Abstract
We have been following clinically and with muscle MRI for the past 3-decades a Finnish family with two patients with distal muscular dystrophy. Previously we demonstrated the cellular defect in these patients to be defective membrane repair and more recently have identified the causative gene to be anoctamin 5 (ANO5). The disorder seen in these patients is characterized by onset in the third decade. First symptoms were burning sensation on the calves and later on calf tightness during running. Muscle weakness and wasting were asymmetric and early involving the calf muscles, later spread to the thigh muscles. Biceps brachi was later manifestation. Clinical course was slow. CK levels were high. Muscle biopsy showed dystrophic pattern and multifocal disruption of the sarcolemmal membrane but no subsarcolemmal vesicle accumulation nor active inflammation. We conclude that the disease seen in our cases is a new separate clinical, genetic and histopathologic entity to include within the classification of autosomal recessive distal muscular dystrophies.
Keywords: Miyoshi myopathy, Distal myopathy Miyoshi-like non-dysferlin, linked, Distal myopathy, Anoctaminopathy, Muscle imaging
1. Introduction
The group of autosomal recessive distal muscular dystrophies encompasses two different entities: Miyoshi myopathy (MM) and Nonaka myopathy. These are well separated entities both on their clinical, histopathological and genetic backgrounds [1]. Miyoshi myopathy (MM) is characterized by early calf muscle involvement and high serum CK levels [2]. Mutations in the dysferlin gene cause MM, limb-girdle muscular dystrophy 2B (LGMD2B), distal anterior compartment myopathy, proximodistal phenotype, late-onset forms and asymptomatic hyperCKemia [3–6]. Recently a new type of dysferlinopathy with congenital onset has been reported [7]. Genetic heterogeneity is recognized in MM. Several Dutch families with a clinical picture mimicking MM were excluded from dysferlin involvement and two families were tentatively linked to chromosome 10p, MMD2 [8,9]. In a Finnish family with a clinical picture mimicking dysferlin MM where dysferlin involvement had been excluded we examined the membrane repair capability of patient cells and demonstrated defective membrane repair as described for dysferlin deficient cells [10]. In this and an additional non-dysferlin MM family we mapped the muscular dystrophy to a new locus, MMD3, on chromosome 11p to a region which overlapped with an autosomal recessive LGMD, LGMD2L [11]. We have recently demonstrated that both MMD3 and LGMD2L are caused by mutations in the anoctamin 5 (ANO5) gene [12]. The ANO5 mutations in the MMD3 families include a homozygous duplicated adenine (c.191dupA) in exon 5 which is present in a Dutch family (case 2 in Ref. [8]) previously reported [8]. This mutation is also observed in LGMD2L (Family IX) [12]. The ANO5 mutation in the family reported here is a homozygous nucleotide substitution (c.2272C>T) in exon 20 resulting in the substitution of a conserved arginine to a cysteine residue (R758C) [12].
The study reports a 25-years follow-up clinical, and muscle imaging study of the Finnish MMD3 family in which the distal muscular dystrophy is linked with defective membrane repair.
2. Case reports
2.1. Patients
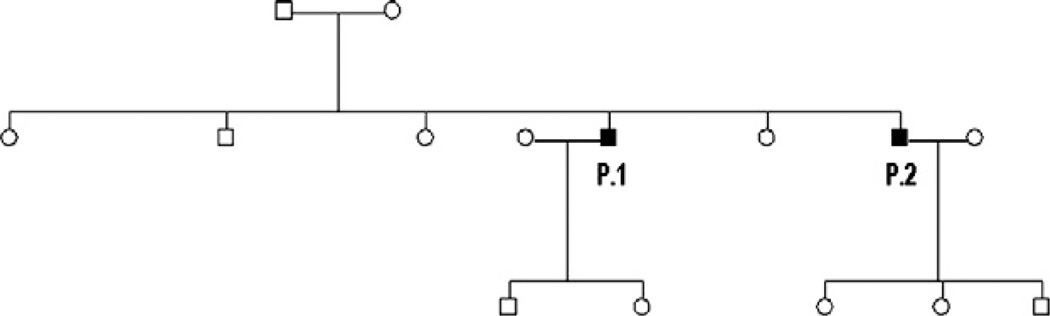
Two affected Finnish brothers aged 44 and 50 years, have been followed from the onset of the first symptoms constantly for a period of 25 years. Although no parental consanguinity was evident in church records going back to the 18th century, remote consanguinity is probable because all maternal and paternal grandparents came from the same isolated rural area. The disease is inherited as autosomal recessive (Fig. 1). At childhood the patients displayed normal psychological and motor development. They enjoyed sports at school and completed the military service period without difficulties.
Fig. 1.
Family pedigree.
Case 1: A 50 year old male.
The patient was seen at age 25 years because of stomach pains. Blood analysis revealed high levels of transaminases. Serum CK levels were found to be highly elevated −12,290 u/l (normal ranges <290 u/l). Upon further clinical assessment EMG and muscle biopsy showed myopathic features. At physical examination the patient showed normal muscle power. He showed calves hypertrophy. After the age of 25 years he began to experience unpleasant muscle sensations in calves, which were unrelated to exercise and relieved by a cold bath. Calf tightness during running was complained at age 44 years. At age 47 years he noticed slight difficulty with climbing the stairs. At present age of 50 years he was able to go on tiptoes with slight difficulty, and showed calves hypertrophy (Fig. 2A). He climbed stairs with slight difficulty without aid. The right biceps muscle was slightly atrophic (Fig. 2B). Muscle strength manual testing revealed mild weakness of right ankle plantar flexion (MRC grade 4) and slight right elbow flexion (MRC grade 4+).
Fig. 2.
P.1 at age 50 years, he is still able to walk on tiptoes even with slight weakness (A). Note the presence of bilateral calf hypertrophy. Slight right biceps brachi atrophy is present (B). P.2 at age 44 years shows a back-kneeing and hyperextension of the ankle (C). He has quadriceps atrophy, more evident in the left side (D) and atrophy of the biceps brachi more prominent in the right side, as well as, right pectoral muscle atrophy (E).
Muscle MRI showed fibro-fatty degeneration of the gastrocnemius medialis muscles at disease duration (DD) 10 years (Fig. 3A). Twelve and 15 years after the first exam showed severe bilateral gastrocnemius medialis and slight right soleus, slight left biceps femoris and moderate right biceps brachi fibro-fatty degeneration.
Fig. 3.
Axial T1-weighted magnetic resonance images of the thighs and legs obtained from P.1 at disease duration (DD) of 10 years shows bilateral gastrocnemius medialis severe fibro-fatty degeneration, while thigh muscles are spared (A). In P.2 fatty degeneration is seen to involve only the posterior muscle compartment of the legs at DD of 9 years (B), and progressively affect the left vastus lateralis and intermedius, left hamstring and adductors, and right adductor magnus muscles of thighs at DD of 15 years (C).
Case 2: A 44 year old male.
The first symptoms appeared at age of 20 years as unpleasant burning sensations in the calves, which were unrelated to exercise, and were alleviated by using ice packs. At the age of 26 years running began to be difficult and clumsy with calf tightness. At the age of 30 years the patient began to rise from a chair supporting himself on one thigh. Climbing stairs was possible by support on one thigh. He rose from the floor using the Gowerś manoeuvre. From the age of 37 years the patient climbs stairs by clinging to the railing with one hand and supporting himself on one thigh. He needs arm support to rise from a chair. Walking became difficult and on a flat, smooth surface his unassisted walking is limited to about 1 km. He has also complained of sudden spontaneous falls. At age of 40 years he experienced burning sensations on the upper limbs. Heavy objects carrying started to be difficult, but fine finger movement and grip were not impaired. At the present age of 44 years, he rose from the floor with Gowerś manoeuvre and from the chair supporting himself on both thighs. He was unable to walk on tiptoes but succeeded on heels. He climbs the stairs using the handrail and supporting one hand on the thigh. Slight difficulty was evident while flexing elbow with 5 kg weight. Slight grip weakness was in the right side. The patient manages to walk less than 500 m without assistance. He showed hyperlordosis and back-kneeing in standing position (Fig. 2C). There was wasting of the quadriceps, mainly on the left side (Fig. 2D). Calf muscles were atrophic as well as the right biceps brachi and pectoralis muscles (Fig. 2E). Muscle manual strength testing showed severe weakness of ankle plantar flexion (MRC grade R2/L1), thigh flexion and adduction (MRC grade R3−/L2), knee extension (MRC grade R3−/L2−), hip flexion and extension (MRC grade 3+), right elbow flexion and grip (MRC grade 4), and right pectoralis (MRC grade 2).
CK values were high ranging from 3822 to 10,471 u/l. Muscle MRI revealed initial fibro-fatty degeneration in the gastrocnemius and soleus muscles at DD nine years (Fig. 2B). The third control performed at DD 15 years showed also involvement of the adductors and biceps femoris muscles, mainly in the left side. The left vastus lateralis and intermedius muscles and the right tensor fascia lata (TFL) were involved (Fig. 2C). While the degeneration in gastrocnemius muscles remained constant or progressed slightly with involvement of the soleus muscle, the quadriceps muscle showed rapid progression of fibro-fatty degeneration at DD of 21 and 24 years, respectively. There was also fibro-fatty degeneration of the long head of the biceps brachi muscle bilaterally.
Muscle biopsies were obtained from the left gastrocmnemius medial from P.1 at DD of 2 years and from P.2 at DD 3 years. Muscle biopsies have been obtained again from the left vastus lateralis from P.1 at DD of 21 years and at DD of 22 years from P.2. Muscle biopsy from P.1 showed fiber size variation, internal nuclei and occasional split fibers (Fig. 4A), while P.2 biopsy showed some picnotic nuclei and nuclear clumps, marked fiber variation, endomysial fibrosis and some fatty infiltration. Necrotic and regenerating fibers were not seen. Immunohistochemical analysis showed normal dysferlin staining in both patients (Fig. 4B). There was little dysferlin accumulation within the fibers. Electron microscopy analysis showed multifocal disruption of the sarcolemmal membrane but no subsarcolemmal vesicle accumulation (Fig. 4C).
Fig. 4.
Biopsy obtained from patient P.1 at DD of 21 years from the left vastus lateralis shows a quite severe dystrophic picture with some picnotic nuclei and nuclear clumps (A). Dysferlin shows a positive sarcolemmal staining on immunohistochemistry (B). Electron microscopy shows normal myofibrillar architecture but there is evidence of multifocal loss of sarcolemmal membrane (arrows). Electron microscopy, original magnification ×5000.
Sensation was normal in both patients. No contractures were detected. No patient had an abnormal ECG or Echocardiogram. Respiratory function evaluation with frequent spirometry and arterial blood gas analysis was normal in both patients.
3. Discussion
The disorder seen in our patients is characterized by onset of unpleasant sensations in calf muscles in the third decade. Then the patients complained of tightness on the calves during running. Difficulty with climbing the stairs appeared later. There was asymmetric muscle involvement. Muscle weakness in the upper limbs appeared in late stages and was asymmetrical and involving mainly the biceps brachi and pectoral muscles. The pattern of muscle involvement determined by a follow-up muscle MRI study was characterized by the involvement in chronological order of gastrocnemius, soleus, hip adductors, hamstrings, and quadriceps and later on TFL and biceps brachi, respectively. Calf hypertrophy was present in early stages. The serum CK levels were very high during the first years of disease. Muscle biopsy findings showed dystrophic features with no active inflammation. Multifocal disruption of the sarcolemmal membrane was seen in electron microscopy analysis.
Intrafamilial variation was present in the family reported here. The disease has progressed more insidiously in the elder brother, while the younger sibling has shown a relatively more rapid progression. At present there is no clear explanation for this clinical variation since we have not analyzed the ANO5 protein levels in the muscle from the two patients. We are predicting that the mutation in this family (homozygous c.2272C>T in exon 5) is loss of function resulting in deficiency of the protein from patient muscle. It is possible that the variation in disease severity between the two brothers may be linked to differential levels of ANO5 in the patient muscle. We are developing ANO5 antibodies to test this. The clinical picture of the Dutch MMD3 patients carrying ANO5 mutation is similar to our patients and is characterized with distal onset, asymmetric calf muscle involvement and very high CK levels. However, no patient presented calf hypertrophy as has been seen in both our patients. Only one Dutch patient had thigh muscle weakness as a later manifestation, another common feature with our patients. The Dutch patients did not complain of any pain, while unpleasant sensations were present in both our cases. The mutation in the Dutch family is a homozygous duplicated adenine (c.191dupA) in exon 5, which is also present as heterozygous in one LGMD2L family [12]. The prominent clinical features of LGMD2L are asymmetric early muscle involvement of proximal lower limb-girdle muscles and high CK levels [11]. LGMD2L patients developed in later stages calf atrophy without associated weakness. The proximal muscles of the lower limbs were involved later in the disease compared to our patients. The biceps brachi muscle was affected in both diseases. Calf hypertrophy was a common feature of both diseases in early stages. Pain was frequently reported by LGMD2L patients, while our patients complained of unpleasant sensations in muscles which were not related to exercise. The clinical course in both diseases was relatively slow. Serum CK levels were very high in both diseases. The muscle histology reported in LGMD2L overlaps with our findings. At present it is not possible to establish genotype–phenotype correlations among the patients with ANO5 mutations.
The disease seen in our patients appears to be different from MM. Muscle asymmetric involvement is not seen in MM and calf pains have been reported only in 17% ofMM patients [8]. In our patients uncomfortable burning muscle sensation preceded the muscle weakness. Distal muscles of the forearms were spared in both patients. There was no involvement of the anterior muscles of the legs even after 24 years of disease. The pelvic and paravertebral muscles were minimally involved. The histopathology of these cases differs from these observed in dysferlinopathy for the lack of inflammation and at EM level for lack of vesicle accumulation under the sarcolemma.
We have shown that in our patients that ANO5 mutations are associated with sarcolemmal lesions and defective membrane repair suggesting that ANO5 may function in muscle membrane repair. Cells from the patients reported in this study are compromised in their ability to repair an injury to their cell membrane despite the fact that the conventional membrane repair pathways are operating normally [10]. Defective membrane repair is thought to be the pathomechanism in the dysferlinopathies [13,14]. Given the clinical similarities of the MMD3 patients with MM which led to their classification as MM patients (see Refs. [8] and [9]) we are predicting that ANO5 functions in the dysferlin membrane repair pathway. Membrane repair is thought to require the fusion of endomembrane derived vesicles accumulating at the membrane rupture site in response to the elevated intracellular calcium levels, to form a patch membrane, which is then inserted to the wound site by exocytosis [15,16]. Dysferlin is predicted to function as the fusogen in the formation of the patch membrane following membrane wounding [13,14]. Recently several studies have shown that dysferlin may also be important for secretion. Dysferlin deficient primary myoblasts when stimulated show reduced cytokine release [17] and they can also assemble the inflammasome complex to secrete IL-1β [18].
ANO5 is a member of the anoctamin protein family several members of which have been shown to function as calcium activated chloride channels (CaCCs) [19–21]. CaCCs are gated by increases in intracellular calcium and they are required for a variety of cellular functions including secretion, cell volume regulation, olfactory and photoreceptor transduction, cardiac membrane excitability, and smooth muscle contraction [19–21]. ANO5 shows high skeletal muscle expression which is increased in differentiating muscle cells [22]. By biochemical fractionation ANO5 has been localized to vesicles and the plasma membrane in muscle cells [22]. Intriguingly rapid increases in chloride currents which are calcium dependent have been detected soon after wounding of starfish oocyte membranes [23]. Wounded starfish oocytes have also been shown to secrete ATP resulting in intercellular calcium signalling [24]. It is possible that ANO5 functions with dysferlin to regulate secretion following calcium induced exocytosis.
4. Conclusions
The disease seen in our patients is clinically, genetically and histopathologically distinct from Miyoshi myopathy. The previously used non-dysferlin linked MM nomenclature may be inappropriate.
Acknowledgements
We are grateful to the family for participating actively in this research. We want to thank the neuropathologist Anders Paetau, MD, PhD (Department of Pathology, University of Helsinki) for examining the muscle biopsies of our patients.
References
- 1.Mastaglia FL, Lamont PJ, Laing NG. Distal myopathies. Curr Opin Neurol. 2005;18:504–510. doi: 10.1097/01.wco.0000175936.23945.b6. [DOI] [PubMed] [Google Scholar]
- 2.Miyoshi K, Kawai H, Iwasa M, et al. Autosomal recessive distal muscular dystrophy as a new type of progressive muscular dystrophy: seventeen cases in eight families including an autopsied case. Brain. 1986;109:31–54. doi: 10.1093/brain/109.1.31. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Myoshi myopathy and limb-girdle muscular dystrophy. Nat Genet. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 4.Bashir R, Britton S, Strachan T, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. doi: 10.1038/1689. [DOI] [PubMed] [Google Scholar]
- 5.Illa I, Serrano-Munuera C, Gallardo E, et al. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49:130–134. [PubMed] [Google Scholar]
- 6.Nguyen K, Bassez G, Krahn M, et al. Phenotypic study in 40 patients with dysferlin gene mutations: high frequency of atypical phenotypes. Arch Neurol. 2007;64:1176–1182. doi: 10.1001/archneur.64.8.1176. [DOI] [PubMed] [Google Scholar]
- 7.Paradas C, González-Quereda L, De Luna N, et al. A new phenotype of dysferlinopathy with congenital onset. Neuromuscul Disord. 2009;19:21–25. doi: 10.1016/j.nmd.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Linssen WH, Notermans NC, Van der Graaf Y, et al. Miyoshi-type distal muscular dystrophy. clinical spectrum in 24 Dutch patients. Brain. 1997;120:1989–1996. doi: 10.1093/brain/120.11.1989. [DOI] [PubMed] [Google Scholar]
- 9.Linssen WH, de Visser M, Notermans NC, et al. Genetic heterogeneity in Miyoshi-type distal muscular dystrophy. Neuromuscul Disord. 1998;8:317–320. doi: 10.1016/s0960-8966(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal J, Marlow G, Summerill G, et al. Defect in a novel membrane repair pathway in patients with a non-dysferlin Miyoshi myopathy. Traffic. 2007;8:77–88. doi: 10.1111/j.1600-0854.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 11.Jarry J, Rioux MF, Bolduc V, et al. A novel autosomal recessive limb-girdle muscular dystrophy with quadriceps atrophy maps to 11p13–p12. Brain. 2007;130:368–380. doi: 10.1093/brain/awl270. [DOI] [PubMed] [Google Scholar]
- 12.Bolduc V, Marlow G, Boycott KM, et al. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet. 2010;86:1–9. doi: 10.1016/j.ajhg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han R, Campbell KP. Dysferlin and muscle membrane repair. Curr Opin Cell Biol. 2007;19:409–416. doi: 10.1016/j.ceb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glover L, Brown RH., Jr Dysferlin in membrane trafficking and patch membrane repair. Traffic. 2007;8:785–794. doi: 10.1111/j.1600-0854.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 15.McNeil PM, Kirchausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 16.McNeil PM, Steinhardt RA. Plasma membrane disruption, repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 17.Chiu YH, Hornsey MA, Klinge L, et al. Attenuated muscle regeneration is a key factor in dysferlin-linked muscular dystrophy. Hum Mol Genet. 2009;18:1976–1989. doi: 10.1093/hmg/ddp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawat R, Cohen TV, Ampong B, et al. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol. 2010;176:2891–2900. doi: 10.2353/ajpath.2010.090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+ activated Cl− channels. J Physiol. 2009;587:2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galietta J. The TMEM16 protein family: a new class of chloride channels. Biophys J. 2009;97:3047–3053. doi: 10.1016/j.bpj.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kunzlemann K, Kongsuphol P, Aldehni F, et al. Bestrophin and TMEM16-Ca(2+)-activated Cl(−) channels with different functions. Cell Calcium. 2009;46:233–241. doi: 10.1016/j.ceca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Mizuta K, Tsutsumi S, Inoue H, et al. Molecular characterization of GDD1/TMEM16E, the gene product responsible for autosomal dominant gnathodiaphyseal dysplasia. Biochem Biophys Res Commun. 2007;357:126–132. doi: 10.1016/j.bbrc.2007.03.108. [DOI] [PubMed] [Google Scholar]
- 23.Fein A, Terasaki M. Rapid increase in plasma membrane chloride permeability during wound resealing of starfish oocytes. J Gen Physiol. 2005;126:151–159. doi: 10.1085/jgp.200509294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covian-Nares JF, Koushik SV, Puhl HL, 3rd, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci. 2010;123:1884–1893. doi: 10.1242/jcs.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]