biophys. Acta. (2001);1551:1–37. doi: 10.1016/s0304-419x(01)00031-2. [DOI] [PubMed] [Google Scholar]
- 22.Roy N., Deveraux O. L., Takahashi R., Salvesen G. S., Reed J. C. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. (1997);16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deveraux Q. L., Reed J. C. IAP family proteins suppressors of apoptosis. Genes Dev. (1999);13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 24.Hwang Y. J., Lee E. J., Kim H. R., Hwang K. A. Molecular mechanisms of luteolin-7-O-glucoside-induced growth inhibition on human liver cancer cells: G2/M cell cycle arrest and caspase-independent apoptotic signaling pathways. BMB Rep. (2013);46:611–616. doi: 10.5483/BMBRep.2013.46.12.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou C., Shen Q., Xue J., Ji C., Chen J. Overexpression of TTRAP inhibits cell growth and induces apoptosis in osteosarcoma cells. BMB Rep. (2013);46:113–118. doi: 10.5483/BMBRep.2013.46.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


