Abstract
We have developed NOD-Rag2null IL-2Rγnull (NR2G) mice similar to NOD-scidIL-2Rγnull (NOG) mice that are known as an excellent host to generate humanized mice. To evaluate the usefulness of NR2G mice as a host for humanized mice, the engraftment rates and differentiation of human cells after human hematopoietic stem cell (HSC) transplantation were compared among NR2G, NOG, and NOD-scid mice. For this purpose, the appropriate irradiation doses to expand the niche for human stem cells in the bone marrow were first determined. As a result, 8 and 2.5 Gy in adult, and 4 and 1 Gy in newborn NR2G and NOG mice, respectively, were found to be appropriate. Next, 5 × 104 human umbilical cord blood CD34+ cells were intravenously inoculated into irradiated adult or newborn of the immunodeficient mice. These HSC transplantation experiments demonstrated that both NR2G and NOG mice showed high engraftment rates compared with NOD-scid mice, although NOG mice showed a slightly higher engraftment rate than that for NR2G mice. However, no difference was found in the human cell populations differentiated from HSCs between NR2G and NOG mice. The HSC transplantation experiments to adults and newborns of two immunodeficient mice also revealed that the HSC transplantation into newborn mice resulted in higher engraftment rate than those into adults. These results showed that NR2G mice could be used as an alternative host to NOG mice to generate humanized mice.
Keywords: humanized mice, immunodeficient mice, NOD-Rag2null IL-2Rγnull mice, NOG mice
Introduction
Immunodeficient mice harboring human cells or tissues are considered useful for analyzing human biology in vivo without the ethical constraints associated with using humans themselves. These mouse models, termed humanized mice, would significantly advance our understanding of various human diseases and would facilitate development of new drugs against human diseases [18].
Recent advances in the development of newly immunodeficient mice such as NOD-scid IL-2Rγnull (NOG/NSG) and BALB/c-Rag2null IL-2Rγnull (BR2G) mice have enabled the generation of humanized mice in which various human immune cells are successfully developed, and have promoted research in human biology and diseases [6, 14, 16]. Indeed, those studies not only showed an extremely high engraftment efficacy but also the development of multi-lineage human cells, including various subsets of T cells after transplantation of human umbilical cord blood (CB)-derived CD34+ stem cells (HSCs) [4].
To generate appropriate humanized mice, the choice of immunodeficient mouse strains, human stem cell source, inoculation route, and mouse age used for cell transplantation are considered to be important. For a stem cell source, Lepus et al. [8] recently compared the engraftment and differentiation of human cells from various sources using three types of immunodeficient mice—NSG, BR2G, and C.B-17-scid/bg mice—and concluded that the use of CD34+ stem cells from fetal liver and CB is suitable for studying human hematopoietic cell lineage development and function in humanized mice. However, the use of cells from aborted fetuses is not always feasible because of ethical issues in some countries. In this sense, the use of stem cells from CB may be more convenient for collection to circumvent ethical issues. For an inoculation route, intravenous inoculation has been used commonly for adult mice; by contrast, intrahepatic or intravenous inoculation has been used for newborn mice [4, 15]. However, it remains unresolved which route generates humanized mice more efficiently because of the different conditions, such as cell sources and mouse strains, used by researchers.
Regarding mouse strains, NOG/NSG and BRG mice are presently generally used for the generation of humanized mice. Pearson et al. [12] recently reported radio-resistant NOD-Rag1null IL-2Rγnull (NR1G) mice, as well as NSG mice, showing high engraftment of human cells. Brehm et al. [2] compared the engraftment of human cells among NSG, NR1G, and BALB/c-Rag1null IL-2Rγnull (BR1G) mice generated based on Rag1null mice and concluded that NSG and NR1G mice showed more efficient engraftment than BR1G mice. Both Prkdc and Rag1/2 genes are responsible to compose T and B cell receptor genes resulting in T and B cell deficiency in mice mutated with both genes. SCID mice mutated with Prkdc gene but not with RAG1/2 genes have disadvantages in which irradiation sensitivity and T/B cell leakiness occurred [1, 3]. Therefore, Introduction of RAG1/2 mutation for Prkdcscid mutation into mice may provide more stable immunodeficient strain of mice for xenotransplantation. We also independently developed NOD-Rag2null IL-2Rγnull (NR2G) mice based on Rag2null mice. In the present study, we evaluated the usefulness of NR2G mice as humanized mice by comparing human cell engraftment and differentiation among NOG, NR2G, and NOD-scid mice after HSC transplantation.
Regarding mouse age, when HSCs are inoculated, adult or newborn mice have been used. Particularly, HSC inoculation into newborn mice is considered to be efficient because Traggiai et al. [16] reported humanized mice by HSC inoculation into newborn BRG mice. However, it is unclear what differences exist in the engraftment and differentiation of human cells from HSCs upon inoculation into newborn and adult mice. To address this issue, we compared the engraftment and differentiation of human cells in the mice when the same lot of CB CD34+ cells was transplanted into newborn and adult NOG, NR2G, and NOD-scid mice.
Materials and Methods
Mice
NOD.CB17-Prkdcscid/ShiJic (NOD-scid), NOD.Cg-Prkdcscid Il2rgtm1Sug/Jic (NOD-scid IL-2Rγnull: NOG), NOD.Cg-Rag2tmlFwa Il2rgtmlSug/ShiJic (NOD-Rag2null IL-2Rγnull: NR2G, here termed to distinguish from NR1G of NOD-Rag1null IL-2Rγnull mice), and BALB/c-Rag2null IL-2Rγnull (BR2G) were used in the present study. NOD-scid mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). NOG and NR2G mice were maintained at the Central Institute for Experimental Animals (CIEA). NR2G mice, first described in the present study, were generated as follows. NOD.Cg-Rag2tm1Fwa/Jic mice were generated by the six-generation backcross-mating of B6.129S1-Rag2tmlFwa/JJic mice [13], which were originally a gift from Dr. Alt F. of Columbia University, into NOD mice using a speed congenic technique combining a marker-assisted selection protocol and in vitro fertilization [15]. NR2G mice were obtained by intercross mating among the offspring (NOD-Rag2+/-IL-2Rγ+/-) between NOD-Rag2null and NOD-IL-2Rγnull mice.
For HSC transplantation experiments, adult mice were obtained by natural mating, and newborn mice were obtained by Cesarean section from recipient IQI females transplanted with NR2G embryos into the oviduct after in vitro fertilization. The newborn mice were nursed by IQI foster mothers until weaning after irradiation and cell transplantation.
The present study was performed in accordance with institutional guidelines and was approved by the Animal Experimentation Committee of CIEA.
Irradiation
Adults and newborns of NOG, NR2G, and BR2G mice were irradiated with 0–12 Gy using an X-ray irradiation device (MBR-1505R; Hitachi Medical Co., Tokyo, Japan) to determine an appropriate dose for the respective mice. The body weight of NOG, NR2G, and BR2G mice was measured each week for 4 or 8 weeks after irradiation using a scale (Pocket Scale 80; BOMSO, Tokyo, Japan).
Transplantation of human HSCs
The same lot of commercially available human CB derived CD34+ cells (Lonza, Basel, Switzerland) was used in the current study. The frozen cells were incubated for a few minutes in a 37°C water bath, and then were moved quickly into phosphate-buffered saline (PBS) containing 2% fetal bovine serum. After washing with PBS, the viability of CD34+ cells was examined by dye exclusion using 2.5% Trypan blue solution, and the cells with more than 80% viability were used for transplantation into the mice. For HSC transplantation into adult mice at 8 to 9 weeks of age, 5 × 104 of CD34+ cells were inoculated intravenously via the tail vein at 24 h after irradiation. Newborn mice were irradiated at a day after birth, and 5 × 104 CD34+ cells were inoculated intravenously via the facial vein at 24 h after irradiation.
Flow cytometry
To identify human cells in mouse peripheral blood (PB), the spleen, and the bone marrow (BM), multi-color flow cytometric analysis was performed using a FACSCanto flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) with FACS Diva software (ver. 5.0.2; Becton Dickinson).
PB was collected periodically from the retro-orbital venous plexus using a capillary pipette (Drummond Scientific, Broomall, PA, USA) coated with heparin (Novo-Heparin 5000 units for Injection; Mochida Pharmaceutical Co., Tokyo, Japan) under anesthesia with isoflurane between 8 and 20 weeks after transplantation.
Mice were sacrificed by exsanguinating under anesthesia between 22 and 23 weeks after HSC transplantation. PB was collected from an abdominal vein, and the femurs, spleens and thymuses were also removed. After preparation of single cell suspensions and following treatment with red blood cell (RBC) lysis solution (0.154 M NH4Cl, 13.770 mM NaHCO3, 0.102 mM EDTA-2Na) to eliminate RBCs, white blood cells (WBCs) were suspended in PBS containing 2% fetal bovine serum. The cells were incubated with human-specific antibodies for 30 min at 4°C under protection from light.
The anti-human antibodies used for staining were fluorescein isothiocyanate (FITC)-conjugated anti-human CD45 (clone HI30; Becton Dickinson), anti-human CD33 (clone HIM3-4; BD Pharmingen, Franklin Lakes, NJ, USA), and anti-human CD4 (clone RPA-T4; eBioscience, Inc., San Diego, CA, USA); phycoerythrin (PE)-conjugated anti-human CD3 (clone UCHT1 555333; BD Pharmingen), PE-Cy7-conjugated anti-human CD3 (clone UCHT1; Beckman Coulter, Brea, CA, USA), and anti-human CD19 (clone J4.119; Beckman Coulter); allophycocyanin (APC)-conjugated anti-human CD8a antibody (clone OKT8; eBioscience, Inc.), anti-mouse CD45 antibody (clone 30-F11; BD Pharmingen), and APC-Cy7-conjugated anti-human CD45 (clone 2D1; BD Pharmingen). The engraftment rate of human cells was expressed as a percentage of human CD45+ cells in the total human CD45+ and mouse CD45+ cells. The ratio of human immune cells was expressed as a percentage of human CD3+, CD19+, CD4+, and CD8+ cells in human CD45+ cells.
Immunochemistry
To identify human cells in the spleen of transplanted mice, the spleens were removed from the mice after blood removal, and tissues were fixed with 10 nM formalin (10 nM Mildform; Wako, Tokyo, Japan), embedded in paraffin, and stained with hematoxylin and eosin (HE), mouse anti-human CD45 antigen monoclonal antibody (clone 2B11+PD7/26; Dako Cytomation, Glostrup, Denmark), rabbit anti-human CD3 monoclonal antibody (clone SP7; Nichirei, Tokyo, Japan), and mouse anti-human CD79a monoclonal antibody (clone JCB117; Nichirei). Briefly, 5-μm-thick sections of femurs and spleens on amino-silane coated glass slides (Matsunami glass, Osaka, Japan) were immunostained by the universal immuno-enzyme polymer method (Nichirei). Each of the anti-human antibodies was incubated overnight at 4°C. Sections were serially incubated with peroxidase-labeled polymer-conjugated goat anti-mouse antibody (Histofine Simplestain Max-PO; Nichirei) for 30 min at room temperature. Immunoreaction products were visualized by incubation with 0.02% 3, 3’-diaminobenzidine (DBA; Dojindo, Kumamoto, Japan) containing 0.006% H2O2. Immunostained sections were counterstained with hematoxylin for visualization of nuclei.
Statistical analysis
Mean values and standard deviations were calculated using the Excel software (Microsoft, Redmond, WA, USA). Significant differences were identified using Student’s t-test, and a P-value less than 0.05 was deemed to indicate statistical significance.
Results
Radiation sensitivity of immunodeficient mice
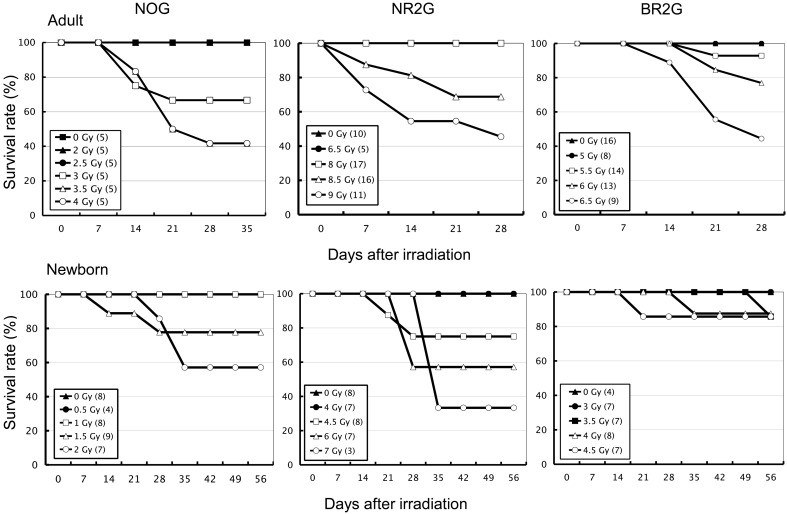
Prior to transplantation experiments of human HSCs, the radiation sensitivity of adult and newborn mice of NOG, NR2G, and BR2G mice was investigated to determine an appropriate irradiation dose for HSC transplantation in each mouse strain. After irradiation into the mice with a dose ranging from 0.5 to 12 Gy, the body weight was periodically measured, and general observation was also performed for 26 days in adults and 56 days in newborns after irradiation, respectively. Non-irradiated mice in each strain were used as controls. As shown in Fig. 1, the minimum irradiation doses for the survival of all mice of each strain were 2.5 Gy, 8 Gy, and 5.5 Gy in adults, and 1 Gy, 4 Gy, and 3.5 Gy in newborns of NOG, NR2G, and BR2G mice, respectively. Thus extremely high resistance to irradiation was observed in NR2G mice.
Fig. 1.
Sensitivity of immunodeficient mice to total-body irradiation. Adult and newborn NOG, NR2G, and BR2G mice were irradiated with 0–12 Gy using an X-ray irradiation device to determine the appropriate dose. The body weight of NOG, NR2G, and BR2G mice was measured every week beginning 4 or 8 weeks after irradiation using a scale.
Engraftment of human cells after HSC transplantation into adults and newborns of immunodeficient mice
The engraftment and differentiation of human cells were investigated when HSCs were transplanted into adult or newborn NOG, NR2G, and NOD-scid mice using the experimental protocol shown in Table 1. The 2.5-Gy dose of irradiation for adult NOD-scid mice used here was described previously [17], and this dose was equivalent with that of adult NOG mice. Therefore, the 1-Gy dose for newborn NOG mice was used for newborn NOD-scid mice.
Table 1. Experimental conditions for human cell engraftment in immunodeficient mice.
| Mouse strain | Age for injection | No. of mice | Dose of irradiation (Gy) |
Route of injection |
|---|---|---|---|---|
| NOG | Adult | 10 | 2.5 | Tail vein |
| Newborn | 6 | 1 | Facial vein | |
| NR2G | Adult | 10 | 8 | Tail vein |
| Newborn | 6 | 4 | Facial vein | |
| NOD-scid | Adult | 9 | 2.5 | Tail vein |
| Newborn | 5 | 1 | Facial vein |
Table 2 summarizes the engraftment rates of human cells into the adults and newborns of three strains of mice at 20 weeks after HSC transplantation. Twenty weeks after cell transplantation into NOG mice, 3 (30%) of 10 adult mice died, but none of the six newborn mice died during the same period. By contrast, all of the transplanted adult NR2G mice survived, but 1 (17%) of 6 newborn mice died. Human cells could be engrafted successfully in all surviving mice. When the engraftment rate of human cells was compared among the three strains of the mice, no significant difference was observed among adult mice of the three mouse strains. However, a significant difference was noted among the mouse strains when human cells were transplanted into newborn mice—namely, engraftment was higher in NOG, NR2G, and NOD-scid mice, in that order.
Table 2. Human cell engraftment in adults or newborns of three strains of immunodeficient mice at 20 weeks after CD34+ cell transfer.
| Age for injection | Mouse strain | No. of mice | Survival rate (%) | Frequency of engrafted mice (%) |
Human CD45+ cells (%)* |
|---|---|---|---|---|---|
| Adult | NOG | 10 | 70 (7/10) | 100 (7/7) | 50.8 ± 17.2 |
| NR2G | 10 | 100 (10/10) | 100 (10/10) | 40.0 ± 12.4 | |
| NOD-scid | 9 | 55.6 (5/9) | 100 (5/5) | 43.4 ± 14.6 | |
| Newborn | NOG | 6 | 100 (6/6) | 100 (6/6) | 69.9 ± 4.5 |
| NR2G | 6 | 83.3 (5/6) | 100 (5/5) | 43.1 ± 9.9 | |
| NOD-scid | 5 | 40 (2/5) | 100 (2/2) | 12.3 ± 2.1 |
*Percentages of human CD45+ cells in mononuclaer cells of peripheral blood.
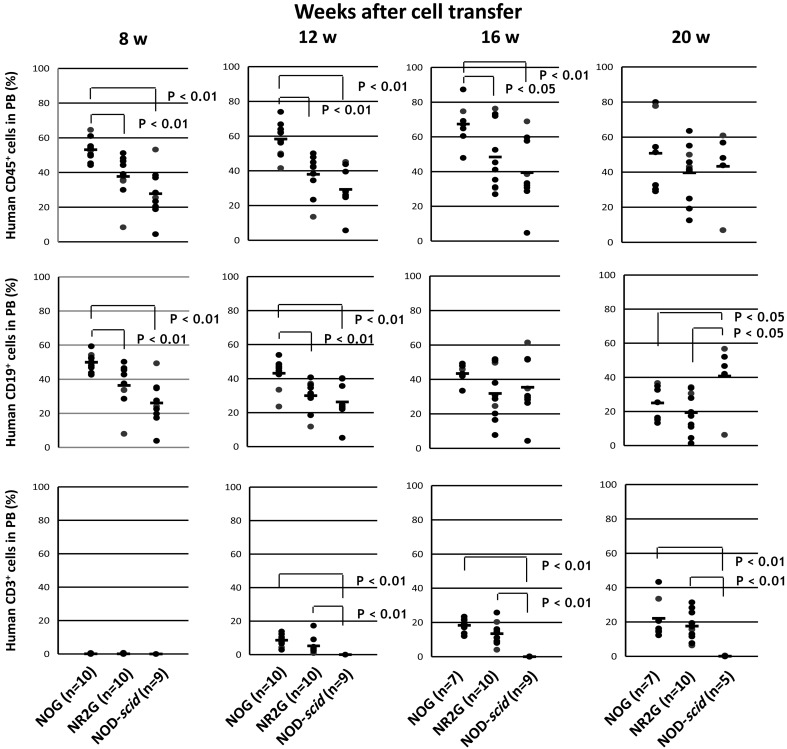
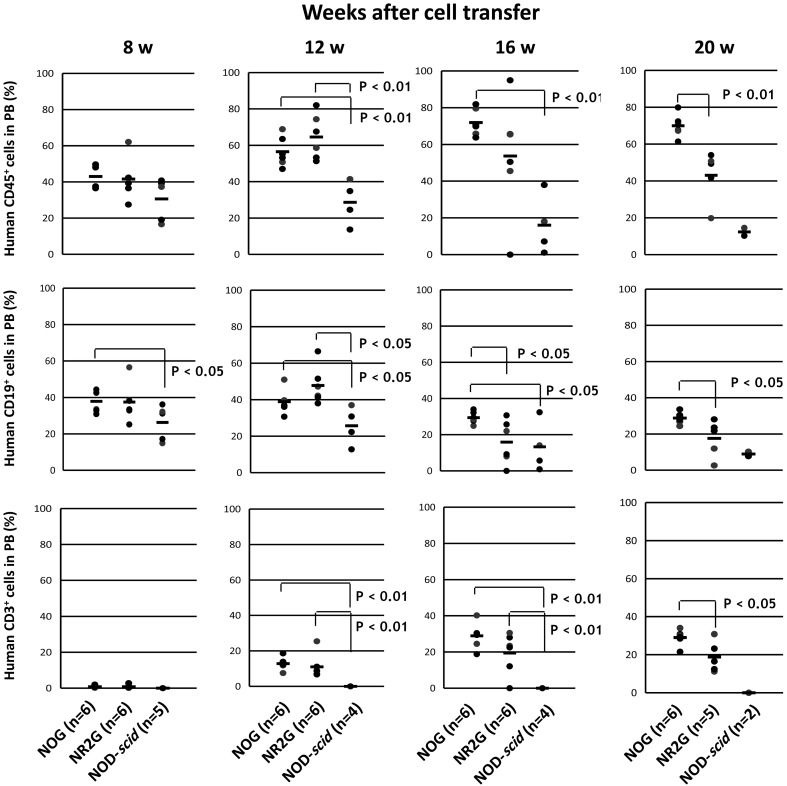
To examine the time course of human cell engraftment and differentiation from HSCs, the peripheral blood of the transplanted mice were collected at 8, 12, 16, and 20 weeks after HSC transplantation into adults and newborns mice, and then were analyzed by flow cytometry (Figs. 2 and 3).
Fig. 2.
Human cells in mouse peripheral blood after HSC transplantation into adult immunodeficient mice. A total of 5 × 104 commercially available human CB CD34+ cells was intravenously inoculated via the tail vein in adult mice at 24 h after irradiation. After HSC transplantation, PB was collected periodically from the retro-orbital venous plexus using a capillary pipette coated with heparin under anesthesia with isoflurane at 8–20 weeks after transplantation. Human cells in mouse PB were analyzed by flow cytometry.
Fig. 3.
Human cells in mouse peripheral blood after HSC transplantation into newborn immunodeficient mice. A total of 5 × 104 commercially available human CB CD34+ cells was intravenously inoculated via the facial vein in newborn mice at 24 h after irradiation. After HSC transplantation, the analysis was done in the same manner as described in Figure 2. The number of NOD-scid mice (n=2) at 20 weeks after HSC transplantation was so small for statistical analysis that the mice were excluded in statistical analysis.
In NOG and NR2G mice, only CD19+ B cells were detected at 8 weeks after HSC transplantation. CD3+ T cells were detected at 12 weeks after transplantation and reached 20% at 16 weeks in the NOG and NR2G mice. By contrast, only CD19+ B cells, not CD3+ T cells, developed during all periods tested in NOD-scid mice. The same results were obtained in transplanted adult and newborn mice, suggesting no significant difference in the differentiation of human cells. Conversely, engraftment of human cells was higher in transplanted NOG mice than in transplanted NR2G mice for both newborns and adults.
Human cells in the BM, the spleen, and PB of HSC transplanted mice
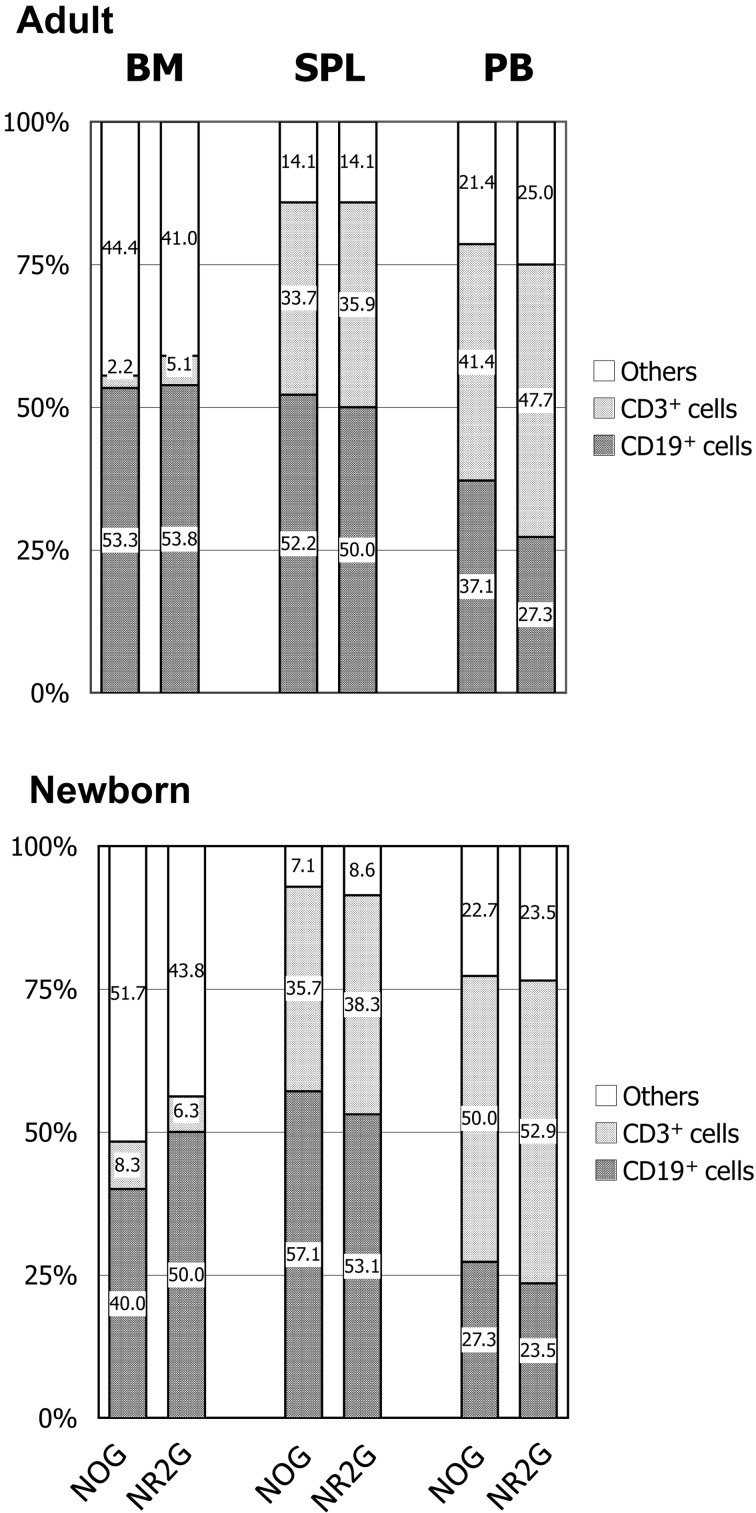
The human cell engraftment rates in the BM, the spleen, and PB at 22–23 weeks after cell transplantation into adult and newborn mice are shown in Supplemental Data Fig. 1. Extremely high engraftment rates were observed in both NOG and NR2G mice regardless of the transplanted age of the mice. Human cell engraftment rates were higher in the spleen, the BM, and PB, in that order, and this tendency was identified regardless of the transplanted age of the mice. The transplanted newborn mice showed higher engraftment rates than transplanted adult mice. However, no difference in the engraftment rate was observed between NOG and NR2G mice, except in PB from the newborn transplanted mice. The percentage of T, B cells and other lineage cells in human CD45+ cells of the BM, the spleen, and PB are shown in Fig. 4. CD3+ T cells were dominant in PB, but CD19+ B cells were dominant in the BM and spleen of both NOG and NR2G mice. Particularly, a few CD3+ cells were detected in the BM. No difference in the T- and B-cell subsets was noted in transplanted adult and newborn mice. For other lineage cells including NK cells and myeloid cells, only a small numbers of them were observed in NOG and NR2G mice, and there was also no difference among them (Data not shown).
Fig. 4.
Human T and B cells in the BM, spleen and PB of HSC-transplanted mice at 20 weeks after HSC transplantation. Figures in the column represent the percentage of each cell type among human CD45+ cells. The number of NOG and NR2G mice used in this figure was 7 and 10 in adult, and 6 and 5 in newborn, respectively.
T-cell subsets in PB, the spleen, and the thymus from HSC transplanted mice
To investigate the differentiation potential of T cell subsets, human CD3+, CD4+, and CD8+ cells were examined in the BM, spleen, and thymus of both strains of mice. The ratios of CD4+ cells were twofold those of CD8+ cells in all organs tested. In the thymus, CD4+CD8+ T cells were dominant, as in humans. Here, no difference in the ratios of these T-cell subsets was observed in all organs of both mouse strains (Table 3). In addition, no difference in T-cell subset differentiation was observed between the adult and newborn transplanted mice. These results indicate that T-cell subsets can develop from HSCs in both strains, regardless of the transplanted age.
Table 3. Percentages of T-cell subsets in the spleen, thymus, and peripheral blood of HSC-transplanted mice.
| Age in injection |
Mouse strain |
No. of mice |
Spleen |
Peripheral blood |
Thymus |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hCD3 | hCD4 | hCD8 | hCD3 | hCD4 | hCD8 | hCD3 | hCD4/CD8 | hCD4 | hCD8 | |||||
| Adult | NOG | 7 | 28.9 ± 2.9 | 64.1 ± 6.1 | 30.0 ± 4.4 | 14.1 ± 4.9 | 70.9 ± 4.2 | 26.5 ± 3.6 | 90.2 ± 12.0 | 62.6 ± 26.4 | 22.0 ± 14.9 | 13.1 ± 10.3 | ||
| NR2G | 10 | 27.7 ± 13.9 | 69.0 ± 8.5 | 28.6 ± 8.8 | 8.2 ± 5.8 | 76.1 ± 8.8 | 22.0 ± 9.0 | 89.3 ± 6.2 | 46.9 ± 29.0 | 29.9 ± 17.5 | 15.5 ± 9.8 | |||
| Newborn | NOG | 6 | 29.5 ± 6.0 | 56.8 ± 8.0 | 35.5 ± 7.5 | 31.4 ± 16.0 | 69.5 ± 3.6 | 28.5 ± 3.0 | 88.4 ± 3.7 | 69.9 ± 13.2 | 13.9 ± 6.5 | 12.4 ± 5.2 | ||
| NR2G | 5 | 24.1 ± 7.1 | 63.9 ± 12.9 | 30.5 ± 10.0 | 17.4 ± 5.8 | 70.8 ± 7.4 | 27.8 ± 6.7 | 78.8 ± 15.0 | 83.1 ± 6.0 | 10.8 ± 3.5 | 4.4 ± 1.7 | |||
Human cells in spleen, thymus and peripheral blood from mice at 20 weeks after HSC transplnatation were analyzed by flow cytometry.
Distribution of human cells in the spleen
To investigate the distribution of human cells in the spleens of NOG and NR2G mice, we performed immunohistological staining of the spleen using anti-human CD45, CD79a, and CD3 antibodies. As shown in Supplemental Data Fig. 2, human CD45+ cells were broadly distributed in the spleen and formed strongly concentrated round-shaped areas. In these areas, CD79a+ B cells were located in the periphery, whereas CD3+ T cells were found in the central regions. Such structures were also observed in NOG and NR2G mice regardless of their transplanted age.
Discussion
In the present study, we established NR2G mice by introducing the Rag2null gene into NOD-IL-2Rγnull mice and investigated the capacity of the NR2G mice as recipients for human cell development after HSC transplantation in comparison to NOG mice, which are currently used extensively for generating humanized mice. In addition, we investigated the differences in human cell development and differentiation when HSCs are transplanted into newborns and adults of these immunodeficient mice.
Prior to HSC transplantation, irradiation sensitivities were determined in NOG, NR2G, and BR2G mice. NR2G mice showed high resistance against irradiation, similar to that in NR1G mice reported by Pearson et al. [12], compared with NOG and NOD-scid mice with the Prkdcscid gene in which DNA double-strand break repair is impaired [3]. However, the irradiation resistance of NR2G mice was higher than that of NR1G mice. Namely, all NR2G mice survived for at least 26 days after 8-Gy irradiation to adult mice and at least 56 days after 4-Gy irradiation to newborn mice. By contrast, 30% of NR1G adult mice died within 28 days after 7-Gy irradiation. The reason for this difference in irradiation resistance between the NR2G and NR1G mice remains unclear. The difference between the targeted Rag2 and Rag1 genes in both mouse types is unlikely to affect irradiation resistance because both genes are closely located on chromosome 2 [10], and the targeting strategy and resulting phenotypes are quite similar between them [9, 13]. One explanation may be the difference in the irradiation source, namely X-ray irradiation for NR2G mice and 137Cs irradiation for NR1G mice. Another explanation may be the difference in environmental factors, including the intestinal flora. Ivanov et al. [7] reported that the intestinal flora influenced the appearance of T helper cells in the intestinal lymphoid apparatus and demonstrated that the responses differed among mice with different flora from different breeders. The difference in the irradiation resistance between the mouse strains may be due to differences in the intestinal flora because damage to the intestine—in which cell turnover is rapid—causes early death after irradiation. Differences in irradiation sensitivity among mouse strains have been reported [11]; however, these differences are not likely to exist between NOD/LtSz and NOD/ShiJic mice because they are closely related in genetics as substrains.
Pearson et al. [12] also reported that NR1G mice showed high human cell engraftment and differentiation after HSC transplantation, similar to that in NSG mice. In the current study, we also compared the degree of human cell engraftment and differentiation among NR2G, NOG, and NOD-scid mice. To eliminate the influence of different sources of CD34+ cells and different inoculation periods on the results of this comparison, we used the same lot of commercially available CD34+ cells and inoculated them into the mice on the same day. Particularly, for the comparison between adults and newborns at the transplanted age, we obtained the newborns by Cesarean section after transplantation of their embryos from in vitro insemination. High engraftment of human cells from HSCs was observed in both NOG and NR2G mice regardless of inoculation age, although the HSC population in the former was slightly higher than that in the latter during the test periods. No fundamental difference in the differentiation of human cells was observed between the two mouse strains. B cells developed earlier than T cells. Namely, B cells were dominant 8 to 12 weeks after cell transplantation, but T cells became dominant from 16 weeks after cell transplantation. By contrast, no T cells developed in NOD-scid mice during the test period. Immunohistochemical staining of human cells in the spleen revealed that differentiated human cells were located in the same area in both NR2G and NOG mice. These results indicate that NR2G mice can be used as an alternative to NOG mice for generating humanized mice.
Regarding the age of HSC transplantation, Traggiai et al. [16] reported the successful generation of humanized mice by intrahepatic inoculation of HSCs into newborn BR2G mice. Ishikawa et al. [5] also reported the high efficiency of human cell development by intravenous HSC inoculation into newborn NSG mice. The present study also revealed that the transplantation of HSCs into newborn mice resulted in a higher engraftment rate than transplantation into adult mice. However, the difference in the engraftment rates between the mouse strains was not marked, and the differentiation of human cells was fundamentally similar, suggesting that researchers can select the age for HSC inoculation into humanized mice according to the purpose of their research.
In conclusion, newly established NR2G mice can be used as an alternative host to NOG mice for generation of humanized adult and newborn mice by inoculation of HSCs.
Supplement_figure
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (S) (#18100005) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and by a grant from Research on Emerging and Re-emerging Infectious Diseases From Ministry of Health, Labour and Welfare, Japan of Japan.
References
- 1.Bosma G.C., Fried M., Custer R.P., Carroll A., Gibson D.M., Bosma M.J.1988. Evidence of functional lymphocytes in some (leaky) scid mice. J. Exp. Med. 167: 1016–1033. doi: 10.1084/jem.167.3.1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brehm M.A., Cuthbert A., Yang C., Miller D.M., DiIorio P., Laning J., Burzenski L., Gott B., Foreman O., Kavirayani A., Herlihy M., Rossini A.A., Shultz L.D., Greiner D.L.2010. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the IL2rgamma(null) mutation. Clin. Immunol. 135: 84–98. doi: 10.1016/j.clim.2009.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulop G.M., Phillips R.A.1990. The scid mutation in mice causes a general defect in DNA repair. Nature 347: 479–482. doi: 10.1038/347479a0 [DOI] [PubMed] [Google Scholar]
- 4.Hiramatsu H., Nishikomori R., Heike T., Ito M., Kobayashi K., Katamura K., Nakahata T.2003. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood 102: 873–880. doi: 10.1182/blood-2002-09-2755 [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L.D., Harada M.2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood 106: 1565–1573. doi: 10.1182/blood-2005-02-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K., Heike T., Nakahata T.2002. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100: 3175–3182. doi: 10.1182/blood-2001-12-0207 [DOI] [PubMed] [Google Scholar]
- 7.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R.2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498. doi: 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepus C.M., Gibson T.F., Gerber S.A., Kawikova I., Szczepanik M., Hossain J., Ablamunits V., Kirkiles-Smith N., Herold K.C., Donis R.O., Bothwell A.L., Pober J.S., Harding M.J.2009. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg immunodeficient mice. Hum. Immunol. 70: 790–802. doi: 10.1016/j.humimm.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mombaerts P., Iacomini J., Johnson R.S., Herrup K., Tonegawa S., Papaioannou V.E.1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68: 869–877. doi: 10.1016/0092-8674(92)90030-G [DOI] [PubMed] [Google Scholar]
- 10.Oettinger M.A., Stanger B., Schatz D.G., Glaser T., Call K., Housman D., Baltimore D.1992. The recombination activating genes, RAG 1 and RAG 2, are on chromosome 11p in humans and chromosome 2p in mice. Immunogenetics 35: 97–101. doi: 10.1007/BF00189518 [DOI] [PubMed] [Google Scholar]
- 11.Onoue M.1960. Studies on the radiosensitivities of four inbred strains of mice. Jpn. J. Radiol. 19: 2366–2370. [Google Scholar]
- 12.Pearson T., Shultz L.D., Miller D., King M., Laning J., Fodor W., Cuthbert A., Burzenski L., Gott B., Lyons B., Foreman O., Rossini A.A., Greiner D.L.2008. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin. Exp. Immunol. 154: 270–284. doi: 10.1111/j.1365-2249.2008.03753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinkai Y., Rathbun G., Lam K.P., Oltz E.M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A.M., et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68: 855–867. doi: 10.1016/0092-8674(92)90029-C [DOI] [PubMed] [Google Scholar]
- 14.Shultz L.D., Lyons B.L., Burzenski L.M., Gott B., Chen X., Chaleff S., Kotb M., Gillies S.D., King M., Mangada J., Greiner D.L., Handgretinger R.2005. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174: 6477–6489. doi: 10.4049/jimmunol.174.10.6477 [DOI] [PubMed] [Google Scholar]
- 15.Suemizu H., Yagihashi C., Mizushima T., Ogura T., Etoh T., Kawai K., Ito M.2008. Establishing EGFP congenic mice in a NOD/Shi-scid IL2Rg(null) (NOG) genetic background using a marker-assisted selection protocol (MASP). Exp. Anim. 57: 471–477. doi: 10.1538/expanim.57.471 [DOI] [PubMed] [Google Scholar]
- 16.Traggiai E., Chicha L., Mazzucchelli L., Bronz L., Piffaretti J.C., Lanzavecchia A., Manz M.G.2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304: 104–107. doi: 10.1126/science.1093933 [DOI] [PubMed] [Google Scholar]
- 17.Ueda T., Tsuji K., Yoshino H., Ebihara Y., Yagasaki H., Hisakawa H., Mitsui T., Manabe A., Tanaka R., Kobayashi K., Ito M., Yasukawa K., Nakahata T.2000. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor [In Process Citation]. J. Clin. Invest. 105: 1013–1021. doi: 10.1172/JCI8583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B., Duan Z., Zhao Y.2009. Mouse models with human immunity and their application in biomedical research. J. Cell. Mol. Med. 13: 1043–1058. doi: 10.1111/j.1582-4934.2008.00347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.