Abstract
The aim of this study was to evaluate with anxiety tests the effect of resveratrol (RSV) on streptozotocin (STZ)-induced diabetic mouse behavioral performance at the second and fourth week of treatment. Confirmed diabetic mice (>250 mg/dl of glucose in blood after STZ injection) were treated with RSV (RDM, n=12) or control treated (DM, n=12) for 4 weeks. DM and RDM were tested in the Open Field Test (OFT) and Elevated Plus Maze (EPM). In the second week of RSV treatment, a higher grooming frequency (P<0.05) and a lower defecation and rearing frequency (P<0.05) were detected in the OFT in the RDM group compared with the DM. There was a higher grooming frequency (P<0.05) and higher percentage of entries in open arms (P<0.05) in the RDM group than in the DM group in the EPM. However, in the fourth week of RSV treatment, the only effect observed was a higher grooming frequency in the RDM group than in the DM group (P<0.05) in the EPM. In conclusion, RSV treatment in diabetic mice provoked anxiolytic-like effects in both tests (OFT and EPM), and these effects were observed in a short time window (2 weeks). It is suggested that RSV may help diabetic animals to adapt to new stressing and anxiety situations and thus to improve their welfare.
Keywords: diabetes, flavonoid, mouse, resveratrol, stress
Introduction
Diabetes mellitus is a complex endocrine-metabolic and multifactorial disorder [59]. Diabetes provokes alterations in the central nervous system (CNS) and adversely affects behavior, causing depression [4], cognitive dysfunction [3, 63], increasing in fear-related behaviors [50], and stress response, as shown by an increase in hypothalamo-pituitary-adrenal axis activity [24, 67]. Studies performed in rodents with streptozotocin-induced diabetes type-1 have shown an increase in anxiety-like behaviors in Open Field Tests (OFT) and the Elevated Plus Maze (EPM) [55, 62].
In the last decade, it has been reported that resveratrol (RSV) has important implications for diabetes treatment [45, 46]. RSV is a natural flavonoid found in the peel of red grapes, red wine, and peanuts and is well known for its antioxidant, cardioprotective, and anticarcinogenic properties [9, 27, 33]. In mice, RSV improves mitochondrial function, energy balance, and life span under caloric restriction by stimulating the Sirt1-mediated PGC-1α deacetylation [8, 38, 48, 64]. Brain PGC-1α deficiency in mice leads to behavioral abnormalities, including profound hyperactivity and neurodegeneration disease [51, 53]. Some works in animal models have shown that RSV provides neuroprotection and learning rescue in CNS degeneration and motor disorders [20, 31, 44]. Xu et al. [81] showed that RSV exerts antidepressant-like effects in mice. These effects might be related to an increase in certain neurotransmitters (for example, serotonin) in different brain areas, such as the hippocampus. It is known that the hippocampus is a brain area associated with anxiety behavior [7, 82]. Also, the serotonergic system is associated with anxiety behavior [11]. In addition, Zhang et al. [83] found that certain natural flavonoids exert anxiolytic effects in rats subjected to posttraumatic stress. Moreover, Schmatz et al. [68] suggested that RSV can improve learning and memory in diabetic rats.
The aim of the present work was to determine the effect of RSV on the behavioral performance in anxiety tests of streptozotocin-induced diabetic mice after 2 and 4 weeks of treatment.
Methods
Animals
Twenty-four male mice (CD1) were used in this study. All mice were housed in a controlled environment with a 12 h light and 12 h dark cycle and average temperature of 22 ± 2°C with free access to chow and water. The mice were weaned at 21 days of age and arranged in groups of four to six animals per cage. The experimental procedures were approved by the local Ethics Committee (CEUA IIBCE, Uruguay), in accordance with national legislation.
Induction of diabetes and determination of body weight and glycemia
Type 1 diabetes was induced in 40-day-old mice by administration of a single intraperitoneal streptozotocin (STZ) injection (150 mg/kg body weight, dissolved in 0.1 mmol/l sodium citrate buffer, pH 4.5, Sigma Chemical Co., St. Louis, MO, USA ). Body weight and glycemia levels were measured each week following STZ injection. Glycemia determinations were carried out with an Accu-Chek Active system (Roche, Mannheim, Germany), and blood samples were obtained from the tail vein. Mice showing glycemia levels higher than 250 mg/dl one week after STZ injection were considered diabetic.
Resveratrol treatment
Two weeks after STZ injection, diabetes was confirmed in all animals, and the mice were randomly divided into two groups: Diabetic Mice (DM, n=12) and Diabetic Mice treated with RSV (RDM, n=12). Time zero (week 0) was considered the moment of the start of RSV treatment. RSV (Enzo Clinical Labs, Farmingdale, NY, USA) was freshly prepared in dimethyl sulfoxide (DMSO) with a concentration of 65 mg/ml and administered by intraperitoneal injection (40 mg/kg) every other day for 4 weeks to animals in the RDM group [28, 84]. For a review, see Athar et al. [5] and Saiko et al. [66]. In parallel, DMSO solution was administered to DM mice as a control.
Behavioral experiments
Mice were brought to the experimental room in their home cages two hours before the behavioral experiments to allow them to acclimate to the test environment. The experimental room was kept at a controlled temperature (22 ± 2°C). DM and RDM mice were tested in the OFT and EPM in the 2nd and 4th weeks of RSV treatment. This experimental design was similar to the one carried out by Doron et al. [29] and Kajiyama et al. [40]. The EPM test was performed two days after the OFT in the corresponding week, similar to as reported by Uriarte et al. [78]. Both behavioral tests were scored and recorded using a Sony HD camera for further analysis.
Open field test
The OFT apparatus consists of a square Plexiglas cage (35 × 35 × 40 cm) with walls to minimize outside light and noise. The animals were individually placed in the center of the OFT apparatus and were left to move freely during a 10 min period. The measured behaviors are described in Table 1. Locomotion data including distance moved (m), duration (s) in peripheral zone (a 5.0 cm region in the outer margin of the box), duration (s) in the center zone (12.0 × 12.0 cm), duration (s) during which the mice were making movements associated with locomotion or displacement (TMLM), and velocity (cm/s) were scored and recorded automatically over the course of 10 min by a camera connected to a computer equipped with the Ethovision XT 7.0 software (Noldus, Wageningen, The Netherlands). After behavioral monitoring, the floor was cleaned with 70% ethanol solution and left to dry before testing the next animal.
Table 1. List of behaviors observed and their respective descriptions.
| Behavior | Description |
| Grooming | Number of times that an animal preened its fur or tail with its mouth or forepaws |
| Rearings | Number of times that a mouse reared up on its hindlimbs irrespective of whether the animal showed on- or off-wall rearing |
| Defecations | Number of fecal boli |
| Urinations | Number of times a mouse urinated |
Elevated plus maze test
The EPM device consisted of two open and two closed arms (open arms, 30 × 5 cm; closed arms, 30 × 5 cm, surrounded by 15-cm-high walls). The apparatus was made of wood and elevated 40 cm above the floor. Mice were placed on the central platform (5 × 5 cm) of the maze, facing towards a closed arm and allowed to explore the maze for 5 min. The behavioral parameters scored during the experimental sessions were frequency of grooming, rearing, defecations, urinations, frequency of entries into closed (ECA) and open arms (EOA), and percentage of open arms entries (%EOA) [32].
Statistical analysis
The behavioral parameters without a normal distribution (frequency of grooming, rearing, defecations, urinations, and frequency of ECA, EOA, and%EOA) were compared between DM and RDM mice using the Mann–Whitney U test. The behaviors with a normal distribution, such as the distance moved, duration in peripheral zone, duration in center zone, TMLM, and velocity, were compared between DM and RDM mice using the unpaired Student’s t-test. Body weight and glycemia were analyzed with an ANOVA for repeated measurements. The model considered the following parameters: group (DM or RDM), time (weeks), and the interaction between group and time as fixed effects and individuals in each group as a random effect. Post hoc comparisons were done with the least significant difference (LSD). The data are expressed as means ± standard error of the mean (SEM). Results were considered significant with an alpha level of 0.05 and were considered to show a tendency between 0.05 and 0.1.
Results
Body weight and glycemia
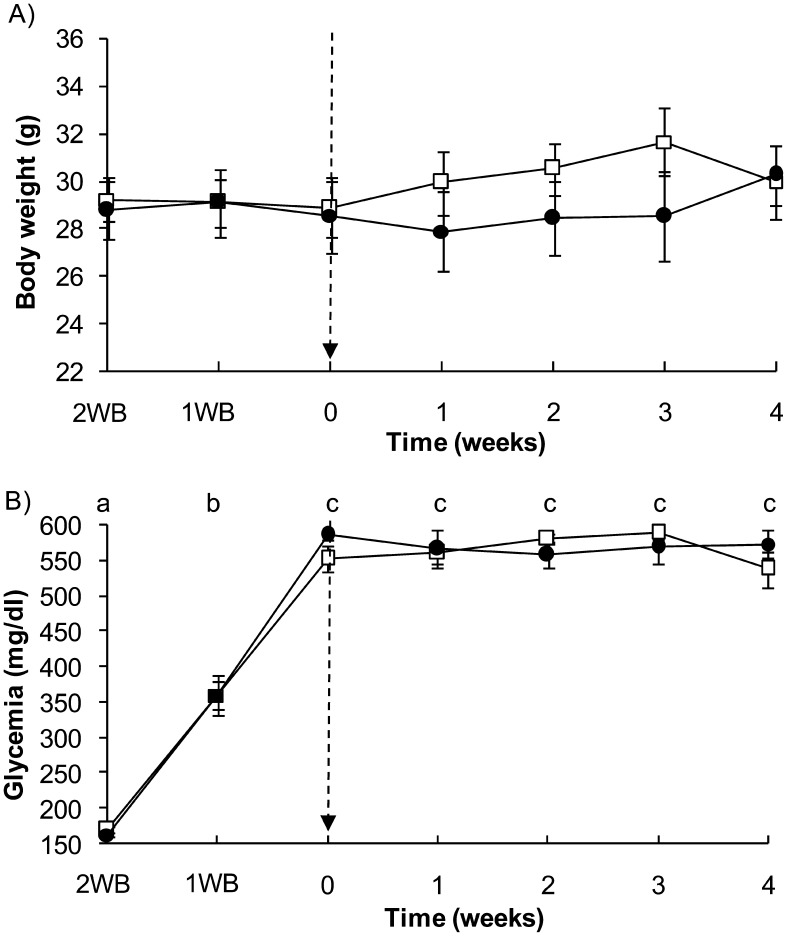
There was no effect of group (P=0.91), time (P=0.81), or interaction between group and time (P=0.15) on body weight of mice (Fig. 1A). Glycemia increased significantly over time after the STZ injection (P<0.0001), reaching the peak values at week 0 (two weeks after STZ injection) and remaining at high levels and unchanged up to four weeks (Fig. 1B). There was no effect of group (P=0.98) or interaction between group and time (P=0.78) on glycemia levels in mice.
Fig. 1.
Body weight (A) and glycemia (B) in diabetic mice with or without RSV treatment (white square and black circle respectively). Two weeks before (2WB) RSV treatment, STZ was injected. The dotted arrow indicates the start of RSV treatment (week 0). Different letters between weeks indicate significant differences at P<0.0001.
Behavioral performance
The effect of RSV treatment on diabetic mice in the OFT is presented in Table 2. After two weeks of treatment, the RDM group showed a higher grooming frequency, lower defecation frequency, and a tendency to have a lower duration in the center zone than the DM group (Table 2). In the 4th week of RSV treatment, only a tendency for a lower TMLM was observed in the RDM group compared with the DM group (Table 2).
Table 2. Behavioral response of DM and RDM mice in the OFT in the 2nd and 4th weeks after RSV treatment.
| Behavior | Resveratrol treatment in diabetic mice |
||||
|---|---|---|---|---|---|
| 2nd week |
4th week |
||||
| DM | RDM | DM | RDM | ||
| Grooming | 2.4 ± 0.2a | 4.7 ± 0.9b | 2.6 ± 0.6 | 2.7 ± 0.5 | |
| Rearing | 47.3 ± 5.3 | 46.5 ± 4.9 | 41.7 ± 6.5 | 28.6 ± 5.3 | |
| Defecations | 3.4 ± 0.4a | 1.9 ± 0.3b | 3.3 ± 0.7 | 1.8 ± 0.5 | |
| Urinations | 0.8 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | |
| Distance moved (m) | 29.6 ± 2.3 | 26.3 ± 1.6 | 25.5 ± 3.1 | 19.5 ± 2.6 | |
| Duration in peripheral zone (s) | 518.9 ± 11.1 | 509.3 ± 19.5 | 532.4 ± 11.4 | 537.8 ± 13.2 | |
| Duration in center zone (s) | 10.4 ± 2.3† | 5.5 ± 1.0 | 5.6 ± 1.2 | 3.5 ± 0.9 | |
| TMLM (s) | 379.6 ± 15.9 | 352.5 ± 16.2 | 310.0 ± 18.9† | 246.6 ± 26.4 | |
| Velocity (cm/s) | 4.9 ± 0.4 | 4.4 ± 0.3 | 4.3 ± 0.5 | 3.3 ± 0.4 | |
TMLM: time during which the mice were making locomotion movement. Different letters between the DM and RDM groups in the same week indicate significant differences (P<0.05, †: P=0.07 − 0.08).
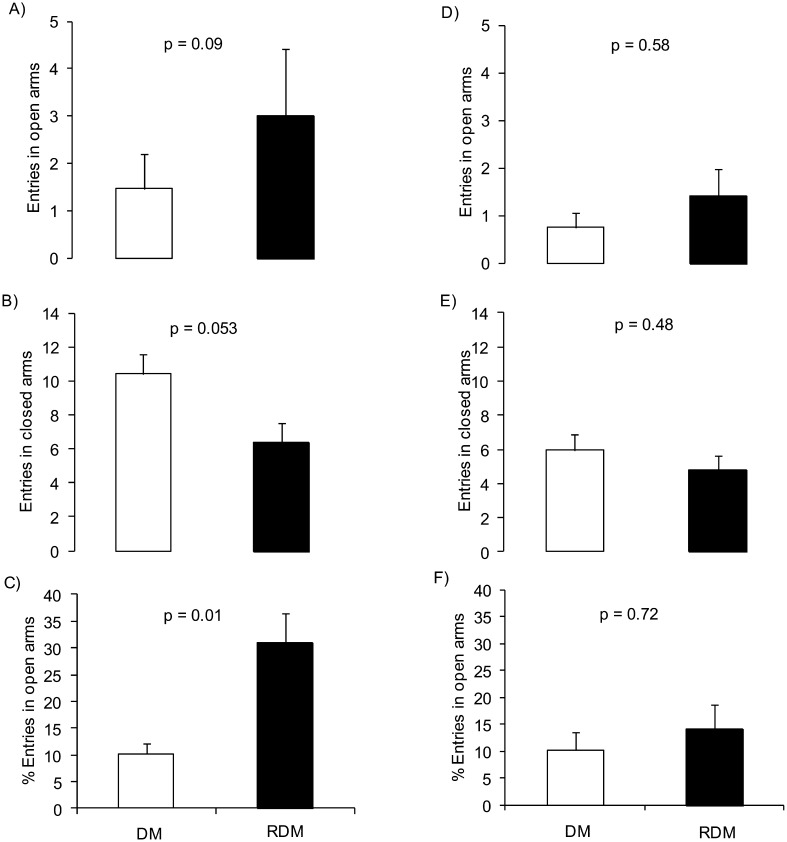
The effect of RSV treatment in diabetic mice on grooming, rearing, defecation, and urination behaviors in the EPM is presented in Table 3. The RDM group showed a higher frequency of grooming and lower frequency of rearing than DM group in the 2nd week of treatment (Table 3). The RDM group had a tendency to show a higher frequency of entries in open arms, lower entries in close arms, and higher percentage of entries in open arms than the DM group after 2 weeks of treatment with RSV (Figs. 2A, B, and C, respectively). In the 4th week of RSV treatment, the only significant effect observed was a higher grooming frequency in the RDM group than in the DM group (Table 3).
Table 3. Behavioral response of DM and RDM mice in the EPM in the 2nd and 4th weeks after resveratrol (RSV) treatment.
| Behavior | Resveratrol treatment in diabetic mice |
||||
|---|---|---|---|---|---|
| 2nd week |
4th week |
||||
| DM | RDM | DM | RDM | ||
| Grooming | 1.4 ± 0.3a | 5.3 ± 1.3b | 2.3 ± 0.8a | 5.2 ± 1.1b | |
| Rearing | 24.5 ± 1.5 a | 11.3 ± 2.4 b | 15.3 ± 2.3 | 15.5 ± 2.5 | |
| Defecations | 0.5 ± 0.2 | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.3 ± 0.2 | |
| Urinations | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.0 | |
Different letters between the DM and RDM groups in the same week indicate significant differences (P<0.05).
Fig. 2.
Frequency of behaviors (mean ± SEM) recorded in the EPM in the 2nd (A, B, and C) and 4th weeks (D, E, and F) in diabetic mice with or without RSV treatment (RDM and DM respectively).
Discussion
In the present report, we provide experimental evidence from anxiety tests suggesting that RSV treatment changes behavioral profiles in mice with STZ-induced diabetes. As mentioned, diabetes was induced in mice by administration of STZ, which produces selective necrosis of pancreatic beta cells and results in an insulin deficiency state. This is a well-characterized animal model used to induce type I diabetes [2,39, 54, 57, 70, 74]. In fact, all diabetic animals used in the present report showed high blood glucose levels (above 250 mg/dl, see Fig. 1B) and developed symptoms of severe diabetes characterized by polyurea and polydypsia. By using this animal model to induce type I diabetes, it was observed that RSV treatment produced anxiolytic-like effects in both OFT and EPM. These effects were shown in the OFT by the higher grooming frequency, lower defecation, and lower rearing frequency in the RDM group compared with the DM group and in the EPM test by a significantly higher grooming frequency, a tendency to show a higher frequency of entries in open arms, lower entries in close arms, and higher percentage of entries in open arms in the RDM group than in the DM group. These results agree with the study of Zhang et al. [83], who found that certain natural flavonoids exert anxiolytic effects in rats subjected to posttraumatic stress disorder in 3–15 days. In the present study, it was also observed that the effect on most of the behaviors was recorded mainly in a short time window (2 weeks) of treatment in both tests. After four weeks of resveratrol treatment, there was no effect on most of the behaviors recorded.
As previously mentioned, diabetic type 1 rodents have anxiety-like behaviors augmented in the EPM [1, 62]. This state of anxiety in diabetic animals is associated with changes in the central dopaminergic and serotonergic systems [1, 15, 25, 47, 61, 76]. Xu et al. [81] observed that RSV increased dopamine and serotonin levels in several mouse brain areas. Therefore, the anxiolytic effect of RSV in diabetic mice may be explained, partly at least, by its actions on the central dopaminergic and serotoninergic systems.
In rodents, an increase in fear response has been associated with an increase in the frequency of defecation in both the OFT and EPM test [10, 21, 35]. According to our results, RSV reduced the defecation frequency in the OFT in the 2nd week of treatment, suggesting that RSV provoked a diminished fear and stress response in diabetic mice. Although the difference was only observed in the OFT, decrease in the frequency of defecation in RDM mice may also be explained by the dopaminergic status [19].
It should be noted, however, that grooming is a behavior that may manifest as body care, self-calming procedure, displacement activity, and comfort in rodents [13, 72, 77]. The greatest grooming increase in the RDM group resulted in a better appearance and body care than in the DM group (data not shown). Even the higher frequency of grooming in the RDM group than in the DM group was maintained until the fourth week of treatment with RSV during the EPM. Since this grooming behavior is maintained over time and is related to body care, it seems to be one of the most important behavioral changes triggered by RSV in diabetic mice. Several studies in rodents have shown that environmental enrichment increases grooming behavior [16, 17, 60]. In a recent study, it was observed that grooming behavior was linked with an anxiolytic state in rodents [10]. The high grooming activity observed in RDM mice may be a way in which the rodent attempts to escape, to resolve, or to adapt to tension or anxiety situations [36, 41, 56, 65]. It has been reported that the dopaminergic status is implicated in the manifestation of grooming behavior [49] and that this behavior is partly regulated by dopamine D1 receptors [12, 23, 28]. Since dopamine D1 agonists induced an increase in grooming [14, 73] and RSV increased dopamine and serotonin levels in different areas of mice brain [81], it may be possible that RSV increased grooming activity in the RDM group due to a dopaminergic effect. On the other hand, it was found that 5-hydroxytryptamine (5-HT) agonists increase grooming and have an anxiolytic effect in rodents [6, 37, 43]. Therefore, similar effects were reported for the serotonergic system. Thus, the increase in grooming frequency caused by resveratrol can also be explained by higher levels of 5-HT in different brain areas, as it has been shown in mice by Xu et al. [81]. However, some authors propose that the grooming behavior in rodents partially explains anxiety states [42]. Taking all the OFT and EPM tests results together, we suggest that the higher grooming frequency in diabetic mice is associated with an anxiolytic state caused by RSV.
While anxiogenic drugs may decrease the rearing frequency [22], another study reported that fluoxetine (anxiolytic drug) can decrease the frequency of this behavior [30]. Beyond the different interpretations it is known that rearing is a normal behavior of the exploration performed by rodents when placed in a new environment [52]. Since the DM group tended to remain longer in the closed arms than the RDM group, it is difficult to analyze rearing behavior results. RDM mice performed less rearing, but this may be related to the shorter period of time they were in these arms.
In our study, RSV did not alter body weight or glycemia in diabetic mice. Our results regarding the effect of RSV on body weight in diabetic mice are in agreement with those reported by Schmatz et al. [68, 69] in diabetic rats. However, Chen et al. [26] observed that diabetic rats treated with RSV had an increased body weight when compared with diabetic rats without treatment. Nevertheless, it is difficult to reach conclusions because these experiments are not totally comparable, as different dosage methods were used: intraperitoneal [68, 69] and oral administration [26]. Our glycemia results in diabetic mice are also consistent with those obtained by Schmatz et al. [68, 69] in rats, and both studies administered RSV intraperitoneally. However, using the same RSV intraperitoneal administration method, Silan [71] observed a blood glucose lowering effect in diabetic rats. This effect was also observed by Thirunavukkarasu et al. [75] and Penumathsa et al. [58] in studies in which resveratrol was given orally to STZ-induced diabetic rats.
The anxiolytic-like effect of RSV in diabetic mice was mainly observed in a short time window (2 weeks). The absence of an RSV effect on anxiety behavior in long-term diabetic animals may be explained in part by chronic hyperglycemia. It is well known that long-term diabetic animals develop chronic hyperglycemia, which may provoke great functional brain changes [80], and these harmful effects may be stronger than the positive RSV effects.
It is important to point out that the animals used in the present report had high levels of glycemia and were not treated with insulin. For this reason, future studies are needed to evaluate if RSV affects anxiety behavior in streptozotocin-induced diabetic mice with insulin treatment. In addition, it will be interesting to know if systems apart from the dopaminergic and serotoninergic ones have an effect on the diabetic animal’s behavior when RSV is administered.
Other important aspects to evaluate could be the doses and alternative routes of RSV administration. In this sense, Vissiennon et al. [79] worked with others flavonoids (such as kaempferol and quercetin) in mice and found that the anxiolytic effect was observed only after oral administration, while this effect was not observed after intraperitoneal administration. The authors suggested that these flavonoids can be transformed to active metabolites by intestinal microbiota. In the same work, the authors also observed the effect of doses of the respective flavonoids (kaempferol and quercetin) on anxiety behavior in mice. Both flavonoids showed an anxiolytic effect at the concentrations of 0.5 and 1.0 mg/kg administered by the oral route, but no anxiolytic effect was observed at lower (0.1 mg/kg) or higher doses (2.0 mg/kg). For the specific case of RSV, it was reported that it provokes changes at the CNS level under oral or intraperitoneal administration [34, 68, 81]. Gacar et al. [34] administered different intraperitoneal doses of RSV in rats and observed that only the higher doses (50 mg/kg) caused the spatial memory in the Morris water maze test to improve, while no effects were observed with lower doses (12.5 and 25 mg/kg). Schmatz et al. [68] suggest that RSV (intraperitoneal administration at 10 and 20 mg/kg) can improve learning and memory in diabetic rats. In addition, Xu et al. [81] observed that RSV (oral administration in mice) increased dopamine and serotonine levels in several mouse brain areas in a dose-dependent manner. However, it will be necessary to perform more studies in diabetic animals using several doses of RSV by different routes of administration to determine its effects on anxiety behavior.
The welfare of an individual was defined by Broom [18] as “its state as regards its attempts to cope with its environment.” Since in our study the RSV had an anxiolytic effect in diabetic mice, it is suggested that RSV may help diabetic animals to adapt to new situations of stress and anxiety and thus to improve their welfare.
In conclusion, RSV provoked anxiolytic-like effects in streptozotocin-induced diabetic mice, and these effects were observed in a short time window (2 weeks) of treatment. It is suggested that RSV may help diabetic animals to adapt to new situations of stress and anxiety and thus to improve their welfare.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The research described in this article was supported by the “Agencia Nacional de Investigación e Innovación” of Uruguay (ANII, FCE_2009_1_2887) and by the PEDECIBA. The authors thank Dr. Natalia Uriarte for critical review of the article, Dr. Cecilia Scorza for the use of their equipment and facilities to perform the behavioral test, Dr. Aldo Calliari for his help with animal handling, and Hector Rodríguez for animal care. This paper was revised for linguistic presicion by Ms. Rita Pessano.
References
- 1.Abraham P.M., Kuruvilla K.P., Mathew J., Malat A., Joy S., Paulose C.S.2010. Alterations in hippocampal serotonergic and INSR function in streptozotocin induced diabetic rats exposed to stress: neuroprotective role of pyridoxine and Aegle marmelose. J. Biomed. Sci. 17: 78. doi: 10.1186/1423-0127-17-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarzadeh A., Norouzian D., Mehrabi M.R., Jamshidi S., Farhangi A., Verdi A.A., Mofidian S.M., Rad B.L.2007. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 22: 60–64. doi: 10.1007/BF02913315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez E.O., Beauquis J., Revsin Y., Banzan A.M., Roig P., De Nicola A.F., Saravia F.2009. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav. Brain Res. 198: 224–230. doi: 10.1016/j.bbr.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 4.Anderson R.J., Freedland K.E., Clouse R.E., Lustman P.J.2001. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 24: 1069–1078. doi: 10.2337/diacare.24.6.1069 [DOI] [PubMed] [Google Scholar]
- 5.Athar M., Back J.H., Tang X., Kim K.H., Kopelovich L., Bickers D.R., Kim A.L.2007. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 224: 274–283. doi: 10.1016/j.taap.2006.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagdy G., Kalogeras K.T., Szemeredi K.1992. Effect of 5-HT1C and 5-HT2 receptor stimulation on excessive grooming, penile erection and plasma oxytocin concentrations. Eur. J. Pharmacol. 229: 9–14. doi: 10.1016/0014-2999(92)90279-D [DOI] [PubMed] [Google Scholar]
- 7.Bannerman D.M., Grubb M., Deacon R.M., Yee B.K., Feldon J., Rawlins J.N.2003. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav. Brain Res. 139: 197–213. doi: 10.1016/S0166-4328(02)00268-1 [DOI] [PubMed] [Google Scholar]
- 8.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., Pistell P.J., Poosala S., Becker K.G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K.W., Spencer R.G., Lakatta E.G., Le Couteur D., Shaw R.J., Navas P., Puigserver P., Ingram D.K., de Cabo R., Sinclair D.A.2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342. doi: 10.1038/nature05354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baur J.A., Sinclair D.A.2006. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5: 493–506. doi: 10.1038/nrd2060 [DOI] [PubMed] [Google Scholar]
- 10.Belviranli M., Atalik K.E., Okudan N., Gökbel H.2012. Age and sex affect spatial and emotional behaviors in rats: the role of repeated elevated plus maze test. Neuroscience 227: 1–9. doi: 10.1016/j.neuroscience.2012.09.036 [DOI] [PubMed] [Google Scholar]
- 11.Belzung C., Griebel G.2001. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 125: 141–149. doi: 10.1016/S0166-4328(01)00291-1 [DOI] [PubMed] [Google Scholar]
- 12.Beninger R.J., Mazurski E.J., Hoffman D.C.1991. Receptor subtype-specific dopaminergic agents and unconditioned behavior. Pol. J. Pharmacol. Pharm. 43: 507–528. [PubMed] [Google Scholar]
- 13.Berridge K.C.1990. Comparative fine structure of action: rules of form and sequence in the grooming patterns of six rodent species. Behaviour 113: 21–56. doi: 10.1163/156853990X00428 [DOI] [Google Scholar]
- 14.Berridge K.C., Aldridge J.W.2000. Super-stereotypy I: enhancement of a complex movement sequence by systemic dopamine D1 agonists. Synapse 37: 194–204. doi: [DOI] [PubMed] [Google Scholar]
- 15.Bitar M., Koulu M., Rapoport S.I., Linnoila M.1986. Diabetes-induced alteration in brain monoamine metabolism in rats. J. Pharmacol. Exp. Ther. 236: 432–437. [PubMed] [Google Scholar]
- 16.Brenes J.C., Padilla M., Fornaguera J.2009. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav. Brain Res. 197: 125–137. doi: 10.1016/j.bbr.2008.08.014 [DOI] [PubMed] [Google Scholar]
- 17.Brenes J.C., Rodríguez O., Fornaguera J.2008. Differential effect of environment enrichment and social isolation on depressive-like behavior, spontaneous activity and serotonin and norepinephrine concentration in prefrontal cortex and ventral striatum. Pharmacol. Biochem. Behav. 89: 85–93. doi: 10.1016/j.pbb.2007.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Broom D.M.1986. Indicators of poor welfare. Br. Vet. J. 142: 524–526. doi: 10.1016/0007-1935(86)90109-0 [DOI] [PubMed] [Google Scholar]
- 19.Bruhwyler J., Chleide E., Liégeois J.F., Delarge J., Mercier M.1991. Effects of specific dopaminergic agonists and antagonists in the open-field test. Pharmacol. Biochem. Behav. 39: 367–371. doi: 10.1016/0091-3057(91)90193-6 [DOI] [PubMed] [Google Scholar]
- 20.Busanello A., Peroza L.R., Wagner C., Sudati J.H., Pereira R.P., Prestes A.S., Rocha J.B., Fachinetto R., Barbosa N.B.2012. Resveratrol reduces vacuous chewing movements induced by acute treatment with fluphenazine. Pharmacol. Biochem. Behav. 101: 307–310. doi: 10.1016/j.pbb.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 21.Candland D.K., Nagy Z.M.1969. The open field: some comparative data. Ann. N. Y. Acad. Sci. 159: 831–851. doi: 10.1111/j.1749-6632.1969.tb12982.x [DOI] [PubMed] [Google Scholar]
- 22.Crawley J.N., Belknap J.K., Collins A., Crabbe J.C., Frankel W., Henderson N., Hitzemann R.J., Maxson S.C., Miner L.L., Silva A.J., Wehner J.M., Wynshaw-Boris A., Paylor R.1997. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl.) 132: 107–124. doi: 10.1007/s002130050327 [DOI] [PubMed] [Google Scholar]
- 23.Cromwell H.C., Berridge K.C., Drago J., Levine M.S.1998. Action sequencing is impaired in D1A-deficient mutant mice. Eur. J. Neurosci. 10: 2426–2432. doi: 10.1046/j.1460-9568.1998.00250.x [DOI] [PubMed] [Google Scholar]
- 24.Chan O., Inouye K., Riddell M.C., Vranic M., Matthews S.G.2003. Diabetes and the hypothalamo-pituitary-adrenal (HPA) axis. Minerva Endocrinol. 28: 87–102. [PubMed] [Google Scholar]
- 25.Chen C.C., Yang J.C.1991. Effects of short and long-lasting diabetes mellitus on mouse brain monoamines. Brain Res. 552: 175–179. doi: 10.1016/0006-8993(91)90677-N [DOI] [PubMed] [Google Scholar]
- 26.Chen K.H., Hung C.C., Hsu H.H., Jing Y.H., Yang C.W., Chen J.K.2011. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-β/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 190: 45–53. doi: 10.1016/j.cbi.2011.01.033 [DOI] [PubMed] [Google Scholar]
- 27.Chen Y., Tseng S.H., Lai H.S., Chen W.J.2004. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery 136: 57–66. doi: 10.1016/j.surg.2004.01.017 [DOI] [PubMed] [Google Scholar]
- 28.Chinen C.C., Frussa-Filho R.1999. Conditioning to injection procedures and repeated testing increase SCH 23390-induced catalepsy in mice. Neuropsychopharmacology 21: 670–678. doi: 10.1016/S0893-133X(99)00061-5 [DOI] [PubMed] [Google Scholar]
- 29.Doron R., Lotan D., Rak-Rabl A., Raskin-Ramot A., Lavi K., Rehavi M.2012. Anxiolytic effects of a novel herbal treatment in mice models of anxiety. Life Sci. 90: 995–1000. doi: 10.1016/j.lfs.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 30.Enginar N., Hatipoğlu I., Firtina M.2008. Evaluation of the acute effects of amitriptyline and fluoxetine on anxiety using grooming analysis algorithm in rats. Pharmacol. Biochem. Behav. 89: 450–455. doi: 10.1016/j.pbb.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 31.Fischer A., Sananbenesi F., Pang P.T., Lu B., Tsai L.H.2005. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48: 825–838. doi: 10.1016/j.neuron.2005.10.033 [DOI] [PubMed] [Google Scholar]
- 32.Fraga M.C., Moura E.G., Silva J.O., Bonomo I.T., Filgueiras C.C., Abreu-Villaça Y., Passos M.C., Lisboa P.C., Manhães A.C.2011. Maternal prolactin inhibition at the end of lactation affects learning/memory and anxiety-like behaviors but not novelty-seeking in adult rat progeny. Pharmacol. Biochem. Behav. 100: 165–173. doi: 10.1016/j.pbb.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 33.Frémont L.2000. Biological effects of resveratrol. Life Sci. 66: 663–673. doi: 10.1016/S0024-3205(99)00410-5 [DOI] [PubMed] [Google Scholar]
- 34.Gacar N., Mutlu O., Utkan T., Komsuoglu Celikyurt I., Gocmez S.S., Ulak G.2011. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 99: 316–323. doi: 10.1016/j.pbb.2011.05.017 [DOI] [PubMed] [Google Scholar]
- 35.Gentsch C., Lichtsteiner M., Feer H.1987. Open field and elevated plus-maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behav. Brain Res. 25: 101–107. doi: 10.1016/0166-4328(87)90003-9 [DOI] [PubMed] [Google Scholar]
- 36.Gispen W.H., Isaacson R.L.1981. ACTH-induced excessive grooming in the rat. Pharmacol. Ther. 12: 209–246. doi: 10.1016/0163-7258(81)90081-4 [DOI] [PubMed] [Google Scholar]
- 37.Graf M., Kantor S., Anheuer Z.E., Modos E.A., Bagdy G.2003. m-CPP-induced self-grooming is mediated by 5-HT2C receptors. Behav. Brain Res. 142: 175–179. doi: 10.1016/S0166-4328(02)00404-7 [DOI] [PubMed] [Google Scholar]
- 38.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., Scherer B., Sinclair D.A.2003. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196. doi: 10.1038/nature01960 [DOI] [PubMed] [Google Scholar]
- 39.Joffe I.I., Travers K.E., Perreault-Micale C.L., Hampton T., Katz S.E., Morgan J.P., Douglas P.S.1999. Abnormal cardiac function in the streptozotocin-induced non-insulin-dependent diabetic rat: noninvasive assessment with doppler echocardiography and contribution of the nitric oxide pathway. J. Am. Coll. Cardiol. 34: 2111–2119. doi: 10.1016/S0735-1097(99)00436-2 [DOI] [PubMed] [Google Scholar]
- 40.Kajiyama Y., Iijima Y., Chiba S., Furuta M., Ninomiya M., Izumi A., Shibata S., Kunugi H.2010. Prednisolone causes anxiety- and depression-like behaviors and altered expression of apoptotic genes in mice hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 34: 159–165. doi: 10.1016/j.pnpbp.2009.10.018 [DOI] [PubMed] [Google Scholar]
- 41.Kalueff A.V., Tuohimaa P.2004. Grooming analysis algorithm for neurobehavioural stress research. Brain Res. Brain Res. Protoc. 13: 151–158. doi: 10.1016/j.brainresprot.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 42.Kalueff A.V., Tuohimaa P.2005. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J. Neurosci. Methods 143: 169–177. doi: 10.1016/j.jneumeth.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 43.Kennett G.A., Whitton P., Shah K., Curzon G.1989. Anxiogenic-like effects of mCPP and TFMPP in animal models are opposed by 5-HT1C receptor antagonists. Eur. J. Pharmacol. 164: 445–454. doi: 10.1016/0014-2999(89)90252-5 [DOI] [PubMed] [Google Scholar]
- 44.Kim D., Nguyen M.D., Dobbin M.M., Fischer A., Sananbenesi F., Rodgers J.T., Delalle I., Baur J.A., Sui G., Armour S.M., Puigserver P., Sinclair D.A., Tsai L.H.2007. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 26: 3169–3179. doi: 10.1038/sj.emboj.7601758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koo S.H., Montminy M.2006. In vino veritas: a tale of two sirt1s? Cell 127: 1091–1093. doi: 10.1016/j.cell.2006.11.034 [DOI] [PubMed] [Google Scholar]
- 46.Kumar A., Sharma S.S.2010. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem. Biophys. Res. Commun. 394: 360–365. doi: 10.1016/j.bbrc.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 47.Lacković Z., Salković M., Kuci Z., Relja M.1990. Effect of long-lasting diabetes mellitus on rat and human brain monoamines. J. Neurochem. 54: 143–147. doi: 10.1111/j.1471-4159.1990.tb13294.x [DOI] [PubMed] [Google Scholar]
- 48.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J.2006. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122. doi: 10.1016/j.cell.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 49.Le Foll B., Diaz J., Sokoloff P.2005. Neuroadaptations to hyperdopaminergia in dopamine D3 receptor-deficient mice. Life Sci. 76: 1281–1296. doi: 10.1016/j.lfs.2004.09.018 [DOI] [PubMed] [Google Scholar]
- 50.Leedom L.J., Meehan W.P., Zeidler A.1987. Avoidance responding in mice with diabetes mellitus. Physiol. Behav. 40: 447–451. doi: 10.1016/0031-9384(87)90029-1 [DOI] [PubMed] [Google Scholar]
- 51.Leone T.C., Lehman J.J., Finck B.N., Schaeffer P.J., Wende A.R., Boudina S., Courtois M., Wozniak D.F., Sambandam N., Bernal-Mizrachi C., Chen Z., Holloszy J.O., Medeiros D.M., Schmidt R.E., Saffitz J.E., Abel E.D., Semenkovich C.F., Kelly D.P.2005. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3: e101. doi: 10.1371/journal.pbio.0030101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lever C., Burton S., O’Keefe J.2006. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 17: 111–133. doi: 10.1515/REVNEURO.2006.17.1-2.111 [DOI] [PubMed] [Google Scholar]
- 53.Lin J., Wu P.H., Tarr P.T., Lindenberg K.S., St-Pierre J., Zhang C.Y., Mootha V.K., Jäger S., Vianna C.R., Reznick R.M., Cui L., Manieri M., Donovan M.X., Wu Z., Cooper M.P., Fan M.C., Rohas L.M., Zavacki A.M., Cinti S., Shulman G.I., Lowell B.B., Krainc D., Spiegelman B.M.2004. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119: 121–135. doi: 10.1016/j.cell.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 54.Marfella R., Di Filippo C., Esposito K., Nappo F., Piegari E., Cuzzocrea S., Berrino L., Rossi F., Giugliano D., D’Amico M.2004. Absence of inducible nitric oxide synthase reduces myocardial damage during ischemia reperfusion in streptozotocin-induced hyperglycemic mice. Diabetes 53: 454–462. doi: 10.2337/diabetes.53.2.454 [DOI] [PubMed] [Google Scholar]
- 55.Miyata S., Yamada N., Hirano S., Tanaka S., Kamei J.2007. Diabetes attenuates psychological stress-elicited 5-HT secretion in the prefrontal cortex but not in the amygdala of mice. Brain Res. 1147: 233–239. doi: 10.1016/j.brainres.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 56.Moody T.W., Merali Z., Crawley J.N.1988. The effects of anxiolytics and other agents on rat grooming behavior. Ann. N. Y. Acad. Sci. 525: 281–290. doi: 10.1111/j.1749-6632.1988.tb38613.x [DOI] [PubMed] [Google Scholar]
- 57.Nielsen L.B., Bartels E.D., Bollano E.2002. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J. Biol. Chem. 277: 27014–27020. doi: 10.1074/jbc.M203458200 [DOI] [PubMed] [Google Scholar]
- 58.Penumathsa S.V., Thirunavukkarasu M., Zhan L., Maulik G., Menon V.P., Bagchi D., Maulik N.2008. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell. Mol. Med. 12: (6A): 2350–2361. doi: 10.1111/j.1582-4934.2008.00251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Permutt M.A., Wasson J., Cox N.2005. Genetic epidemiology of diabetes. J. Clin. Invest. 115: 1431–1439. doi: 10.1172/JCI24758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pietropaolo S., Branchi I., Cirulli F., Chiarotti F., Aloe L., Alleva E.2004. Long-term effects of the periadolescent environment on exploratory activity and aggressive behaviour in mice: social versus physical enrichment. Physiol. Behav. 81: 443–453. doi: 10.1016/j.physbeh.2004.02.022 [DOI] [PubMed] [Google Scholar]
- 61.Ramanathan M., Jaiswal A., Bhattacharya S.1997. Brain monoamines and metabolites during early streptozotocin-induced diabetes in rats. Biog. Amines 13: 55–63. [Google Scholar]
- 62.Ramanathan M., Jaiswal A.K., Bhattacharya S.K.1998. Differential effects of diazepam on anxiety in streptozotocin induced diabetic and non-diabetic rats. Psychopharmacology (Berl.) 135: 361–367. doi: 10.1007/s002130050523 [DOI] [PubMed] [Google Scholar]
- 63.Reijmer Y.D., van den Berg E., Ruis C., Kappelle L.J., Biessels G.J.2010. Cognitive dysfunction in patients with type 2 diabetes. Diabetes Metab. Res. Rev. 26: 507–519. doi: 10.1002/dmrr.1112 [DOI] [PubMed] [Google Scholar]
- 64.Rogina B., Helfand S.L.2004. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 101: 15998–16003. doi: 10.1073/pnas.0404184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sachs B.D.1988. The development of grooming and its expression in adult animals. Ann. N. Y. Acad. Sci. 525: 1–17. doi: 10.1111/j.1749-6632.1988.tb38591.x [DOI] [PubMed] [Google Scholar]
- 66.Saiko P., Szakmary A., Jaeger W., Szekeres T.2008. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. 658: 68–94. doi: 10.1016/j.mrrev.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 67.Saravia F.E., Gonzalez S.L., Roig P., Alves V., Homo-Delarche F., De Nicola A.F.2001. Diabetes increases the expression of hypothalamic neuropeptides in a spontaneous model of type I diabetes, the nonobese diabetic (NOD) mouse. Cell. Mol. Neurobiol. 21: 15–27. doi: 10.1023/A:1007165127420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmatz R., Mazzanti C.M., Spanevello R., Stefanello N., Gutierres J., Corrêa M., da Rosa M.M., Rubin M.A., Chitolina Schetinger M.R., Morsch V.M.2009. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 610: 42–48. doi: 10.1016/j.ejphar.2009.03.032 [DOI] [PubMed] [Google Scholar]
- 69.Schmatz R., Perreira L.B., Stefanello N., Mazzanti C., Spanevello R., Gutierres J., Bagatini M., Martins C.C., Abdalla F.H., Daci da Silva Serres J., Zanini D., Vieira J.M., Cardoso A.M., Schetinger M.R., Morsch V.M.2012. Effects of resveratrol on biomarkers of oxidative stress and on the activity of delta aminolevulinic acid dehydratase in liver and kidney of streptozotocin-induced diabetic rats. Biochimie 94: 374–383. doi: 10.1016/j.biochi.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 70.Shiomi T., Tsutsui H., Ikeuchi M., Matsusaka H., Hayashidani S., Suematsu N., Wen J., Kubota T., Takeshita A.2003. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J. Am. Coll. Cardiol. 42: 165–172. doi: 10.1016/S0735-1097(03)00509-6 [DOI] [PubMed] [Google Scholar]
- 71.Silan C.2008. The effects of chronic resveratrol treatment on vascular responsiveness of streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 31: 897–902. doi: 10.1248/bpb.31.897 [DOI] [PubMed] [Google Scholar]
- 72.Spruijt B.M., van Hooff J.A., Gispen W.H.1992. Ethology and neurobiology of grooming behavior. Physiol. Rev. 72: 825–852. [DOI] [PubMed] [Google Scholar]
- 73.Stoessl A.J.1994. Dopamine D1 receptor agonist-induced grooming is blocked by the opioid receptor antagonist naloxone. Eur. J. Pharmacol. 259: 301–303. doi: 10.1016/0014-2999(94)90657-2 [DOI] [PubMed] [Google Scholar]
- 74.Sulaiman M., Matta M.J., Sunderesan N.R., Gupta M.P., Periasamy M., Gupta M.2010. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 298: H833–H843. doi: 10.1152/ajpheart.00418.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thirunavukkarasu M., Penumathsa S.V., Koneru S., Juhasz B., Zhan L., Otani H., Bagchi D., Das D.K., Maulik N.2007. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic. Biol. Med. 43: 720–729. doi: 10.1016/j.freeradbiomed.2007.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trulson M.E., Himmel C.D.1985. Effects of insulin and streptozotocin-induced diabetes on brain norepinephrine metabolism in rats. J. Neurochem. 44: 1873–1876. doi: 10.1111/j.1471-4159.1985.tb07182.x [DOI] [PubMed] [Google Scholar]
- 77.Tuli J.S., Smith J.A., Morton D.B.1995. Stress measurements in mice after transportation. Lab. Anim. 29: 132–138. doi: 10.1258/002367795780740249 [DOI] [PubMed] [Google Scholar]
- 78.Uriarte N., Ferreira A., Rosa X.F., Sebben V., Lucion A.B.2008. Overlapping litters in rats: effects on maternal behavior and offspring emotionality. Physiol. Behav. 93: 1061–1070. doi: 10.1016/j.physbeh.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 79.Vissiennon C., Nieber K., Kelber O., Butterweck V.2012. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—are they prodrugs? J. Nutr. Biochem. 23: 733–740. doi: 10.1016/j.jnutbio.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 80.Wessels A.M., Scheltens P., Barkhof F., Heine R.J.2008. Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur. J. Pharmacol. 585: 88–96. doi: 10.1016/j.ejphar.2007.11.080 [DOI] [PubMed] [Google Scholar]
- 81.Xu Y., Wang Z., You W., Zhang X., Li S., Barish P.A., Vernon M.M., Du X., Li G., Pan J., Ogle W.O.2010. Antidepressant-like effect of trans-resveratrol: Involvement of serotonin and noradrenaline system. Eur. Neuropsychopharmacol. 20: 405–413. doi: 10.1016/j.euroneuro.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 82.Zarrindast M.R., Babapoor-Farrokhran S., Babapoor-Farrokhran S., Rezayof A.2008. Involvement of opioidergic system of the ventral hippocampus, the nucleus accumbens or the central amygdala in anxiety-related behavior. Life Sci. 82: 1175–1181. doi: 10.1016/j.lfs.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 83.Zhang L.M., Yao J.Z., Li Y., Li K., Chen H.X., Zhang Y.Z., Li Y.F.2012. Anxiolytic effects of flavonoids in animal models of posttraumatic stress disorder. Evid. Based Complement. Alternat. Med. 2012: 623753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zunino S.J., Storms D.H., Newman J.W., Pedersen T.L., Keen C.L., Ducore J.M.2012. Resveratrol given intraperitoneally does not inhibit the growth of high-risk t(4;11) acute lymphoblastic leukemia cells in a NOD/SCID mouse model. Int. J. Oncol. 40: 1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]