Abstract
Human tumor tissue line models established in the severely immunodeficient NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic (NOD/Shi-scid, IL-2Rγnull or NOG) mouse are important tools for oncology research. During the establishment process, a lymphoproliferative lesion (LPL) that replaces the original tumor cells in the site of transplantation occurs. In the present study, we studied the impact of the LPL on the establishment process and the characteristics of the lesion, investigated the systemic distribution of the lesion in the mouse, and evaluated the potential of a simple identification method. The incidence of the lesion varied among tumor types, and the lesion was found to be the leading cause of unsuccessful establishment with gastric and colorectal cancer. The lesion consisted of a varying population of proliferating lymphoid cells that expressed CD20. The cells were positive for Epstein-Barr virus (EBV)-related antigens, and EBV DNA was detected. There was systemic distribution of the lesion within the NOG mouse, and the most consistent gross finding was splenomegaly. Additionally, identification of LPL-affected cases was possible by detecting splenomegaly in the 1st and 2nd generation mice at necropsy. From our findings the lesion was judged to arise from EBV-infected B cells originating from the donor, and monitoring splenomegaly at necropsy was thought effective as a simple method for identifying the lesion at an early stage of the establishment process.
Keywords: donor tissue, Epstein-Barr virus, lymphoproliferative lesion, NOG mouse
Introduction
As the level of immunodeficiency of the host is known to affect the efficiency of xenotransplantation, several types of immunodeficient mice have been developed [6, 11]. A relatively novel type of mouse, the NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic mouse (NOD/Shi-scid, IL-2Rγnull or NOG mouse) was created by inducing null alleles for the common cytokine receptor γ-chain on a NOD-scid background [6, 11]. The mouse lacks functional T, B and NK cells and has reduced innate immunity including defects in dendritic and macrophage function, complement hemolytic activity, and other deficiencies [6, 11]. The severely immunodeficient state of this mouse is thought to contribute to better efficiency of xenotransplantation, and indeed, in a recent study, the engraftment rate of hematopoietic cells in the spleen and bone marrow of irradiated NOG mice was 86.4% compared with 65.2% in the NOD.CB17-Prkdcscid (NOD/scid) mouse [9].
We anticipated that tumor cells would readily engraft into immunodeficient mice, since the hallmarks of tumor cells include growth autonomy and the ability to invade [4, 8]. Based on this hypothesis, we attempted to establish stable human tumor tissue lines by serial transplantation of surgically excised human tumor tissues in NOG mice [3]. We were able to establish in vivo tissue lines that reproduce the features of their original patient tissues with several types of tumor tissues [3]. However, the rate of establishment was, at highest, approximately only one-third of the total number of transplanted tissues, and we found that one of the major causes for unsuccessful establishment was the proliferation of lymphoid cells (lymphoproliferative lesion, LPL) that replaced the original tumor cells at the site of implantation [3]. When conducting serial passage for the establishment of tumor tissue lines, the presence of a palpable mass is used as an indicator of tumor growth. But when an LPL occurs, the lesion also forms a palpable mass and so hinders the correct judgment of tumor intake.
The pathobiology of the LPL is unknown. But in humans, there is a well-known condition that involves the proliferation of lymphocytes infected by the Epstein-Barr virus (EBV) according to the condition of the immune system [2, 5]. There is a possibility that infected lymphocytes could acutely proliferate when transplanted into the severely immunodeficient NOG mouse. Therefore, we considered that EBV-infected lymphocytes originating from transplanted tumor tissue may be related to onset of the LPL in NOG mice.
Because of the high incidence of the lesion and also the fact that the phenomenon can be misleading during the establishment process, we considered that it would be important to study the LPL when establishing tumor tissue lines in NOG mice. In the present study, we 1) considered the impact of the LPL on the establishment process, 2) attempted to characterize this lesion for better understanding of its biology including the association with EBV, 3) investigated the systemic distribution of the lesion in the host to discover a means of identifying the LPL, and 4) evaluated the potential of a simple identification method at necropsy.
Materials and Methods
Animals
The NOG mice used to produce the xenograft tissues were provided by the breeding facility of the Central Institute for Experimental Animals (CIEA, Kanagawa, Japan), at the age of 5–6 weeks. All animals were housed in plastic cages within a bioBubble® system (bioBubble, Fort Collins, CO, USA) in a pathogen-free environment and at a temperature of 23 ± 1°C with 60–80% humidity, and a 12 h light/12 h dark cycle. Mice were fed pelleted chow (CE-2; Clea Japan Inc., Tokyo, Japan) and tap water ad libitum.
All studies and procedures involving animal subjects were approved by the Animal Care and Use Committee at PharmaLogicals Research. The animals used in this experiment were treated in accordance with the Animal Research Guideline of PharmaLogicals Research.
Transplantation of human tumor tissues into NOG mice
After an adaptation period, animals between 6 and 12 weeks of age were surgically transplanted with human tumor tissues, which were obtained at PharmaLogicals Research, Pte., Ltd.., under ether anaesthesia by the method described previously [3]. Briefly, each tissue was kept in Hanks’ balanced salt solution containing 5% penicillin, streptomycin, and a neomycin antibiotics mixture until transplantation. For tissue transplantation, tissue specimens were cut into small pieces, and approximately 200 mm3 of the tissue pieces was embedded into the subcutis with a transplant needle. The tissues were transplanted and observed until the tumor mass was approximately 1 cm3 in size or until the case was judged to have no tumor growth. All mice were terminated within 13 months after they underwent transplant.
The surgically excised tissues were obtained from consenting patients as approved by the ethical committee at PharmaLogicals Research and Parkway Laboratory Services in Singapore.
Tissue preparation and histopathological examination
At the end of the transplantation period, necropsy was carried out after exsanguination from the abdominal artery under deep anaesthesia. Transplanted tumor and the host spleen, liver, kidney, lung, heart and grossly present masses were sampled for histopathological examination and fixed in 4% paraformaldehyde. The tissues were then processed by the AMeX method [3, 12, 13]. Thin sections were prepared, stained with hematoxylin and eosin and then examined histopathologically.
Analysis of the outcome of transplanted human tumor tissues
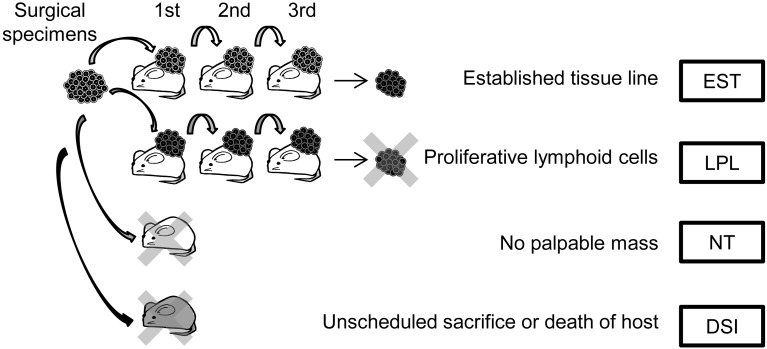
To examine the impact of the LPL on the tumor tissue line establishment process, the outcome of human tumor tissues transplanted into NOG mice to establish tumor tissue lines in vivo was examined. A total of 50 colorectal cancer, 32 gastric cancer, 76 breast cancer, and 24 lung cancer tissues were examined. Tissue lines were considered established (EST) when a palpable mass was formed for 3 serial passages (Fig. 1). Next, we determined the cases in which the mass turned out to be composed of an LPL (Fig. 1). Other outcomes included cases in which no palpable masses were formed (no tumor; NT) or cases in which the procedure was terminated because of the state of the host (found dead, sick or developed infection; DSI) (Fig. 1).
Fig. 1.
Analysis of the outcome of transplanted human tumor tissues. The transplanted tumor tissues were passaged through 3 generations for establishment (EST). In some cases, the palpable mass formed after transplantation consisted of a lymphoproliferative lesion (LPL) that was thought to replace the original tumor cells. In other cases, establishment was unsuccessful because no palpable mass was formed after transplantation (NT) or because of unscheduled death of the mouse (DSI).
Characterization of the LPL in the palpable mass
We analyzed 7 tissues from 7 original patient tissues that were transplanted with the objective of carrying out the establishment process of tumor tissue lines in the NOG mouse. A palpable mass was formed after transplantation in these cases, and the masses were passaged for 2 to 5 generations, but were found to be affected by the LPL when examined histopathologically. The morphological characteristics of the LPL in the mass of the host were determined histopathologically, and the expression of cell surface markers was examined by immunohistochemistry (IHC). Additionally, Epstein-Barr virus (EBV)-related antigens were examined by IHC, and the presence of EBV DNA was examined by PCR. The primary antibodies used for IHC were mouse antibodies raised against human CD3 (clone F7.2.38, DakoCytomation, Glostrup, Denmark), human CD20 (clone L26, DakoCytomation), human CD68 (clone KP1, DakoCytomation), EBV-encoded nuclear antigen 2 (EBNA2, clone PE2, DakoCytomation), and Latent membrane protein 1 (LMP1, clone CS1-4, DakoCytomation). Staining was performed using the Ventana HX Discovery system (Ventana Medical Systems, Inc., Tucson, AZ, USA). For PCR analysis, isolation of DNA was carried out using a QIAamp DNA Micro Kit (Qiagen GmbH, Hilden, Germany). Amplification of the target sequence for BamC was carried out using a primer set (Virus, Epstein-Barr virus Bam C, Primer set kit; Maxim Biotech, Inc., Rockville, MD, USA) according to the instructions of the manufacturer with a Perkin Elmer 9600 Gene Amp PCR system (Perkin Elmer, Waltham, MA, USA). The PCR products were evaluated using an the Agilent 2100 Bioanalyzer and a DNA500 LabChip kit (Agilent Technologies, Santa Clara, CA, USA).
Examination of the systemic distribution of the LPL in NOG mice
The systemic distribution of the LPL in the host was examined to investigate the possibility of identifying the lesion at necropsy. Six mice that developed palpable masses affected by an LPL after transplantation with 1st generation colorectal cancer tissue were examined for gross findings. The palpable mass, spleen, liver, kidney, lung and other tissues with gross abnormalities were examined histopathologically. The spleen and mass were tested for EBV by PCR.
Retrospective evaluation of a simple identification method for LPL
Colorectal cancer cases were selected for this objective because the number of established tissue lines was relatively abundant for the tissue type, making it possible to compare the palpable mass of the transplantation site between the affected and non-affected cases. We selected 7 LPL (LPL group) and 8 EST (EST group) cases and examined the gross findings and organ weight of the spleen of mice transplanted with 1st and 2nd generation tissues. These findings were then compared with the microscopic findings of the spleen and palpable mass.
Results
Analysis of the outcome of transplanted human tumor tissues
The percentage of EST cases was relatively low, as in our previous study (Table 1). In non-EST cases, LPL was found to occur in all 4 tumor types examined. The incidence of the lesion varied among the tumor types. The incidence in colorectal and gastric cancer was as high as 40%, while the incidence in lung cancer was lower and was markedly low in breast cancer. LPL was the leading cause for unsuccessful establishment of tissue lines with colorectal cancer and gastric cancer. For lung cancer and breast cancer, the leading cause was NT. DSI mainly thought to be due to bacterial infection was another persistent cause and accounted for approximately 20% of unsuccessful establishment for all examined tumor types.
Table 1. Outcome of transplanted tissue in NOG mice.
| Fate | Tumor type |
|||
|---|---|---|---|---|
| Colorectal | Gastric | Breast | Lung | |
| ESTa) | 14 (28) | 4 (13) | 2 (3) | 2 (8) |
| LPLb) | 19 (38) | 13 (41) | 2 (3) | 4 (17) |
| NTc) | 3 (6) | 9 (28) | 64 (84) | 13 (54) |
| DSId) | 14 (28) | 6 (19) | 8 (11) | 5 (21) |
| Total examined | 50 | 32 | 76 | 24 |
Numbers indicate the number of cases (the numbers in the parentheses indicate the percentage to the total number of cases for each tumor type). a) Established as tumor tissue lines, b) replaced by lymphoproliferative lesion, c) no tumor tissue detected in the transplantation site, d) passage terminated because of death or infection in host.
Characterization of the LPL in the palpable mass
In the LPL cases that were passaged for several generations, a varying population of proliferating lymphoid cells, ranging from small lymphocytes or plasma cells to large atypical lymphoid cells with pleomorphic nuclei and abundant basophilic cytoplasm resembling lymphoblasts, was observed histopathologically (Fig. 2a). The LPLs were found to totally replace the original tumor cells within the mass. The mass was EBV DNA positive by PCR in all examined cases, and the EBV-related antigens EBNA2 and LMP1 were expressed in the lymphoid cells (Figs. 2b, c). The cells also expressed CD20 (Fig. 2d), but not CD3 or CD68 (data not shown), which shows that they were of B cell origin.
Fig. 2.
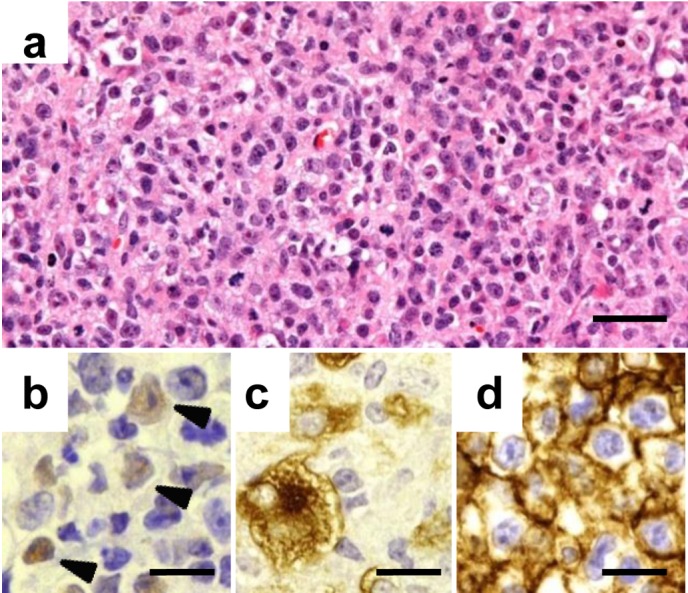
Characterization of the LPL in the palpable mass. A mixed population of lymphoid cells is observed in the palpable mass of an affected case (a). The lymphoid cells are positive for EBNA2 (b), LMP-1 (c), and human CD20 (d) by immunohistochemistry. Arrowheads indicate the positive cells in b. Bars=25 µm (a) or 15 µm (b, c, d). Hematoxylin and eosin stain (a) and labeled streptavidin-biotin method (b, c, d).
Examination of the systemic distribution of the LPL in NOG mice
We examined the palpable mass of the first generation formed in 6 mice transplanted with colorectal cancer that turned out to mainly consist of proliferating lymphoid cells. There were no remaining original tumor cells in the masses of 5 mice and only a very small number in the mass of 1 mouse. The morphology of the proliferating lymphoid cells was similar to that mentioned above, and EBV was also detected in the mass by PCR.
At necropsy, splenomegaly was observed in all cases. In addition nodules in sites such as the axillary area, the thoracic cavity, and the intestinal wall were observed in 4 cases. White foci of the liver were also found in 1 case.
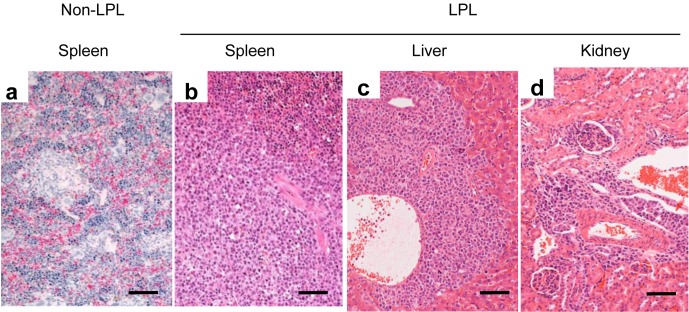
Histopathologically, a LPL was observed in the spleen, the nodules, and in the liver (Fig. 3). These findings coincided with the macroscopical findings. In addition, the lesion was seen in the kidney (Fig. 3d) and lung (data not shown).
Fig. 3.
The systemic distribution of the LPL in NOG mice. An LPL completely replacing the spleen structure (b) compared with a non-affected spleen (a) is shown. An LPL is also seen in the Glisson’s sheath of the liver and in the perivascular area in the cortex of the kidney. Bar=50 µm. Hematoxylin and eosin stain.
In the spleen, the LPL ranged from foci seen peripheral to the central artery to a diffuse lesion that completely replaced the original spleen structure (Figs. 3a, b). EBV was detected by PCR in all cases. Thus the LPL was found to not only affect the transplantation site but also to be distributed systemically within the host. The organ that was most consistently affected was the spleen, and this finding was accompanied by splenomegaly at gross examination.
Retrospective evaluation of a simple identification method for LPL
Based on the aforementioned findings, we decided to investigate the potential of gross examination of the spleen as a means to identify LPL-affected cases at an early stage of the establishment process. We examined and compared spleen tissues of the 1st and 2nd generations in 7 cases of LPL and 8 cases of EST originating from human colorectal cancer (Table 2).
Table 2. Comparison of gross and histopathological findings in mass and spleen for an early detection method.
| LPLa) |
ESTb) |
||||||
|---|---|---|---|---|---|---|---|
| 1stc) | 2ndd) | 1st | 2nd | ||||
| No. examined | 7 | 7 | 8 | 8 | |||
| Mass | Histological findings | LPL | 7 | 7 | 0 | 0 | |
| Spleen | Histological findings | LPL | 7 | 7 | 2 | 0 | |
| Gross findings | Enlarged | 6 | 6 | 3e) | 0 | ||
| Organ Weight (mg) | Mean | 122 | 389 | 90 | 25 | ||
| Maximum | 303 | 822 | 233 | 37 | |||
| Median | 72 | 477 | 42 | 24 | |||
| Minimum | 34 | 35 | 17 | 19 | |||
a), lymphoproliferative lesion; b), established tissue line; c), 1st generation of serial passage; d), 2nd generation of serial passage; e), severe extramedullary hematopoiesis was observed in 1 animal. Numerals indicate the number of animals.
Splenomegaly characterized by enlargement of the spleen and high organ weight was found in several cases in the LPL and EST groups. In the 1st generation, splenomegaly was observed in 6/7 cases in the LPL group and in 3/8 cases in the EST group. Histopathologically, the spleen and mass of all cases with (6/7 cases) or without (1/7 cases) splenomegaly in the LPL group were affected by LPLs. But in the EST group, an LPL was only observed in the spleen in 2/3 cases with splenomegaly.
In the 2nd generation, splenomegaly was observed only in the LPL group (6/7 cases). Histopathologically, LPL was also observed in the spleen and mass in all cases in the LPL group. In the EST group, no LPLs were observed in the 2nd generation, including the cases that had splenomegaly in the 1st generation.
The cases with splenomegaly in the 2nd generation were all found to be affected by LPL. The cases that did not have splenomegaly in either generations were free of the lesion. In 4 cases, splenomegaly was observed in the 1st generation but not in the 2nd generation, and these cases included 1 LPL case and 3 EST cases (Table 2).
Discussion
Tumor tissue lines established in the NOG mouse capture the morphological features of their original surgical tissues, a characteristic that can be repeatedly produced when transplanted into a new host [3, 7]. Kobayashi et al. discovered that cancer stem cells are enriched in colon cancer tissue lines that we previously established and succeeded in establishing cancer stem cell lines from these cells [7]. Such recent developments show that the tissue lines can significantly contribute to the advancement of cancer research, so we believe that there will be a growing need for human tissue lines established in NOG mice, and that refinement of the establishment process is necessary.
The tumor cells of the original tissue type were completely or partially replaced by lymphoid cells in the LPL, so the lesion was found to have a significant impact on the establishment process, especially with gastrointestinal tumors. The LPL was characterized by an abnormal and progressive proliferation of lymphoid cells. The proliferating cells mainly consisted of a mixed population of lymphoid cells and were of B cell origin. EBV DNA and EBV-associated proteins were found in these cells. Therefore, the LPL was thought to arise from EBV-infected lymphocytes originating from the transplanted tissue.
Lymphoproliferative disorders are associated with EBV in humans [2, 5]. EBV is kept in check by the immune response of the intact immune system, but in immunosuppressed states, such as in cases of organ transplantation, EBV-driven B lymphocytes proliferate [2, 5]. B cells, especially memory B cells, are a well-known host for EBV infection and are thought to be transplanted along with the tumor cells in the NOG mouse [10, 14]. The host immune system is severely impaired in this mouse, so the environment is thought to be favorable for proliferation of infected B cells which was thought to cause a condition similar to human lymphoproliferative disorders.
Considering that more than 90% of the human population is infected with EBV and that infection persists for life, transplantation of infected lymphocytes is thought to commonly occur during the tissue line establishment process [10, 14]. Thus, it is inevitable that LPLs would occur at a certain rate. Based on this, we considered that if there was a simple way to identify the lesion at an early stage of the establishment process, it would be beneficial in reducing the number of animals necessary to establish tissue lines, which would enhance the efficiency of the process.
To accomplish this, we attempted to find a method that would enable identification of an LPL at necropsy. We thought that this would be possible because we found that the spleen of the host was consistently affected in cases in which a palpable mass contained an LPL and that the presence of an LPL correlated well with splenomegaly. We found that there was a clear difference between the findings in the spleen of EST and LPL cases in both the 1st and 2nd generations. In LPL cases, there was proliferation of lymphoid cells in the transplantation site as well as in the spleen, along with splenomegaly in most of the 1st and 2nd generation mice, but in EST cases, this was observed only in the spleen of a small number of 1st generation animals. From these results, we concluded that splenomegaly was in fact a good indicator of the presence of an LPL at necropsy.
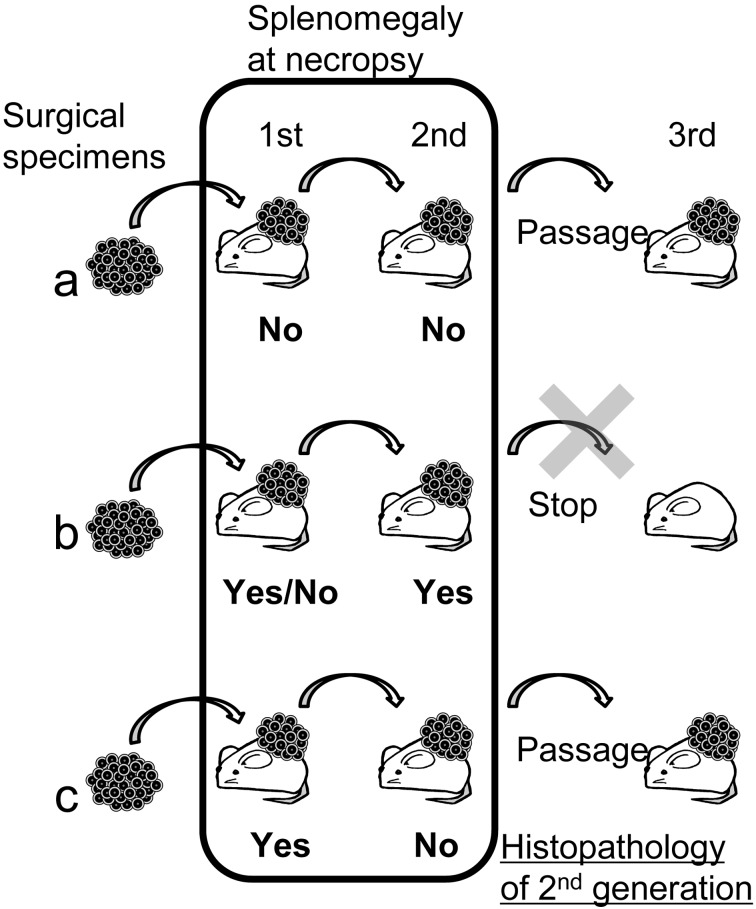
Based on our present findings, we recommend that the judgment for passage be made according to the presence of splenomegaly at necropsy in 1st and 2nd generation mice (Fig. 4). In cases with no splenomegaly in either the 1st or 2nd generation, then the judgment would be to proceed with passage to the 3rd generation (Fig. 4a). If there is splenomegaly in the 2nd generation, then the chances of the case being affected by an LPL is high, so the judgment would be to not proceed with passage (Fig. 4b). In cases with splenomegaly in only the 1st generation, we would recommend proceeding with passage but to immediately carry out histopathology of the mass at the 2nd generation; if the presence of an LPL is confirmed microscopically, the 3rd generation should be terminated (Fig. 4c). If the mass is free of LPLs then the 3rd generation should be continued (Fig. 4c). Although we have recommended a simple method, the most sensitive method for detection of this lesion is histopathological examination. So if the study scale is relatively small and histopathological examination can be performed immediately for all cases, then a decision to continue the 2nd generation should be made after microscopic examination of the 1st generation.
Fig. 4.
Recommendation of a simple identification method for LPL. If there is no splenomegaly in the 1st or 2nd generation then passage should proceed to the 3rd generation (a). If there is splenomegaly in the 2nd generation, passage should be stopped, and the line should be terminated (b). If there is splenomegaly in the 1st generation but not in the 2nd generation, then passage to the 3rd generation should proceed, but immediate histopathological examination should be carried out to for a definite diagnosis (c).
Since the LPL results from immunodeficiency, methods of early attrition and prevention will become more and more important as novel immunodeficient animals are developed.
One possible solution for prevention of LPL is the administration of rituximab, a therapeutic antibody directed against the CD20 antigen that is currently widely used for the treatment of clinical lymphoma and also for posttransplant lymphoproliferative disorder in humans [1, 2, 5]. We have obtained preliminary data with 1 original patient tissue of colorectal cancer that was transplanted into 4 mice and treated with either rituximab (n=2) or saline (n=2). In the saline-treated mice, an LPL was detected in both mice with splenomegaly. On the other hand, no LPLs were detected in either rituximab-treated mouse. Moreover, when the tissues were passaged from the rituximab-treated mice, positive parenchymal cell intake of tumor cells was observed. We believe that this is promising data that may prove useful in future studies.
Acknowledgments
We would like to express our thanks to Dr. M. Watanabe, Dr. T. Watanabe, and Ms. Y.L. Yang for their skillful assistance, Dr. H. Yamada-Okabe for critical discussions concerning human tissue models, and Mr. R. Nomura for his continuous support throughout the study. We would also like to thank Dr. T. Yamazaki for his continuous encouragement and support for research at PharmaLogicals Research since 2002, which is when he realized the importance of human cancer tissue models generated in NOG mice for biological insights, including insights concerning cancer stem cells.
References
- 1.Cang S., Mukhi N., Wang K., Liu D.2012. Novel CD20 monoclonal antibodies for lymphoma therapy. J. Hematol. Oncol. 5: 64. doi: 10.1186/1756-8722-5-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evens A.M., Roy R., Sterrenberg D., Moll M.Z., Chadburn A., Gordon L.I.2010. Post-transplantation lymphoproliferative disorders: diagnosis, prognosis, and current approaches to therapy. Curr. Oncol. Rep. 12: 383–394. doi: 10.1007/s11912-010-0132-1 [DOI] [PubMed] [Google Scholar]
- 3.Fujii E., Suzuki M., Matsubara K., Watanabe M., Chen Y.J., Adachi K., Ohnishi Y., Tanigawa M., Tsuchiya M., Tamaoki N.2008. Establishment and characterization of in vivo human tumor models in the NOD/SCID/γ(c)(null) mouse. Pathol. Int. 58: 559–567. doi: 10.1111/j.1440-1827.2008.02271.x [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A.2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Heslop H.E.2009. How I treat EBV lymphoproliferation. Blood 114: 4002–4008. doi: 10.1182/blood-2009-07-143545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M., Kobayashi K., Nakahata T.2008. NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr. Top. Microbiol. Immunol. 324: 53–76. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi S., Yamada-Okabe H., Suzuki M., Natori O., Kato A., Matsubara K., Jau Chen Y., Yamazaki M., Funahashi S., Yoshida K., Hashimoto E., Watanabe Y., Mutoh H., Ashihara M., Kato C., Watanabe T., Yoshikubo T., Tamaoki N., Ochiya T., Kuroda M., Levine A.J., Yamazaki T.2012. LGR5-positive colon cancer stem cells interconvert with drug-resistant LGR5-negative cells and are capable of tumor reconstitution. Stem Cells 30: 2631–2644. doi: 10.1002/stem.1257 [DOI] [PubMed] [Google Scholar]
- 8.Kumar V., Cotran R.S., Robbins S.L.2003. Robbins Basic Pathology, 7th ed. Saunders, Pennsylvania. [Google Scholar]
- 9.McDermott S.P., Eppert K., Lechman E.R., Doedens M., Dick J.E.2010. Comparison of human cord blood engraftment between immunocompromised mouse strains. Blood 116: 193–200. doi: 10.1182/blood-2010-02-271841 [DOI] [PubMed] [Google Scholar]
- 10.Niller H.H., Wolf H., Minarovits J.2008. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity 41: 298–328. doi: 10.1080/08916930802024772 [DOI] [PubMed] [Google Scholar]
- 11.Pearson T., Greiner D.L., Shultz L.D.2008. Humanized SCID mouse models for biomedical research. Curr. Top. Microbiol. Immunol. 324: 25–51. [DOI] [PubMed] [Google Scholar]
- 12.Sato Y., Mukai K., Watanabe S., Goto M., Shimosato Y.1986. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am. J. Pathol. 125: 431–435. [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki M., Katsuyama K., Adachi K., Ogawa Y., Yorozu K., Fujii E., Misawa Y., Sugimoto T.2002. Combination of fixation using PLP fixative and embedding in paraffin by the AMeX method is useful for histochemical studies in assessment of immunotoxicity. J. Toxicol. Sci. 27: 165–172. doi: 10.2131/jts.27.165 [DOI] [PubMed] [Google Scholar]
- 14.Thorley-Lawson D.A., Allday M.J.2008. The curious case of the tumour virus: 50 years of Burkitt’s lymphoma. Nat. Rev. Microbiol. 6: 913–924. doi: 10.1038/nrmicro2015 [DOI] [PubMed] [Google Scholar]