Abstract
In this study, we showed that Mycobacterium abscessus MAB2560 induces the maturation of dendritic cells (DCs), which are representative antigen-presenting cells (APCs). M. abscessus MAB2560 stimulate the production of pro-inflammatory cytokines [interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-1β, and IL-12p70] and reduce the endocytic capacity and maturation of DCs. Using TLR4-/- DCs, we found that MAB2560 mediated DC maturation via Toll-like receptor 4 (TLR4). MAB2560 also activated the MAPK signaling pathway, which was essential for DC maturation. Furthermore, MAB2560-treated DCs induced the transformation of naïve T cells to polarized CD4+ and CD8+ T cells, which would be crucial for Th1 polarization of the immune response. Taken together, our results indicate that MAB2560 could potentially regulate the host immune response to M. abscessus and may have critical implications for the manipulation of DC functions for developing DC-based immunotherapy. [BMB Reports 2014;47(9): 512-517]
Keywords: Dendritic cells, MAB2560, MAPKs, Mycobacterium abscessus, Th1 polarization
INTRODUCTION
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) that play major roles in innate and adaptive immune responses (1). DCs express a variety of co-stimulatory surface molecules and MHC class I and II, and are classified into immature DCs and mature DCs according to their phenotype. Immature DCs capture and internalize pathogens via pattern recognition receptors (PRRs) (2).
Nontuberculous mycobacteria (NTM) are ubiquitously distributed in the environment (3) and cause a wide spectrum of human infections, such as skin and soft tissue infections, lung infections, and opportunistic infections (4), in immunocompromised patients such as the HIV-infected individuals (5). Mycobacterium abscessus infections present a therapeutic challenge because M. abscessus strains are resistant to most antibiotics and linked to high death rate (6). APC-mediated innate and adaptive immune responses are crucial in the protection against M. abscessus infections (7).
Synthetic agonists of TLR3, TLR4, TLR5, TLR7, TLR8, and TLR9 have been recently identified as suitable immunostimulants of APCs (8). Moreover, on the basis of the efficacy of TLR agonists used thus far, the use of TLR ligands as adjuvants in humans is likely to be developed in the future. Therefore, DC maturation and activation by various microbial through TLRs signaling is the decisive link between innate and adaptive immune responses and is pivotal to the generation of protective immunity.
Recently, Shin et al. have shown that M. abscessus induces the activation of Raw264.7, a macrophage cell line, through TLR2 (7). Several ligands of TLR2 and TLR4 from mycobacteria have been discovered including LpqH (9), LprA (10), LprG (11), lipomannan (12), certain lipoarabinomannan (LAM) species (13), phosphatidyl-myo-inositol mannoside (14), PE_PGRS (15), HBHA (16), and CobT (17). Most of the identified ligands of TLR2 were purified from M. tuberculosis; however, little is known about the ligands purified from M. abscessus. MAB2560 consists of 201 amino acids and is the hypothetical protein of M. abscessus. However, there is still little information on MAB2560, and its function in M. abscessus remains unknown.
Taken together, our results suggest that MAB2560 is an effective Th1 polarizing adjuvant and that immune stimulation appears to be mediated through activation of DCs by TLR4-mediated MAPKs pathways.
RESULTS
MAB2560 is nontoxic to DCs and enhances DC maturation
To examine the immunological effect of MAB2560 on DCs, we purified soluble recombinant MAB2560 using the Escherichia coli expression system under endotoxin-free experimental conditions. Using the LAL endotoxin assay kit (GenScript USA, Inc., Piscataway, NJ, USA), we confirmed that endotoxin contamination of MAB2560 had not occurred (<15 pg/ml). As shown in Supplementary Fig. 1A, we detected a purified band of MAB2560 in the range of 21 kDa. Next, we investigated the cytotoxicity of MAB2560 against DCs using Annexin V and propidium iodide (PI) staining. We observed no marked change in the percentage of dead cells in DCs stimulated with MAB2560 (up to 2.5 μg/ml concentration) (Supplementary Fig. 1B). Thus, MAB2560 had no effect on cell death. Furthermore, proteinase K- or heat-treated MAB2560 lost its activity to enhance the levels of CD86 in DCs. However, MAB2560 was resistant to polymyxin B treatment, indicating that LPS contamination was not responsible for the observed effects (Supplementary Fig. 1).
Fig. 1. MAB2560 induces the maturation of DCs. Immature DCs were treated with MAB2560 (2.5 μg/ml) or LPS (50 ng/ml) for 24 h. (A) The expression of surface markers was analyzed by flow cytometry stained with anti-CD80, anti-CD86, anti-MHC class I, or anti-MHC class II in CD11c+ gated cells. The percentages are indicated in the histogram. Results are representative of three experiments with similar data. (B) ELISA was performed to determine the levels of IL-6, TNF-α, IL-1β, IL-12p70, and IL-10 in MAB2560- or LPS-treated DCs. Data are means and SEM of 3 experiments. **P < 0.01 and ***P < 0.001 compared to medium control. (C) The endocytic capacity of DCs using FITC-conjugated dextran uptake systems was assessed at 37℃ and 4℃ (as a negative control) by flow cytometric analysis. The percentage of FITC-dextran in CD11c+ cells is indicated in the dot plot diagram. Results are representative of three experiments with similar data.
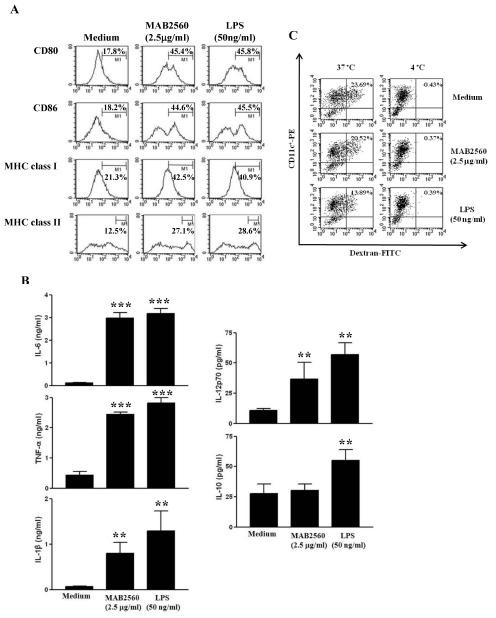
To investigate the effect of MAB2560 on the maturation of DCs, we evaluated the co-stimulatory molecules and MHC classes, which are involved in T cell activation. Fig. 1A shows that MAB2560-treated DCs had increased levels of CD80, CD86, and MHC class I and II. LPS, which is a well-known activator of DC maturation, served as a positive control. Next, we measured the production of pro- and anti-inflammatory cytokines in MAB2560-treated DCs. Fig. 1B shows that TNF-α, IL-1β, and IL-6 levels significantly increased in MAB2560-treated DCs. whereas the secretion of IL-12, which drives Th1 polarization, was significantly enhanced in MAB2560-treated DCs, and the production of IL-10, which inhibits the function of Th1 immune responses, was not significantly enhanced. Generally, immature DCs have a higher antigen endocytic capacity than mature DCs. Therefore, we examined the influence of MAB2560 on the endocytic capacity of DCs, using the dextran-FITC uptake experiment. MAB2560-treated DCs had diminished endocytic capacity, as expected for mature DCs (Fig. 1C). On the basis of these results, we inferred that MAB2560 is a potent inducer of DC maturation.
TLR4 is required for the MAB2560-induced maturation of DCs
We investigated whether MAB2560 acts through TLRs in DCs. To test the ability of MAB2560 to activate DCs via TLRs, we measured the expression of surface molecules and the production of pro-inflammatory cytokines, such as TNF-α and IL-6, in MAB2560-treated WT, TLR2-/-, and TLR4-/- DCs. MAB2560 increased the expression of surface molecules (Fig. 2A) and the production of pro-inflammatory cytokines (Fig. 2B) in WT and TLR2-/- DCs. On the other hand, these effects were strongly decreased in TLR4-/- DCs, indicating that MAB2560 is a potent agonist of DC maturation that acts through a TLR4-depedent mechanism. Next, we asked whether MAB2560 could bind to TLR4. Thus, we analyzed the interaction between MAB2560 and TLR4 using the BLItz system. His-tagged recombinant TLR4/MD2 was labeled with an anti-penta-HIS biosensor. Association was started by dipping the TLR4-tagged HIS sensor in buffers of Pam3CSK4, LPS, MAB2560, or PBS (Supplementary Fig. 3A). MAB2560 bound to TLR4 in a dose-dependent manner, and the concentration-binding rate curve between TLR4 and MAB2560 was calculated (Supplementary Fig. 3B). Dissociation was started by dipping the biosensor into PBS. The calculated values of Ka, Kd, and KD between MAB2560 and TLR4/MD2 were 1.738e6 (1/Ms), 2.281e-2 (1/S), and 1.31 nM, respectively. These results indicated that the MAB2560-mediated DC maturation occurred through binding to the TLR-4, rather than the TLR-2, signaling cascade.
Fig. 2. MAB2560 induces the maturation of DCs via TLR4. DCs derived from WT, TLR2-/-, or TLR4-/- mice were treated with MAB2560 (2.5 μg/ml) or LPS (50 ng/ml) for 24 h. (A) Histograms show the expression of CD80, CD86, MHC class I, or MHC class II in CD11c+-gated cells. The percentages are indicated in the histogram. Results are representative of three experiments with similar data. (B) ELISA was performed to determine the levels of IL-6 and TNF-α in MAB2560- or LPS-treated DCs. Data are means and SEM of 3 experiments.
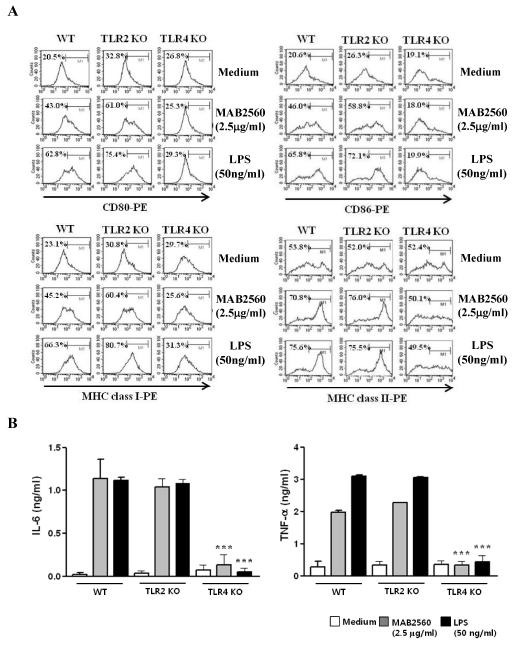
Fig. 3. The activation of DCs by MAB2560 enhances the proliferation of CD8+ and CD4+ T-cells. (A) OVA257-264-specific CD8+ T cells were isolated, stained with CFSE, and then co-cultured with immature DCs, OVA257-264-pulsed DCs, OVA257-264-pulsed MAB2560-treated DCs, or OVA257-264-pulsed LPS-treated DCs for 72 h. Histograms showing CD8+ T-cell proliferation as assessed by flow cytometry. Results are representative of three experiments with similar data. (B) OVA323-339-specific CD4+ T cells were isolated, stained with CFSE, and then co-cultured with immature DCs, OVA323-339-pulsed DCs, OVA323-339-pulsed MAB2560-treated DCs, or OVA323-339-pulsed LPS-treated DCs for 72 h. Histograms showing CD8+ T-cell proliferation as assessed by flow cytometry. Results are representative of three experiments with similar data. (C) The production of IFN-γ was measured in each culture supernatant from (A) and (B) at 72 h by ELISA.

MAB2560-treated DCs induce the proliferation of CD8+ and CD4+ T-cells
Generally, T cells are stimulated by antigens presented by mature DCs. Naïve T cells are then induced to proliferate and differentiate into effector or helper T cells. To confirm whether MAB2560-treated DCs induce T-cell proliferation, we performed a mixed lymphocyte reaction (MLR) assay using OT-I T cell receptor (TCR) transgenic CD8+ T cells and OT-II TCR transgenic CD4+ T cells, which express a TCR specific for the MHC class I-restricted OVA peptide 257-264 Ag (OVA257-264) and the MHC class II-restricted OVA peptide 323-339 Ag (OVA323-339) in DCs, respectively. MAB2560 treatment enhanced the proliferation of CD8+ and CD4+ T-cells, similar to the LPS-treated group (positive control) (Fig. 3A). These results demonstrate that MAB2560 is a potent stimulus for the proliferation of CD8+ and CD4+ T cells via DC activation. In addition, we investigated IFN-γ production under MLR conditions. A higher level of IFN-γ produced by T-cells primed with MAB2560-treated DCs (Fig. 3B), indicating that matured DCs stimulated by MAB2560 directly drive naïve T cell polarization toward a Th1 phenotype.
MAPKs are involved in the maturation of DCs
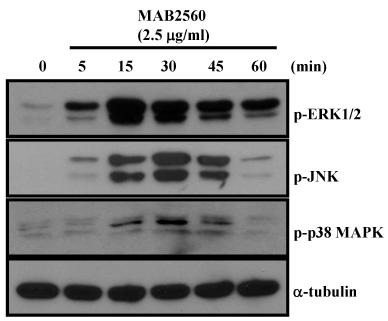
MAPK signaling pathways play a pivotal role in DC maturation (18). Thus, we investigated whether MAB2560 influences MAPKs activation in DCs. MAB2560 activated MAPK pathways, such as p38 MAPK, JNK, and ERK1/2 (Fig. 4). Furthermore, we tested whether MAPK inhibitors specifically suppresses the MAB2560-induced activation of MAPKs. We found that specific inhibitors of MAPKs specifically inhibited the MAB2560-induced activation of MAPKs (Supplementary Fig. 4). These results showed that MAB2560 activates the MAPK signaling pathway, and we inferred that DC maturation is through the activation of the above-mentioned signaling cascade.
Fig. 4. MAB2560 activates MAPKs in DCs. For MAPK activation analysis, DCs were treated with MAB2560 (2.5 μg/ml) for 0, 5, 15, 30, 45, and 60 min. Cell lysates were prepared and immunoblotted with antibodies against p-p38 MAPK, p-JNK, p-ERK1/2, and α-tubulin. Results are representative of three experiments with similar data.
DISCUSSION
In this study, MAB2560 was purified and identified from an M. abscessus strain in our group for the first time; however, the function of MAB2560 in immune systems has not been studied yet. The recognition of TLRs by various stimuli in DCs is critical for the switch from an immature to a mature phenotype for the leading the effective systemic immune responses. M. abscessus has been recently suggested induce the activation of APCs (7) and human cells (19) via TLR2. However, M. abscessus-derived proteins with TLR agonistic effects have not yet been identified. Here, we describe the biological activity and cellular immune responses of M. abscessus MAB2560 in DC-mediated T cell immunity and show that TLR4 signaling is involved in the maturation and activation of DCs stimulated by MAB2560.
Several studies have reported that MAPKs, especially ERK1/2 and p38, are key modulators of granuloma formation during NTM infection (20). Consistent with these reports, our result showed the activation of MAPKs in MAB2560-treated DCs. Thus, the activation of MAPKs by MAB2560 is a potent mechanism underlying the enhancement of IL-12 production in DCs. Taken together, our findings suggest that MAB2560 enhances DC maturation via TLR4 and elicits MAPKs-mediated production of IL-12. The induction of IL-12 production is then responsible for activating and polarizing the naïve CD4+ T cells toward Th1 cells that produce IFN-γ. The production of IFN-γ in PBMCs is represents to be severely compromised in patients with tuberculosis (21). In this regard, IFN-γ is critically important in the host defense against to M. abscessus (22).
In this study, MAB2560 establishes a link between the innate DCs function and the adaptive Th1 immunity to M. abscessus. The characteristic of the bacterial pathogens that are encountered by the adaptive immune response tailors the balance of the Th1/Th2 response to a particular pathogen (23). Our results suggest that M. abscessus MAB2560 stimulates DCs and specifically produces the Th1 immunity ex vivo. The enhancement of IL-12 production by DCs and IFN-γ by T cells is strongly evaluated by MAB2560 stimulation.
In conclusion, MAB2560 is a potent protein that induces DCs maturation and Th1-mediated responses. Understanding the mechanism about how MAB2560 modulates the function of DCs may contribute to vaccine development against M. abscessus infection. Further understanding of the mechanism by which MAB2560 regulates the DC function may aid in the development of effective M. abscessus vaccines and an effective immunotherapy adjuvant for other infectious diseases. Moreover, identification of mycobacterial antigens as an adjuvant that induces the activation of APCs may affect the development of immunotherapy in the future.
MATERIALS AND METHODS
Animals
Female (4-6 weeks old) C57BL/6 (H-2Kb and I-Ab) mice were purchased from the Korean Institute of Chemistry Technology (Daejeon, Korea). C57BL/6 OT-I T-cell receptor (TCR) transgenic mice, C57BL/6 OT-II TCR transgenic mice, C57BL/6J TLR2-/- (B6.129-Tlr2tm1Kir/J), and C57BL/10 TLR4-/- (C57BL/ 10ScNJ) were purchased at 6 to 8 weeks of age from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in a specific pathogen-free environment and used in accordance with the institutional guidelines for animal care.
Reagents and antibodies
Recombinant murine granulocyte-macrophage colony-stimulating factor (rmGM-CSF) and rmIL-4 were purchased from R&D Systems (Minneapolis, MN). Cytokine enzyme-linked immunosorbent assay (ELISA) kits for murine IL-12p70, TNF-α, IL-10, IL-1β, and IL-6 were purchased from R&D Systems. Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs) used to detect the expression of CD11c (HL3), CD80 (16-10A1), CD86 (GL1), IAb β-chain (AF-120.1), and H2Kb (AF6-88.5) by flow cytometry, as well as isotype-matched control mAbs and the biotinylated anti-CD11c (N418) mAb, were purchased from R&D Systems. Antibodies against phospho-ERK, ERK, phospho-p38, phospho-JNK, and tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Expression and purification of MAB2560 from E. coli
MAB2560 gene was amplified by PCR using M. abscessus genomic DNA (ATCC19977) as a template and the following primers: forward, 5'-CAT ATG ACG AGC GTT GAC GTG CCC TCG-3', and reverse, 5'-AAG CTT TAG GCC GGA GGG CAC GAC CTT-3'. The PCR product was cut with NdeI and HindIII and then inserted into pET28a vector cut with the same restriction enzymes. The recombinant plasmid was transformed into E. coli BL21 cells carrying bacteriophage DE3 for protein overexpression. Recombinant MAB2560 was produced from E. coli (rEC-MAB2560). Recombinant E. coli (pET22b-MAB2560) cells were incubated in LB medium (100 μg/ml ampicillin) at 37℃ for 12 h. Next, the cells were treated with 1 mM isopropyl-β-D-thio-galactoside (IPTG) and incubated at 37℃ for 6 h. The cells were lysed in lysis buffer (1 M DDT, lysozyme, PMSF) and sonicated. Vector control cells (pET-BL21) were treated in the same manner. The products from each cell was separated by 13.5% SDS-PAGE and visualized by Coomassie blue staining. Lastly, 6X His-tagged MAB2560 attached to a Ni-NTA column was purified using imidazole (50 and 100 mM) and treated with Tachypleus amebocyte lysate (TAL) assay for eliminating the endotoxin in the fusion protein.
Generation and culture of murine bone marrow-derived dendritic cells
Primary culture of DCs was performed as previously described (24, 25).
Flow cytometric analysis of the expression of surface molecules
Flow cytometic analysis to detect the expression of CD80, CD86, MHC class I and MHC class II in CD11c positive cells were experimented according to the procedure of Park et al. (23).
Antigen uptake quantitation
Briefly, 2×105 cells were equilibrated at 37℃ or 4℃ for 45 min and then pulsed with 1 mg/ml FITC-conjugated dextran, which was stopped with cold staining buffer. Washed cells were stained with PE-conjugated anti-CD11c and analyzed using a FACSCalibur flow cytometer.
Cytokine assay
Murine IL-12p70, IL-10, IL-1β, IL-6, and TNF-α were measured using an ELISA kit according to the manufacturer’s instructions.
Western blot analysis
Western bot analysis was experimented according to the procedure of Lee et al. (24).
Mixed lymphocyte reaction
Mixed lymphocyte reaction analysis was experimented according to the procedure of Lee et al. (24).
Statistical analysis
All experiments were repeated at least 3 times with consistent results. Unless otherwise stated, data are expressed as mean ± SEM. Analysis of variance was used to compare experimental groups with control values, while comparisons between multiple groups were made using Tukey’s multiple comparison tests (Prism 3.0 GraphPad software). A P value of less than 0.05 was considered to indicate statistical significance.
Acknowledgments
This work was supported by a grant from Pusan National University (PNU; Bio-Scientific Research Grant PNU-2008-101-102).
References
- 1.Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. (1998);392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Janeway C. A., Jr., Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin. Immunol. (1998);10:349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- 3.Falkinham J. O.,, 3rd Nontuberculous mycobacteria in the environment. Clin. Chest. Med. (2002);23:529–551. doi: 10.1016/S0272-5231(02)00014-X. [DOI] [PubMed] [Google Scholar]
- 4.Griffith D. E., Aksamit T., Brown-Elliott B. A., Catanzaro A., Daley C., Gordin F., Holland S. M., Horsburgh R., Huitt G., Iademarco M. F., Iseman M., Olivier K., Ruoss S., von Reyn C. F., Wallace R. J.,, Jr., Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care. Med. (2007);175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 5.Horsburgh C. R.,, Jr., Selik R. M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am. Rev. Respir. Dis. (1989);139:4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- 6.Brown-Elliott B. A., Wallace R. J.,, Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. (2002);15:716–746. doi: 10.1128/CMR.15.4.716-746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin D. M., Yang C. S., Yuk J. M., Lee J. Y., Kim K. H., Shin S. J., Takahara K., Lee S. J., Jo E. K. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. (2008);10:1608–1621. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal S., Agrawal A., Doughty B., Gerwitz A., Blenis J., Van Dyke T., Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. (2003);171:4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 9.Noss E. H., Pai R. K., Sellati T. J., Radolf J. D., Belisle J., Golenbock D. T., Boom W. H., Harding C. V. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. (2001);167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 10.Pecora N. D., Gehring A. J., Canaday D. H., Boom W. H., Harding C. V. Mycobacterium tuberculosis LprA is a lipoprotein agonist of TLR2 that regulates innate immunity and APC function. J. Immunol. (2006);177:422–429. doi: 10.4049/jimmunol.177.1.422. [DOI] [PubMed] [Google Scholar]
- 11.Gehring A. J., Dobos K. M., Belisle J. T., Harding C. V., Boom W. H. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. (2004);173:2660–2668. doi: 10.4049/jimmunol.173.4.2660. [DOI] [PubMed] [Google Scholar]
- 12.Quesniaux V. J., Nicolle D. M., Torres D., Kremer L., Guerardel Y., Nigou J., Puzo G., Erard F., Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J. Immunol. (2004);172:4425–4434. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- 13.Jones B. W., Means T. K., Heldwein K. A., Keen M. A., Hill P. J., Belisle J. T., Fenton M. J. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. (2001);69:1036–1044. [PubMed] [Google Scholar]
- 14.Gilleron M., Quesniaux V. F., Puzo G. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus Calmette Guerin and mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. (2003);278:29880–29889. doi: 10.1074/jbc.M303446200. [DOI] [PubMed] [Google Scholar]
- 15.Bansal K., Elluru S. R., Narayana Y., Chaturvedi R., Patil S. A., Kaveri S. V., Bayry J., Balaji K. N. PE_PGRS antigens of Mycobacterium tuberculosis induce maturation and activation of human dendritic cells. J. Immunol. (2010);184:3495–3504. doi: 10.4049/jimmunol.0903299. [DOI] [PubMed] [Google Scholar]
- 16.Jung I. D., Jeong S. K., Lee C. M., Noh K. T., Heo D. R., Shin Y. K., Yun C. H., Koh W. J., Akira S., Whang J., Kim H. J., Park W. S., Shin S. J., Park Y. M. Enhanced efficacy of therapeutic cancer vaccines produced by co-treatment with Mycobacterium tuberculosis heparin-binding hemagglutinin, a novel TLR4 agonist. Cancer Res. (2011);71:2858–2870. doi: 10.1158/0008-5472.CAN-10-3487. [DOI] [PubMed] [Google Scholar]
- 17.Byun E. H., Kim W. S., Kim J. S., Won C. J., Choi H. G., Kim H. J., Cho S. N., Lee K., Zhang T., Hur G. M., Shin S. J. Mycobacterium paratuberculosis CobT activates dendritic cells via engagement of Toll-like receptor 4 resulting in Th1 cell expansion. J. Biol. Chem. (2012);287:38609–38624. doi: 10.1074/jbc.M112.391060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi F., Smith K. D., Ozinsky A., Hawn T. R., Yi E. C., Goodlett D. R., Eng J. K., Akira S., Underhill D. M., Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. (2001);410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 19.Sampaio E. P., Elloumi H. Z., Zelazny A., Ding L., Paulson M. L., Sher A., Bafica A. L., Shea Y. R., Holland S. M. Mycobacterium abscessus and M. avium trigger Toll-like receptor 2 and distinct cytokine response in human cells. Am. J. Respir. Cell. Mol. Biol. (2008);39:431–439. doi: 10.1165/rcmb.2007-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sim Y. S., Kim S. Y., Kim E. J., Shin S. J., Koh W. J. Impaired Expression of MAPK Is Associated with the Downregulation of TNF-alpha, IL-6, and IL-10 in Mycobacterium abscessus Lung Disease. Tuberc. Respir. Dis. (Seoul) (2012);72:275–283. doi: 10.4046/trd.2012.72.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ordway D., Henao-Tamayo M., Smith E., Shanley C., Harton M., Troudt J., Bai X., Basaraba R. J., Orme I. M., Chan E. D. Animal model of Mycobacterium abscessus lung infection. J. Leukoc. Biol. (2008);83:1502–1511. doi: 10.1189/jlb.1007696. [DOI] [PubMed] [Google Scholar]
- 22.Vilcek J., Klion A., Henriksen-DeStefano D., Zemtsov A., Davidson D. M., Davidson M., Friedman-Kien A. E., Le J. Defective gamma-interferon production in peripheral blood leukocytes of patients with acute tuberculosis. J. Clin. Immunol. (1986);6:146–151. doi: 10.1007/BF00918747. [DOI] [PubMed] [Google Scholar]
- 23.Lee J. S., Shin S. J., Collins M. T., Jung I. D., Jeong Y. I., Lee C. M., Shin Y. K., Kim D., Park Y. M. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein activates dendritic cells and induces a Th1 polarization. Infect. Immun. (2009);77:2979–2988. doi: 10.1128/IAI.01411-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S. J., Noh K. T., Kang T. H., Han H. D., Shin S. J., Soh B. Y., Park J. H., Shin Y. K., Kim H. W., Yun C. H., Park W. S., Jung I. D., Park Y. M. The Mycobacterium avium subsp. Paratuberculosis protein MAP1305 modulates dendritic cell-mediated T cell proliferation through Toll-like receptor-4. BMB Rep. (2014);47:115–120. doi: 10.5483/BMBRep.2014.47.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noh K. T., Son K. H., Jung I. D., Kang H. K., Hwang S. A., Lee W. S., You J. C., Park Y. M. Protein kinase C delta (PKCdelta)-extracellular signal-regulated kinase 1/2 (ERK1/2) signaling cascade regulates glycogen synthase kinase-3 (GSK-3) inhibition-mediated interleukin-10 (IL-10) expression in lipopolysaccharide (LPS)-induced endotoxemia. J. Biol. Chem. (2012);287:14226–14233. doi: 10.1074/jbc.M111.308841. [DOI] [PMC free article] [PubMed] [Google Scholar]


