Abstract
Reproductive engineering techniques, such as in vitro fertilization (IVF) and cryopreservation of embryos or spermatozoa, are essential for preservation, reproduction, and transportation of genetically engineered mice. However, it has not yet been elucidated whether these techniques can be applied for the generation of genome-edited mice using engineered nucleases such as transcription activator-like effector nucleases (TALENs). Here, we demonstrate the usefulness of frozen oocytes fertilized in vitro using frozen sperm for TALEN-mediated genome editing in mice. We examined side-by-side comparisons concerning sperm (fresh vs. frozen), fertilization method (mating vs. IVF), and fertilized oocytes (fresh vs. frozen) for the source of oocytes used for TALEN injection; we found that fertilized oocytes created under all tested conditions were applicable for TALEN-mediated mutagenesis. In addition, we investigated whether the ages in weeks of parental female mice can affect the efficiency of gene modification, by comparing 5-week-old and 8–12-week-old mice as the source of oocytes used for TALEN injection. The genome editing efficiency of an endogenous gene was consistently 95–100% when either 5-week-old or 8–12-week-old mice were used with or without freezing the oocytes. Thus, our report describes the availability of freeze-thawed oocytes and oocytes from female mice at various weeks of age for TALEN-mediated genome editing, thus boosting the convenience of such innovative gene targeting strategies.
Keywords: Cryopreservation, Female age, Genome editing, Knockout mouse, TALEN
Introduction
Recently, transcription activator-like effector nucleases (TALENs) and other customizable site-specific nucleases have been widely used for genetic engineering in various cells and animals [4, 15]. Such nuclease-mediated double-strand breaks at the specified genomic region are mainly repaired by error-prone non-homologous end-joining, resulting in disruption of gene functions caused by randomly induced insertions and deletions.
Knockout and knockin mice have conventionally been produced by an embryonic stem cell-mediated strategy depending on homologous recombination between genomic DNA and a targeting construct [1]. In these situations, direct production of various genome-edited mice by zygote injection with customized nucleases such as TALENs has been taking the place of conventional gene targeting owing to its simplicity and quickness [2, 5, 11, 12, 16, 17, 19]. However, it has never been ascertained whether reproductive engineering techniques such as in vitro fertilization (IVF) and cryopreservation of embryos or spermatozoa can be used for genome editing in mice.
Fertilized oocytes for TALEN injection are generally obtained by mating a male mouse and a superovulated female mouse, and used without cryopreservation. As this method constantly requires many male mice accommodated in separate cages, occupation of breeding space and the burden of breeding costs often plague researchers and facilities. In addition, fertilized oocytes must be collected each time on the experimental day. However, if frozen oocytes can be used for the injection, the breeding costs will be reduced and the working efficiency will be greatly improved. Moreover, if it is possible to use frozen oocytes manufactured by IVF, the hurdles for creating genome-edited mice will be further lowered.
Here, we investigated whether frozen oocytes created by mating and IVF using fresh or freeze-thawed sperm can be used for TALEN-mediated gene disruption. We used ICR female mice and male mice harboring a single-copy transgene encoding β-lactamase (bL), which is not expressed in mice and is expected to have no influence on mouse development following TALEN-mediated gene disruption. In addition, we examined endogenous genome editing with TALENs targeting the C-type lectin domain family 4, member b1 (Clec4b1) gene using fresh and freeze-thawed fertilized oocytes, which were obtained by mating male mice and 5-week-old or 8–12-week-old female mice. The pups were analyzed by screening methods described in a previous report [9] and the genome editing efficiency was evaluated accordingly.
Materials and Methods
Mice harboring the bL transgene
Ayu 8104, a mouse strain harboring the bL transgene, was originally generated by insertion of a gene trap vector into the mouse Crk gene [3]. Single-copy insertion was previously confirmed by Southern blot analysis [3]. Homozygous Crk mutant mice did not exhibit any obvious abnormalities. The mutant allele was subsequently transferred to ICR mice. The parental mice for TALEN injection had the homozygous bL gene with the complete ICR background.
Fertilized oocytes of ICR female mice mated with Ayu 8104 male mice
To obtain fertilized oocytes for injection of bL TALENs, ICR female mice were induced to superovulate using 7.5 IU of pregnant mare serum gonadotropin (PMSG) (Serotropin; Aska Pharmaceutical, Tokyo, Japan) and 7.5 IU of human chorionic gonadotropin (hCG) (Veterinary Puberogen; Novartis Animal Health, Tokyo, Japan) at 10 weeks of age. The mice were then mated with the Ayu 8104 male mice described above. Fertilized oocytes were collected from 15 females displaying vaginal plugs. Fertilized oocytes forming male and female pronuclei were used for bL TALEN injection with or without cryopreservation. Cryopreservation of fertilized oocytes was performed by a simple vitrification method [8, 10].
IVF with fresh or cryopreserved sperm of Ayu 8104 mice
Sperms were obtained from two Ayu 8104 male mice at 4 months of age. A cauda epididymidis was removed from each of the two males and combined to exclude any influence on IVF and embryogenesis by individual male differences, and then used for IVF with fresh sperm or cryopreserved sperm. The procedures for sperm preparation were in accord with the previous report [8].
ICR female mice were induced to superovulate at 10 weeks of age by the same procedures described above. IVF was performed according to the previous report [8]. The nine or eight female mice were euthanized for IVF with fresh or freeze-thawed sperm, respectively. The cumulus-oocyte complexes (COCs) were introduced into the drop of CARD MEDIUM. After preincubation of the sperm, the fresh or freeze-thawed sperm was added to the drop containing COCs. After 3–6 hours, the inseminated oocytes were rinsed and the fertilized oocytes forming male and female pronuclei were cryopreserved. The frozen oocytes were thawed and used for bL TALEN injection.
Fertilized oocytes of C57BL/6N mice
To obtain fertilized oocytes for Clec4b1 TALEN injection, mature or immature C57BL/6N female mice were sperovulated, and then mated with C57BL/6N male mice. Mature females were used at 8–12 weeks of age. For immature females, PMSG and hCG (5 IU each) were administered at 5 weeks of age. The fertilized oocytes were collected and used for Clec4b1 TALEN injection with or without cryopreservation.
Platinum TALEN plasmid construction and mRNA preparation
Platinum TALEN plasmids were constructed as previously described [14]. Briefly, cloned TALE repeats were assembled into ptCMV-153/47-VR vectors using the two-step Golden Gate cloning method. The bL TALEN target sequence is indicated in Suppl. Fig. 1A. The TALEN target sequence in Mus musculus C-type lectin domain family 4, member b1 (Clec4b1) is indicated in Suppl. Fig. 1B. TALEN mRNAs were synthesized from plasmids linearized by SmaI digestion and purified as previously described [13].
Microinjection of TALEN mRNAs
TALEN mRNAs were diluted in RNase-free PBS at 150 ng/µl for each TALEN, and then used for the microinjection as previously described [9].
Analyses for mutant screening
Screening of mutants was performed by heteroduplex mobility assay (HMA), restriction fragment length polymorphism (RFLP) analysis and DNA sequencing, as we described previously [9]. Briefly, genomic DNA was extracted from the tail of each pup. Genomic PCR for amplification of the bL gene was performed under the following conditions: 95°C for 2 min; followed by 35 cycles of 95°C for 30 s, 66°C for 30 s, and 72°C for 20 s. The PCR primers for the bL gene were 5′-GTATTCAACATTTCCGTGTCGCC-3′ and 5′-GATAATACCGCGCCACATAGCAG-3′. Genomic PCR for amplification of the Clec4b1 gene was performed under the following conditions: 94°C for 2 min; followed by 35 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 20 s. The PCR primers for the Clec4b1 gene were 5′-GGCTATCTCTGTGGTATTTAGCTC-3′ and 5′-CCACTTGTCTGCATGGTTAC-3′. Each PCR product was subjected to agarose gel electrophoresis and ethidium bromide staining for the HMA.
For RFLP analysis, the PCR products were precipitated with ethanol. Each sample was then digested at 37°C overnight in 10 µl of digestion solution containing 1×CutSmart buffer and 1.25 U of HphI (New England Biolabs, Tokyo, Japan) or Hpy188III (New England Biolabs) for the bL and Clec4b1 genes, respectively. The digested products were subjected to agarose gel electrophoresis and ethidium bromide staining.
For DNA sequence analysis, the PCR products were subcloned and sequenced as previously described [9].
Results and Discussion
TALEN-mediated transgene disruption using freeze-thawed oocytes fertilized in vitro
To assess the availability of reproductive engineering techniques for mouse genome editing, we first conducted TALEN-mediated transgene disruption using the previously described gene-trapped mouse strain Ayu 8104 [3] (Fig. 1A), followed by three-step screening of mutants by HMA, RFLP analysis, and DNA sequencing, as described previously [9].
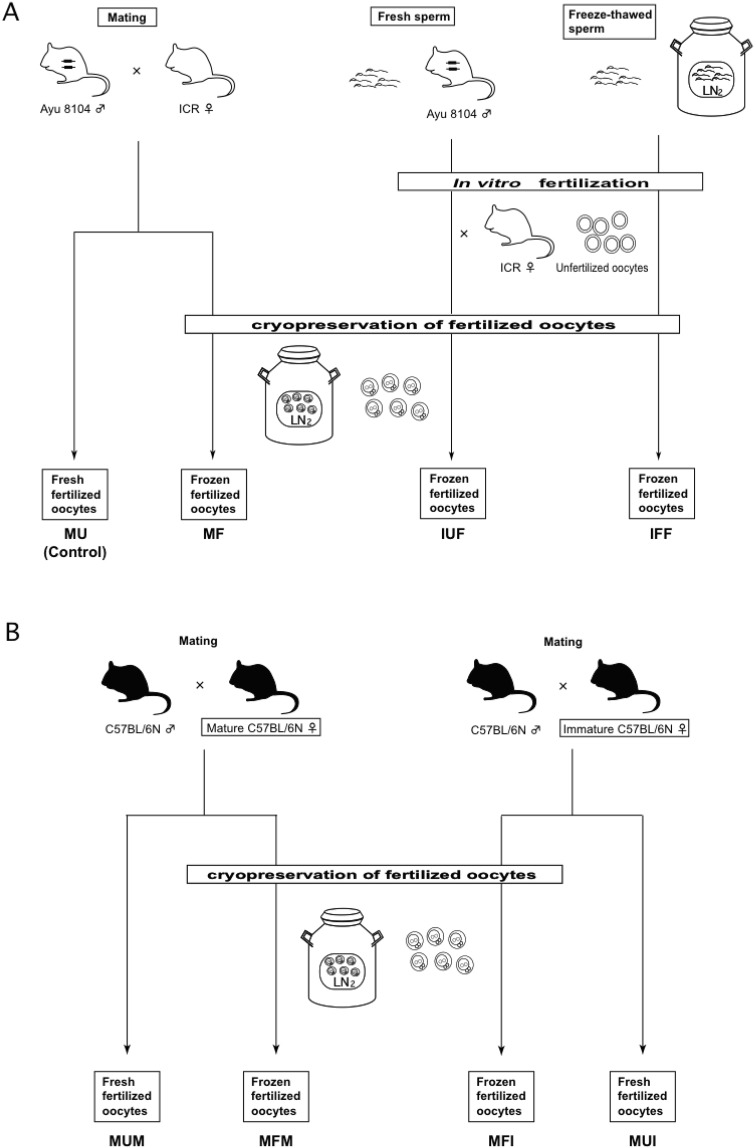
Fig. 1.
Schematic overview of this study. (A) Scheme of the examinations targeting the transgene. Four kinds of fertilized oocytes were used for injection of the TALEN mRNAs. The first two kinds of oocytes were produced by IVF treatment using fresh or frozen sperm, and then cryopreserved. After thawing the oocytes, microinjection of the TALEN mRNAs was performed. The other two kinds of oocytes were fresh or freeze-thawed oocytes collected from female mice mated with male mice. (B) Scheme of the examinations targeting the endogenous gene. Four kinds of fertilized oocytes were used. Sexually mature or immature female mice were mated with male mice, and then fertilized oocytes were collected and used for injection with or without cryopreservation.
As a control, we collected fertilized oocytes from ICR female mice mated with Ayu 8104 male mice, and the bL TALEN mRNAs were injected into fresh fertilized oocytes (mated unfrozen oocytes: MU). At the same time, half of the fertilized oocytes were cryopreserved, and then thawed and used for injection of the TALEN mRNAs at a later occasion (mated frozen oocytes: MF).
After freeze-thawing of oocytes, sufficient numbers of normal oocytes could be used for injection and sufficient numbers of pups could be analyzed in both groups (Table 1 and Suppl. Table 1). Genomic DNA was extracted from all pups and used for genomic PCR amplification around the TALEN target site. The individual products were subjected to agarose gel electrophoresis for the HMA, followed by RFLP analysis. To confirm mutations of amplicons suggestive of mutant mice, sequence analyses were conducted (Suppl. Fig. 2). All of the sequenced amplicons were determined to have mutations, including deletions ranging from a single base to several dozen bases. Overall, the percentages of mutant mice in the MU and MF groups were 80.0% (8/10) and 33.3% (7/21), respectively (Table 1 and Suppl. Table 2).
Table 1. TALEN-mediated bL gene disruption in Ayu 8104 mice.
| Group | Fertilization | Sperm | Fertilized oocytes | Injected | Transferred | Newborns | Analyzed pups | Mutants |
|---|---|---|---|---|---|---|---|---|
| MU (control) | Mating | - | Unfrozen | 56 | 40 | 10 (25.0%) | 10 | 8 (80.0%) |
| MF | Mating | - | Frozen | 52 | 48 | 21 (43.8%) | 21 | 7 (33.3%) |
| IUF | IVF | Unfrozen | Frozen | 89 | 72 | 22 (30.6%) | 20 | 10 (50.0%) |
| IFF | IVF | Frozen | Frozen | 53 | 31 | 6 (19.4%) | 6 | 2 (33.3%) |
In the MU group, the number of newborns was fewer than in the MF group. On the other hand, the percentage of mutants in the MU group was higher than in the MF group. These differences might be caused by the condition of injection needles, because slight differences in the thickness of needles can affect cell damage and quantity of injected mRNA. Further examinations are needed for the statistical comparison.
Next, we examined injection of the bL TALEN mRNAs into freeze-thawed oocytes produced by IVF using fresh or cryopreserved sperm of the Ayu 8104 strain (IVF with unfrozen sperm, followed by freeze-thawing: IUF; IVF with frozen sperm, followed by freeze-thawing: IFF) (Fig. 1A). Although the percentages of normal oocytes were slightly decreased in the IUF and IFF groups, 60.8% (135/222) and 70.6% (84/119), respectively, compared with the MF group and the survival rate after injection was relatively low in the IFF group, we were definitely able to obtain pups for genotyping (Table 1 and Suppl. Table 1).
As a result of the HMA, eight pups in the IUF group and two pups in the IFF group were selected as candidates for mutant mice (Suppl. Table 2). The next screening step, RFLP analysis, revealed that three pups in the IUF group were supposed to be mutant mice (Suppl. Table 2). Sequence confirmation finally identified that 50.0% of pups (10/20) in the IUF group and 33.3% of pups (2/6) in the IFF group harbored mutations (Table 1, Suppl. Fig. 2 and Suppl. Table 2). These results suggested that cryopreserved oocytes had sufficient capability for mutant mouse generation using TALENs.
TALEN-mediated endogenous gene disruption using oocytes from female mice at different weeks of age
We further tested TALEN-mediated endogenous gene targeting using fresh or freeze-thawed oocytes of C57BL/6N mice for the general production of genome-edited mice using cryopreserved oocytes (Fig. 1B). When performing IVF, according to our previous examination regarding the number of ovulated oocytes, the fertility rate and the birth rate using mature and immature C57BL/6 female mice for IVF, fewer oocytes were ovulated in mature females compared with immature females (24 ± 12 vs. 37 ± 5 oocytes/female). However, the fertility rate and the birth rate in using mature females were higher than in using immature females (fertility rate: 86% vs. 96%, birth rate: 31% vs. 52%) (unpublished data). Therefore, to certify the availability of female mice of various ages as sources of cryopreserved fertilized oocytes in TALEN-mediated genome editing, sexually mature females at 8–12 weeks of age and sexually immature females at 5 weeks of age were used (mated unfrozen mature oocytes: MUM; mated unfrozen immature oocytes: MUI; mated frozen mature oocytes: MFM; mated frozen immature oocytes: MFI).
The survival rates after freeze-thawing and microinjection of TALENs were sufficiently high in all groups, but the numbers of newborns were slightly decreased in the MFM and MFI groups (Table 2 and Suppl. Table 3 ). The first screening by the HMA showed that approximately half of the screened pups had some sort of mutation in all groups (Suppl. Table 4). The remaining pups were subsequently analyzed by RFLP, with the surprising result that all of the pups analyzed appeared to be mutant mice except for one pup in the MUM group and another one pup in the MUI group (Suppl. Table 4). Excluding the amplicons from these two pups, all of the PCR products were sequenced and confirmed to be mutants (Suppl. Fig. 3 and Suppl. Table 4). Taking the above results together, the rates of mutant mice in the individual groups were 95.0% (19/20) in the MUM group, 95.0% (19/20) in the MUI group, 100% (6/6) in the MFM group, and 100% (12/12) in the MFI group (Table 2 and Suppl. Table 4 ).
Table 2. TALEN-mediated Clec4b1 gene disruption in C57BL/6N mice.
| Group | Fertilization | Fertilized oocytes | Ages in weeks | Injected | Transferred | Newborns | Analyzed pups | Mutants |
|---|---|---|---|---|---|---|---|---|
| MUM | Mating | Unfrozen | 8–12 (Mature) | 103 | 90 | 21 (23.3%) | 20 | 19 (95.0%) |
| MUI | Mating | Unfrozen | 5 (Immature) | 80 | 66 | 20 (30.3%) | 20 | 19 (95.0%) |
| MFM | Mating | Frozen | 8–12 (Mature) | 64 | 59 | 7 (11.9%) | 6 | 6 (100.0%) |
| MFI | Mating | Frozen | 5 (Immature) | 66 | 63 | 12 (19.0%) | 12 | 12 (100.0%) |
Future perspectives for utilization of cryopreservation in mouse genome editing
Since the survival of frozen mouse embryos was reported by Whittingham in 1972 [18], embryo cryopreservation has been broadly used for preserving and transporting genetically engineered mice. In this study, we report the availability of freeze-thawed oocytes for injection of TALEN mRNAs. Cryopreserved oocytes produced not only by mating but also by IVF with fresh or freeze-thawed sperm were proven to be utilizable for TALEN-mediated genome editing. In addition, there were no differences in the pattern of mutations among four groups except some insertions detected in three groups in which freeze-thawed oocytes were used. Generally, sufficient numbers of sperm to perform IVF can be taken from one sexually mature male mouse when it has normal fecundity. Moreover, it is not necessary to prepare living male mice if cryopreserved sperm can be used for IVF. We further revealed that the genome editing efficiency for targeting an endogenous gene was enormously high using both frozen and fresh oocytes derived from mature and immature female C57BL/6N mice. The background strain of genetically engineered mice is typically C57BL/6 and the survival rate after freeze-thawing is, in general, very good and stable [6, 10].
Therefore, we think that cryopreserved oocytes are significantly useful and can improve the work performance for TALEN- or CRISPR/Cas9-mediated genome editing in mice, not only for routine production of knockout mice using the C57BL/6 strain, but also for various other situations such as adding a new gene modification into an already-established genetically engineered mouse strain, especially when it is difficult to prepare many male mice for mating or when only cryopreserved sperm of the intended male mice is available. Since it has been reported that transgenic mice can also be produced using cryopreserved oocytes [7], reproductive engineering techniques will likely play a significant role in generating a variety of genetically engineered mice.
Supplementary
Acknowledgments
We thank Ms. Kazuko Kuroda and Ms. Reika Yoshimatsu for their excellent technical assistance. We thank Dr. Kimi Araki for kindly providing the Ayu 8104 mice. This study was supported by KAKENHI (24591016) to M. Ohmuraya from the Japan Society for the Promotion of Science.
References
- 1.Capecchi M.R.2005. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat. Rev. Genet. 6: 507–512. doi: 10.1038/nrg1619 [DOI] [PubMed] [Google Scholar]
- 2.Davies B., Davies G., Preece C., Puliyadi R., Szumska D., Bhattacharya S.2013. Site specific mutation of the Zic2 locus by microinjection of TALEN mRNA in mouse CD1, C3H and C57BL/6J oocytes. PLoS ONE 8: e60216. doi: 10.1371/journal.pone.0060216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imaizumi T., Araki K., Miura K., Araki M., Suzuki M., Terasaki H., Yamamura K.1999. Mutant mice lacking Crk-II caused by the gene trap insertional mutagenesis: Crk-II is not essential for embryonic development. Biochem. Biophys. Res. Commun. 266: 569–574. doi: 10.1006/bbrc.1999.1869 [DOI] [PubMed] [Google Scholar]
- 4.Joung J.K., Sander J.D.2013. TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14: 49–55. doi: 10.1038/nrm3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma K., Wang J., Shen B., Qiu L., Huang X., Li Z.2014. Efficient targeting of FATS at a common fragile site in mice through TALEN-mediated double-hit genome modification. Biotechnol. Lett. 36: 471– 479. [DOI] [PubMed] [Google Scholar]
- 6.Nakagata N.1995. Studies on cryopreservation of embryos and gametes in mice. Exp. Anim. 44: 1–8. doi: 10.1538/expanim.44.1 [DOI] [PubMed] [Google Scholar]
- 7.Nakagata N.1996. Use of cryopreservation techniques of embryos and spermatozoa for production of transgenic (Tg) mice and for maintenance of Tg mouse lines. Lab. Anim. Sci. 46: 236–238. [PubMed] [Google Scholar]
- 8.Nakagata N., Takeo T., Fukumoto K., Kondo T., Haruguchi Y., Takeshita Y., Nakamuta Y., Matsunaga H., Tsuchiyama S., Ishizuka Y., Araki K.2013. Applications of cryopreserved unfertilized mouse oocytes for in vitro fertilization. Cryobiology 67: 188–192. doi: 10.1016/j.cryobiol.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa Y., Yamamoto T., Suzuki K., Araki K., Takeda N., Ohmuraya M., Sakuma T.2014. Screening methods to identify TALEN-mediated knockout mice. Exp. Anim. 63: 79–84. doi: 10.1538/expanim.63.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao K., Nakagata N., Katsuki M.1997. Simple and efficient vitrification procedure for cryopreservation of mouse embryos. Exp. Anim. 46: 231–234. doi: 10.1538/expanim.46.231 [DOI] [PubMed] [Google Scholar]
- 11.Panda S.K., Wefers B., Ortiz O., Floss T., Schmid B., Haass C., Wurst W., Kühn R.2013. Highly efficient targeted mutagenesis in mice using TALENs. Genetics 195: 703–713. doi: 10.1534/genetics.113.156570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu Z., Liu M., Chen Z., Shao Y., Pan H., Wei G., Yu C., Zhang L., Li X., Wang P., Fan H.Y., Du B., Liu B., Liu M., Li D.2013. High-efficiency and heritable gene targeting in mouse by transcription activator-like effector nucleases. Nucleic. Acids. Res. 41: e120. doi: 10.1093/nar/gkt258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakuma T., Hosoi S., Woltjen K., Suzuki K., Kashiwagi K., Wada H., Ochiai H., Miyamoto T., Kawai N., Sasakura Y., Matsuura S., Okada Y., Kawahara A., Hayashi S., Yamamoto T.2013. Efficient TALEN construction and evaluation methods for human cell and animal applications. Genes Cells 18: 315–326. doi: 10.1111/gtc.12037 [DOI] [PubMed] [Google Scholar]
- 14.Sakuma T., Ochiai H., Kaneko T., Mashimo T., Tokumasu D., Sakane Y., Suzuki K., Miyamoto T., Sakamoto N., Matsuura S., Yamamoto T.2013. Repeating pattern of non-RVD variations in DNA-binding modules enhances TALEN activity. Sci. Rep. 3: 3379. doi: 10.1038/srep03379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakuma T., Woltjen K.2014. Nuclease-mediated genome editing: At the front-line of functional genomics technology. Dev. Growth Differ. 56: 2–13. doi: 10.1111/dgd.12111 [DOI] [PubMed] [Google Scholar]
- 16.Sung Y.H., Baek I.J., Kim D.H., Jeon J., Lee J., Lee K., Jeong D., Kim J.S., Lee H.W.2013. Knockout mice created by TALEN-mediated gene targeting. Nat. Biotechnol. 31: 23–24. doi: 10.1038/nbt.2477 [DOI] [PubMed] [Google Scholar]
- 17.Wefers B., Meyer M., Ortiz O., Hrabé de Angelis M., Hansen J., Wurst W., Kühn R.2013. Direct production of mouse disease models by embryo microinjection of TALENs and oligodeoxynucleotides. Proc. Natl. Acad. Sci. USA 110: 3782–3787. doi: 10.1073/pnas.1218721110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittingham D.G., Leibo S.P., Mazur P.1972. Survival of mouse embryos frozen to –196° and –269°C. Science 178: 411–414. doi: 10.1126/science.178.4059.411 [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Huang Z., Ren J., Zhang Z., He P., Li Y., Ma J., Chen W., Zhang Y., Zhou X., Yang Z., Wu S.Q., Chen L., Han J.2013. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23: 994–1006. doi: 10.1038/cr.2013.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.