Abstract
Shiitake mushrooms (Lentinula edodes) containing β-glucans may be beneficial for human health; they have been used in the treatment of cancer, hypertension, and high cholesterol levels. The objective of this study was to determine the β-glucan content in different sections of the fruiting bodies and mycelia of ten shiitake mushroom cultivars. The measured β-glucan content ranged from 20.06 ± 1.76% to 44.21 ± 0.13% in the pileus sections, and from 29.74 ± 1.40% to 56.47 ± 4.72% in the stipe sections. The results of this study indicate that the variance in β-glucan content dependent on the shiitake cultivar, and that the β-glucan content is higher in the stipe than in the pileus.
Keywords: β-Glucan, Cultivar, Lentinula edodes, Shiitake mushroom
The shiitake mushroom, Lentinula edodes, is one of most important edible mushrooms cultivated in the world. L. edodes contains various vitamins including vitamins B1, B2, B12, C, D, and niacin, providing the highest level of, vitamin D of any plant food [1]. The mushroom is also a source of proteins, fats, minerals, and (1-3)-(1-6)-β-glucans polysaccharides [2, 3].
β-Glucans are polymers of glucose linked by glycosidic bonds, that are found at high levels in the cell wall of fungi, yeast, oats, and barley. β-Glucans derived from L. edodes are comprised of five linearly linked (1→3)-β-glucose residues and two side chain branches of (1→6)-β-glucopyranoside [4]. β-Glucans bind to lymphocyte surfaces or serum-specific proteins, that activate macrophages, helper T-cells, natural killer cells, and other effector cells. The activation of these effectors results in an increase in the production and release of antibodies, interleukins, and interferon [5].
Mushrooms containing β-glucans have been used to decrease blood cholesterol levels; the β-glucans increase intestinal viscosity and decrease cholesterol absorption, thereby promoting its excretion [6, 7, 8].
β-Glucans increase the resistance of the intentinal mucosa to inflammation and inhibit the development of intestinal ulcers [9, 10]. In addition, it has been reported that β-glucans are effective in treating cancer [11, 12, 13], infectious diseases [14], hypertension [15, 16], diabetes [17, 18], and high blood pressure [19].
Kim et al. [20] described the β-glucan content in whole fruiting bodies of the shiitake mushroom; however, the β-glucan content in the pileus and stipes of fruiting bodies, and in the mycelia of different shiitake cultivars has yet to be examined [21, 22].
The objective of this study was to determine the β-glucan content in two distinct morphological sections of the fruiting body (pileus and stipe) and in the mycelia of ten different shiitake cultivars in order to extend our understanding of the nutritional potential of shiitake mushrooms. The fruiting bodies and mycelia of ten shiitake cultivars registered in Korea were used for this study; Samlim No. 2, Samlim No. 4, Samlim No. 7, Samlim No. 10, Gaeulhyang, Soohyangko, Dasanhyang, Chunjang No. 1, Chunjang No. 2, and Poongnyunko; these were developed by the Korea Forest Research Institute (KFRI). The shiitake mushroom samples used in this study are illustrated in Fig. 1.
Fig. 1.

Fruiting bodies of Lentinula edodes cultivars used in this work: A, Samlim No. 2; B, Samlim No. 4; C, Samlim No. 7; D, Samlim No. 10; E, Gaeulhyang; F, Soohyangko; G, Dasanhyang; H, Chunjang No. 1; I, Poongnyunko; J, Chunjang No. 2.
To prepare the samples, mushrooms were dried in a dry oven at 55℃ for 4 days, separated into pileus and stipes, and then pulverized. Mycelia were maintained on potato dextrose agar (Becton Dickinson, Sparks, MD, USA) plates and subcultured every 2 mon. The plates were incubated at 25℃ for approximately 30 days, at which point the mycelia were harvested from the potato dextrose agar and then dried, and pulverized.
The β-glucan content of the dried shiitake mushroom fruiting bodies was determined using a mushroom and yeast specific β-glucan kit (Megazyme International, Wicklow, Ireland) [23]. The enzyme kit contains exo-1,3-β-glucanase, β-glucosidase, amyloglucosidase and invertase; glucose determination reagent (glucose oxidase peroxidase, and 4-aminoantipyrine), and glucose standard solution. Measurement of total glucan content was conducted by hydrolyzing the shiitake samples with 37% hydrochloric acid (v/v) for 45 min at 30℃ followed by an additional 2 hr at 100℃. Subsequent to neutralization with 2M potassium hydroxide, glucose hydrolysis was performed using a mixture of exo-1,3-β-glucanase and β-glucosidase in sodium acetate buffer (pH 5.0) for 1 hr at 40℃. The absorbance of the resulting color complex was measured at 510 nm using a spectrophotometer (Shimadzu Co., Kyoto, Japan). The α-glucan content was determined using the method described above following enzymatic hydrolysis with amyloglucosidase and invertase. The β-glucan content was calculated by subtracting the α-glucan content from the total glucan content. Glucan content was expressed as percentage (w/w) of mushroom dry weight.
All measurements were taken a minimum of three times, and the experimental data were analyzed using the ANOVA test. The resulting measurements were compared using SPSS ver. 12.0 statistical software (SPSS Inc., Chicago, IL, USA). All data are expressed as mean ± standard deviation (SD). The level of statistical significance was set at p < 0.05.
Table 1 details the glucan content (total, α-glucan, and β-glucan) in the pileus and stipe sections of the dried fruiting bodies and mycelium of ten different shiitake cultivars. Glucan content varied considerably among the shiitake cultivars. As described in Table 1, the total glucan content in the pileus ranged from 29.16 ± 3.13% to 46.30 ± 0.31%, whereas the α-glucan content ranged from 0.70 ± 0.60% to 8.17 ± 0.18%. α-Glucans are present in the polysaccharide fraction of mushrooms [24]; they increase insulin sensitivity and ameliorate insulin resistance in peripheral target tissues, thus exerting an anti-diabetic effect [25].
Table 1.
The glucan content in the fruiting bodies and mycelium of ten Lentinula edodes cultivars
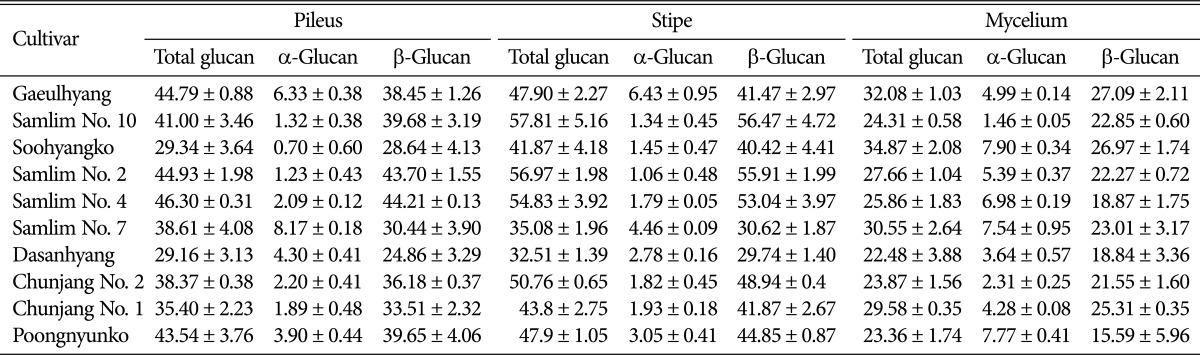
β-Glucan (% w/w) = Total glucan (% w/w) - α-glucan (% w/w).
All values are expressed as mean ± SD of triplicate measurements.
The β-glucan content in the pileus ranged from 20.06 ± 1.76% to 44.21 ± 0.13%. The highest level of β-glucans, 44.21 ± 0.13%, was observed in strain "Samlim No. 4"; the "Dasanhyang" strain demonstrated the lowest levels of β-glucan. The total glucan content in the stipe section varied from 32.51 ± 1.39% to 57.81 ± 5.16%, whereas the α-glucan content ranged from 1.06 ± 0.48% to 6.43 ± 0.95%. The level of β-glucan content in the stipe section ranged from 29.74 ± 1.40% to 56.47 ± 4.72%. The highest β-glucan content, 56.47 ± 4.72%, was observed in strain "Samlim No. 10".
Kozarski et al. [26] analyzed the chemical composition, including the levels of total polysaccharides, total glucans, α-glucan, and β-glucan, in polysaccharide extracts from Lentinula edodes. Their results established the total glucan level as 42.6 g, the α-glucan level as 1.4 g, and the β-glucan level as 41.2 g, per 100 g dry weight of extract, consistent with the findings of this study.
Kim et al. [20] reported that the β-glucan content of shiitake mushrooms, divided based on size, ranged from 26% to 27%; however, the variance between the groups was not significant. Handayani et al. [21] demonstrated that the β-glucan soluble fiber content of shiitake mushrooms was 30 g per 100 g of dry mushroom; this measurement, was used as a nutritional value baseline in other experiments. Gil-Ramirez et al. [27] studied the β-glucan content of several mushroom samples from a local market in Spain, using dry mushroom powder in their β-glucan extraction procedure; their results indicated that the β-glucan content L. edodes is 20 mg/100 mg. The values measured in this study were higher than previously published results. This discrepancy might be due to the shiitake mushroom strain types used growth conditions, degree of fruiting body maturity, use of whole fruiting bodies vs. separate section (pileus, stipes), or the use of fresh vs. dried fruiting bodies [20, 21, 22, 27, 28]. De Andrade et al. [29] reported that the nutritional properties (raw protein, ashes, and raw fiber values) of L. edodes vary according to strain, processing post-harvest, developmental stage of the basidiomata, and the type of substrate used.
β-Glucan is a soluble fiber derived from the cell walls of fungi. In 2013, Nasiri et al. [30] reported that the fiber content in the pileus and stipe of Iranian mushrooms was 33.11% and 38.08%, respectively; the fiber content in the pileus was lower than that in the stipe, in accordance with the results of recent studies [30]. Similarly, Shimizu et al. [31] found that the stipe of L. edodes in Japan contained higher amounts of β-glucan than the pileus; the β-glucan content of the pileus and stipe was determined as 22.8% and 49.5%, respectively [31].
The total and α-glucan content in mycelia ranged from 22.48 ± 3.88% to 34.87 ± 2.08% and from 1.46 ± 0.05% to 7.90 ± 0.34%, respectively. The β-glucan content in mycelia ranged from 15.59 ± 5.96% to 27.09 ± 2.11%. The highest β-glucan content level measured, 27.09 ± 2.11%, was observed in the "Gaeulhyang" strain. The β-glucan content in fruiting bodies was higher than that in the mycelia. These results concur with a previous study by Mölleken et al. [32], who reported levels of 9.5% and 4.2% for fruiting bodies and mycelia, respectively.
Our results demonstrate that the stipe contains higher amounts of β-glucan than the pileus. In addition, the β-glucan content was lowest in the mycelia except in the "Soohyangko" strain.
In this study, we presented a quantitative analysis of β-glucan levels in three sections of the mushroom: stipe, pileus and mycelium. The β-glucan content of ten different shiitake cultivars was determined using enzymatic methods. The highest β-glucan content was observed in the stipe section of the shiitake fruiting bodies, with values ranging between 29.74 ± 1.40% and 56.47 ± 4.72%. The variance in β-glucan content was dependent on shiitake cultivar, and the β-glucan content was higher in the stipe than in the pileus. Shiitake cultivars with elevated β-glucan content could be used as a nutritional source for the modern food industry. However, mycelia of shiitake mushrooms that exhibit relatively low levels of β-glucan, ranging from 15.59 ± 5.96% to 27.09 ± 2.11%, would not provide a suitable nutritional supply.
References
- 1.Mattila P, Suonpää K, Piironen V. Functional properties of edible mushrooms. Nutrition. 2000;16:694–696. doi: 10.1016/s0899-9007(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 2.Longvah T, Deosthale YG. Compositional and nutritional studies on edible wild mushroom from northeast India. Food Chem. 1998;63:331–334. [Google Scholar]
- 3.Mattila P, Salo-Väänänen P, Könkö K, Aro H, Jalava T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J Agric Food Chem. 2002;50:6419–6422. doi: 10.1021/jf020608m. [DOI] [PubMed] [Google Scholar]
- 4.Chihara G. Immunopharmacology of lentinan, a polysaccharide isolated from Lentinus edodes: its application as a host defense potentiator. Int J Orient Med. 1992;17:57–77. [Google Scholar]
- 5.Yap AT, Ng ML. Immunopotentiating properties of lentinan(1-3)-b-D-glucan extracted from culinary-medicinal shiitake mushroom. Int J Med Mushrooms. 2003;5:19–39. [Google Scholar]
- 6.Bernardshaw S, Hetland G, Grinde B, Johnson E. An extract of the mushroom Agaricus blazei Murill protects against lethal septicemia in a mouse model of fecal peritonitis. Shock. 2006;25:420–425. doi: 10.1097/01.shk.0000209526.58614.92. [DOI] [PubMed] [Google Scholar]
- 7.Bobek P, Nosálová V, Cerná S. Effect of pleuran (beta-glucan from Pleurotus ostreatus) in diet or drinking fluid on colitis in rats. Nahrung. 2001;45:360–363. doi: 10.1002/1521-3803(20011001)45:5<360::AID-FOOD360>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Cheung PC. Plasma and hepatic cholesterol levels and fecal neutral sterol excretion are altered in hamsters fed straw mushroom diets. J Nutr. 1998;128:1512–1516. doi: 10.1093/jn/128.9.1512. [DOI] [PubMed] [Google Scholar]
- 9.Zeman M, Nosálová V, Bobek P, Zakálová M, Černa S. Changes of endogenous melatonin and protective effect of diet containing pleuran and extract of black elder in colonic inflammation in rats. Biologia (Bratisl) 2001;56:695–701. [Google Scholar]
- 10.Nosál'oá V, Bobek P, Cerná S, Galbavý S, Stvrtina S. Effects of pleuran (beta-glucan isolated from Pleurotus ostreatus) on experimental colitis in rats. Physiol Res. 2001;50:575–581. [PubMed] [Google Scholar]
- 11.Mizuno M, Minato K, Ito H, Kawade M, Terai H, Tsuchida H. Anti-tumor polysaccharide from the mycelium of liquid-cultured Agaricus blazei mill. Biochem Mol Biol Int. 1999;47:707–714. doi: 10.1080/15216549900201773. [DOI] [PubMed] [Google Scholar]
- 12.Vetvicka V, Yvin JC. Effects of marine beta-1,3 glucan on immune reactions. Int Immunopharmacol. 2004;4:721–730. doi: 10.1016/j.intimp.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Li X, Xu X, Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr Res. 2005;340:1515–1521. doi: 10.1016/j.carres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Markova N, Kussovski V, Drandarska I, Nikolaeva S, Georgieva N, Radoucheva T. Protective activity of Lentinan in experimental tuberculosis. Int Immunopharmacol. 2003;3:1557–1562. doi: 10.1016/S1567-5769(03)00178-4. [DOI] [PubMed] [Google Scholar]
- 15.Kabir Y, Yamaguchi M, Kimura S. Effect of shiitake (Lentinus edodes) and maitake (Grifola frondosa) mushrooms on blood pressure and plasma lipids of spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 1987;33:341–346. doi: 10.3177/jnsv.33.341. [DOI] [PubMed] [Google Scholar]
- 16.Kabir Y, Kimura S, Tamura T. Dietary effect of Ganoderma lucidum mushroom on blood pressure and lipid levels in spontaneously hypertensive rats (SHR) J Nutr Sci Vitaminol (Tokyo) 1988;34:433–438. doi: 10.3177/jnsv.34.433. [DOI] [PubMed] [Google Scholar]
- 17.Lo HC, Tsai FA, Wasser SP, Yang JG, Huang BM. Effects of ingested fruiting bodies, submerged culture biomass, and acidic polysaccharide glucuronoxylomannan of Tremella mesenterica Retz.:Fr. on glycemic responses in normal and diabetic rats. Life Sci. 2006;78:1957–1966. doi: 10.1016/j.lfs.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Kiho T, Morimoto H, Sakushima M, Usui S, Ukai S. Polysaccharides in fungi. XXXV. Anti diabetic activity of an acidic polysaccharide from the fruiting bodies of Tremella aurantia. Biol Pharm Bull. 1995;18:1627–1629. doi: 10.1248/bpb.18.1627. [DOI] [PubMed] [Google Scholar]
- 19.Wang HX, Ooi VE, Ng TB, Chiu KW, Chang ST. Hypotensive and vasorelaxing activities of a lectin from the edible mushroom Tricholoma mongolicum. Pharmacol Toxicol. 1996;79:318–323. doi: 10.1111/j.1600-0773.1996.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Lim J, Bae IY, Park HG, Lee HG, Lee S. Particle size effect of Lentinus edodes mushroom (CHAMSONG-I) powder on the physicochemical, rheological, and oil-resisting properties of frying batters. J Texture Stud. 2010;41:381–395. [Google Scholar]
- 21.Handayani D, Meyer BJ, Chen J, Tang P, Kwok PC, Chan HK, Huang XF. The comparison of the effect of oat and Shiitake mushroom powder to prevent body weight gain in rats fed high fat diet. Food Nutr Sci. 2012;3:1009–1019. [Google Scholar]
- 22.Manzi P, Pizzoferrato L. Beta-glucans in edible mushrooms. Food Chem. 2000;68:315–318. [Google Scholar]
- 23.Megazyme International Ireland Ltd, authors. Mushroom and yeast beta-glucan: assay procedure. Wicklow: Megazyme International Ireland Ltd; 2002. [Google Scholar]
- 24.Zhang P, Cheung PC. Evaluation of sulfated Lentinus edodes alpha-(1→3)-D-glucan as a potential antitumor agent. Biosci Biotechnol Biochem. 2002;66:1052–1056. doi: 10.1271/bbb.66.1052. [DOI] [PubMed] [Google Scholar]
- 25.Hong L, Xun M, Wutong W. Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KKAy mice. J Pharm Pharmacol. 2007;59:575–582. doi: 10.1211/jpp.59.4.0013. [DOI] [PubMed] [Google Scholar]
- 26.Kozarski M, Klaus A, Nikšić M, Vrvić MM, Todorović N, Jakovljević D, Van Griensven LJ. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J Food Compost Anal. 2012;26:144–153. [Google Scholar]
- 27.Gil-Ramirez A, Clavijo C, Palanisamy M, Soler-Rivas C, Ruiz-Rodriguez A, Marín FR, Reglero G, Pérez M. Edible mushrooms as potential sources of new hypocholesterolemic compounds; Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products; 2011 Oct 4-7; Arcachon, France. pp. 110–119. [Google Scholar]
- 28.Rop O, Mlcek J, Jurikova T. Beta-glucans in higher fungi and their health effects. Nutr Rev. 2009;67:624–631. doi: 10.1111/j.1753-4887.2009.00230.x. [DOI] [PubMed] [Google Scholar]
- 29.de Andrade MC, de Almeida Minhoni MT, Zied DC. Nutritional evaluation of shiitake mushroom [Lentinula edodes (Berk.) Pegler] in function of the strain and type of cultivated eucalyptus. Cienc Tecnol Aliment. 2008;28:916–921. [Google Scholar]
- 30.Nasiri F, Tarzi BG, Bassiri AR, Hoseini SE, Aminafshar M. Comparative study on the main chemical composition of button mushroom's (Agaricus bisporus) cap and stipe. J Food Biosci Technol. 2013;3:41–48. [Google Scholar]
- 31.Shimizu K, Fujita R, Kondo R, Sakai K, Kaneko S. Morphological features and dietary functional components in fruit bodies of two strains of Pholiota adiposa grown on artificial beds. J Wood Sci. 2003;49:193–196. [Google Scholar]
- 32.Mölleken H, Nitschke J, Modick H, Malolepszy T, Altenbach HJ. A new colorimetric method to quantify β-1,3-1,6-glucans in comparison with total β-1,3-glucans and a method to quantify chitin in edible mushrooms. Food Chem. 2011;127:791–796. doi: 10.1016/j.foodchem.2010.12.149. [DOI] [PubMed] [Google Scholar]


