Abstract
HIV-affected couples have unique challenges that require access to information and reproductive services which prevent HIV transmission to the uninfected partner and offspring while allowing couples to fulfill their reproductive goals. In high HIV prevalent regions of sub-Saharan Africa, HIV-affected couples require multipurpose prevention technologies (MPTs) to enhance their reproductive healthcare options beyond contraception and prevention of HIV/sexually transmitted infections (STIs) to include assistance in childbearing. The unique characteristics of the condom and its accepted use in conjunction with safer conception interventions allow HIV-serodiscordant couples an opportunity to maintain reproductive health, prevent HIV/STI transmission, and achieve their reproductive goals while timing conception. Rethinking the traditional view of the condom and incorporating a broader reproductive health perspective of HIV-affected couples into MPT methodologies will impact demand, acceptability, and uptake of these future technologies.
Keywords: HIV-serodiscordant couples, safer conception, HIV prevention, assisted reproductive services, multipurpose prevention technology
Introduction
HIV-serodiscordant couples, “a married or cohabiting couple in which one partner is HIV-infected and the other is HIV-uninfected,” are an important source of new HIV infections in sub-Saharan Africa (SSA)1,2 where it is estimated that 23.5 million people are HIV-infected.3 For example, in Kenya, the national prevalence of HIV infection is estimated at 5.6% with an estimated 260,000 HIV-serodiscordant couples.4 HIV-infected individuals have reproductive desires that cannot be ignored and they knowingly risk HIV transmission in order to conceive.5-7 In HIV-serodiscordant partnerships in which pregnancy occurs, the risk of HIV acquisition nearly doubles for the uninfected partner compared to partnerships in which pregnancy does not occur.5 Furthermore, providing fertile HIV-infected women the possibility of preserving their fertility and a safer option for conception is empowering given the stigma and isolation they may already encounter as a result of their HIV status, particularly in cultures where reproduction defines ones value in society.8-10 The reproductive desires and intentions of HIV-infected individuals have not been adequately addressed, particularly in low-resource environments,11 in relation to decreasing the risk of unintended pregnancy and transmission of HIV, other sexually transmitted infections (STIs), and resultant infertility. Infertility is a global public health problem with great implications in SSA, specifically in HIV-affected couples (Figure 1). Therefore, the reproductive intentions and prolonged periods of unprotected intercourse to achieve pregnancy among HIV-infected individuals may reduce the impact of HIV prevention efforts unless comprehensive reproductive services includingas multipurpose technologies (MPTs) equally address contraception, childbearing desires and prevention of HIV/STIs.12, 13 Redefining our view of the condom as a model MPT with integration of comprehensive HIV and reproductive care counseling and education with provision of safer conception strategies will successfully achieve the goal of prevention.
Figure 1.
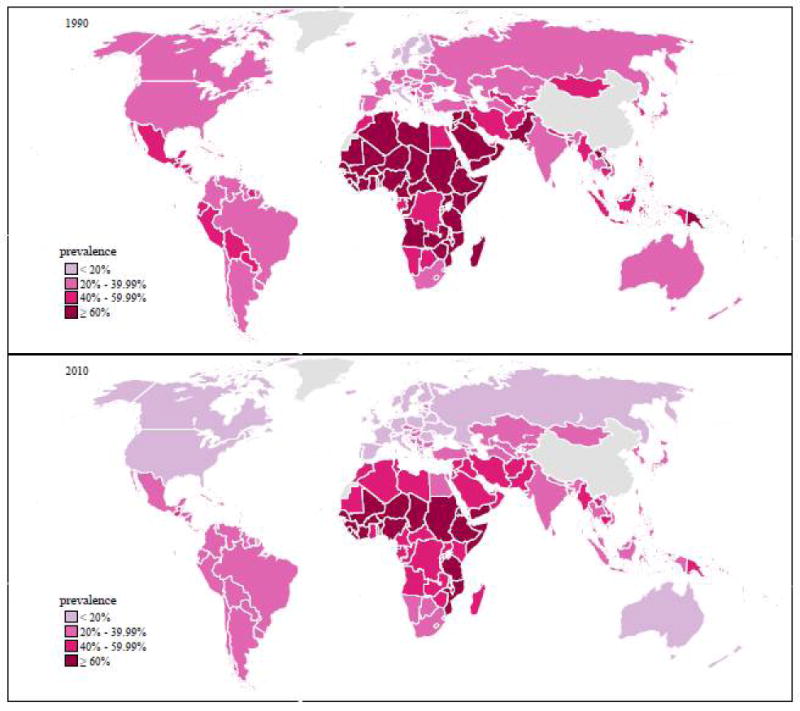
Webfigure 14:Exposure to secondary infertility (out of all women) in 1990 (top) and 2010 (bottom). In fertility exposure is indexed on the female partner, age-standarized prevalence amount all women.
At the International Conference on Population Development in 1994, the accepted definition of reproductive health, implied that women and men have the right to be “informed [about] and have access to safe, effective, affordable and acceptable methods of family planning…and appropriate healthcare services that will enable women to go through pregnancy and childbirth and provide couples with the best chance of having a healthy infant”.14 This definition of reproductive health has shaped and defined the health strategies of various governmental and non-governmental organizations including that of the World Health Organization’s (WHO) Department of Reproductive Health and Research. The vision of the WHO’s Reproductive Health and Research division, which was adopted by United Nations member states in 2004, is “the attainment by all people of the highest possible level of sexual and reproductive health.” Their intent is to conduct and support research initiatives and develop public health policies that strive for a world in which women and men “have access to sexual and reproductive health information services.” This is premised on the concept of reproductive rights and assurance of choice when meeting the needs of diverse populations, particularly those that have been neglected and at-risk. The current reproductive health paradigm addresses universal access in the following five areas: maternal and newborn health, “family planning,” preventing unsafe abortion, management of reproductive tract and sexually transmitted infections, including HIV, and promotion of sexual health. Linking the WHO’s Sexual and Reproductive Health (SRH) and HIV prevention objectives and programs will enhance development of MPTs for the empowerment of women through the prevention of HIV/STIs and provision of reproductive options.
Despite the intention of public health policies that provide universal access to reproductive healthcare services, the reproductive desire to have children by HIV-affected individuals and couples have not been adequately addressed. Furthermore, access to safe assisted fertility interventions have been neglected within the global reproductive health agenda and “family planning” discussions for HIV-serodiscordant couples.15 Comprehensive reproductive services for HIV-affected couples should not only provide contraceptive services but also assisted fertility services for those desiring children. According to the WHO SRH Guidelines for women living with HIV/AIDS men and/or women with HIV/AIDS “may be more likely to have difficulty getting pregnant and to request assistance. These women should be given full support for counseling and advised of their options, including adoption and assisted reproduction, if available.”16 Both simple and complex assisted reproductive techniques, for HIV-affected couples with underlying infertility or subfertility, can provide a means for conception that prevents partner transmission. Prior to the use of fertility services, healthcare providers should consider educating and counseling HIV-affected couples on fertility awareness methods and performing a fertility evaluation in the couple to assess for underlying infertility, which may help direct them to the appropriate reproductive services. Simple fertility methods include timed vaginal insemination (TVI) and sperm washing (SW) with intrauterine insemination (IUI); and where indicated and economically feasible more complex interventions such as in-vitro fertilization (IVF) with or without intracytoplasmic sperm injection (ICSI) as HIV prevention interventions. In HIV-serodiscordant couples, a water-based lubricated male condom is a critical fertility care technology for safe collection of semen that avoids sexual HIV transmission in conjunction with any of the above methods. These reproductive interventions avoid extended exposure with unprotected intercourse in couples desiring children.
All these methods require the consistent use of male condoms, or if found acceptable female condoms, which would decrease the incidence of unintended pregnancy and HIV/STI transmission. Most importantly, the range of reproductive methods offered with the consistent use of condoms will allow HIV-infected men and women the option of choosing the method best suited to their current situation and reproductive health priority, either for contraception or safer conception. As a result of rebranding the condom there is new motivation to increase their consistent use. Overall, options that address the full complement of reproductive health needs will enhance uptake and acceptance of MPT methodologies. Pharmaceutical methods, such as the use of antiretroviral therapy in the HIV-infected partner, have been demonstrated to decrease the risk of sexual HIV transmission.17, 18 In addition, antiretroviral therapy may be administered to the uninfected partner in an HIV-serodiscordant relationship during the periconception period as pre-exposure prophylaxis (PrEP).19 A recent report from the United Kingdom has shown that timed unprotected intercourse along with the use of PrEP by the HIV-uninfected female in the periovulatory period demonstrated feasibility and decreased the risk of sexual and perinatal HIV transmission.21 Thus, safer conception practices that utilize either a reproductive and/or pharmaceutical intervention should be part of the arsenal of options offered to HIV-serodiscordant couples, which will assist in decreasing the risk of sexual and perinatal HIV transmission to uninfected partners and developing fetus. MPTs are being developed to address a complimentary component to provide contraception, however they can be innovatively modified to address the needs of safer conception.
Worldwide, the gap in access and provision of reproductive healthcare services for HIV-infected individuals affects their quality of life and social status.11 An expanded reproductive health paradigm, which includes MPTs, is needed to enhance awareness, options, and access to reproductive services for HIV-affected women and men.
MPTs and Safer Conception
The MPTs (i.e. antiretroviral, monoclonal antibodies, and contraceptive impregnated gels, rings and barrier devices) in development are not readily available on the market for high risk individuals or HIV-serodiscordant couples to use for prevention of HIV/STI or in conjunction with safer conception interventions. None of the proposed products can be used to prevent transmission/acquisition of STIs while allowing HIV-serodiscordant couples the option of safely conceiving with safer conception interventions. The only product that may provide HIV-serodiscordant couples the opportunity to fulfill their reproductive right of childbearing when they desire and also prevent HIV transmission/acquisition is the condom (male or female). Furthermore, the proposed and ongoing pre-clinical and clinical studies of these products have yet to evaluate the impact of antiretroviral, monoclonal antibodies, and contraceptive agents on the reproductive tract (i.e. impact on sperm, endometrial environment for implantation, and embryo development). Despite some of the promising evidence supporting the use of MPTs, their availability, accessibility and acceptability are still in question. If high risk individuals and HIV-affected couples are presented with options that meet their full reproductive and HIV/STI prevention needs, demand and acceptability will likely increase. To ensure a successful platform, the MPT development strategies need to address prevention, contraceptive and fertility needs of high risk individuals and HIV-affected couples.
The State of Affairs: Reproductive Guidelines for HIV-Affected Couples
At the beginning of the HIV epidemic in 1985 in the United States of America (USA), the Centers for Disease Control and Prevention (CDC) discouraged HIV-infected women from having children because of the poor prognosis associated with HIV infection and the risk of perinatal transmission. Advances in HIV prevention and treatment have allowed HIV-infected individuals to live longer and pursue their reproductive goals hence there are now recommendations guiding individuals in this process.21 The American College of Obstetrics and Gynecology (ACOG) recommends that in HIV-serodiscordant partnerships with an HIV-infected man, “assisted conception with sperm washing for intrauterine insemination or intracytoplasmic sperm injection may be safer than timed unprotected intercourse with regard to HIV transmission.”22 Similarly, the American Society for Reproductive Medicine (ASRM) recommends that “when an affected couple requests assistance to have their own genetically related child, they are best advised to seek care at institutions with the facilities that can provide the most effective evaluation treatment and follow-up.”23 Furthermore, ASRM asserts that in couples with an HIV-infected man, the use of sperm preparation techniques coupled with IUI or ICSI have been demonstrated to be highly effective in preventing seroconversion of HIV-uninfected women and offspring.23 In HIV-serodiscordant couples with an HIV-infected female, TVI in the periovulatory period is a low cost intervention that couples can utilize to prevent sexual HIV transmission while attempting conception. A collected semen sample obtained after either natural coitus with a water-based lubricated condom or ejaculation into a clean cup is inseminated with a syringe into the vagina during the periovulatory period. However, advanced assisted reproductive techniques may still be indicated in the presence of underlying infertility.19, 24 - 26 The position statements of these organizations are also in agreement with that of the European Society of Human Reproduction and Embryology.27 In Spain, some clinicians endorse timed unprotected intercourse in HIV-serodiscordant couples desiring children when the following conditions are met: an undetectable serum HIV-RNA, antiretroviral therapy in the infected partner, absence of genital tract infections, and a normal fertility evaluation in the couple.28, 29 However, some argue that timed unprotected intercourse should not be endorsed in high risk HIV-serodiscordant couples who wish to conceive. Unprotected sexual intercourse in high risk situations when the above conditions cannot be assured is discouraged; therefore, preconception counseling, SW-IUI or ICSI are ideal options when the male partner is HIV-infected.30, 31 Most recently, there have been clinical and laboratory innovations resulting in lower cost IVF interventions, which may be adapted for low-resource environments.32
Reproductive Services in High-Resource Countries
The use of assisted reproductive services such as SW-IUI or IVF should not only be considered as methods of enhancing fertility or addressing underlying infertility but as critical components of the HIV prevention armamentarium coupled with consistent condom use.33 In the USA, there have been no reported cases of HIV transmission to the HIV-uninfected female partner with the use of SW and ICSI.33, 34Similarly, there have been no reported cases of HIV transmission with the use of SW-IUI worldwide.34 - 36 In high-resource countries (World Bank classifications), assisted reproductive services for HIV-affected couples are available and have been determined to be effective and safe in retrospective studies.37 However, these services are not accessible and/or affordable to the majority of couples in need, specifically in the public healthcare arena in low-resource settings.38 HIV-serodiscordant couples receiving assisted reproductive services in Italy, believed that it was “not right to withhold something as important to procreation [from] people because they have a disease”8 because it provided a safer alternative to natural conception, although not risk free. Overall, couples living with HIV believe that society has a moral obligation to help them find solutions that will assist in overcoming their barriers to access and providing choice through information on safer conception alternatives to natural conception.8
In the USA, less than three percent of assisted reproductive practices registered with ASRM provide services to couples in whom one or both partners are HIV-infected.23 The limited access and barriers to services has been attributed to concerns about transmission to clinical personnel and contamination of gametes and embryos stored on clinical premises; however, there have been no reported cases of occupational HIV transmission to personnel, gametes or embryos in the clinical setting that would support limiting services to HIV-affected couples.23 The current costs of this level of assisted reproductive services has made it an unattainable service for a significant proportion of HIV-affected couples – in high, low and middle income countries. Current estimates in high income countries can range from $10,000 - $17,000 per cycle of advanced assisted reproductive services and can be as high as $25,000.39 In low and middle income countries, the International Federation for Fertility Services (IFFS) has found that the average cost is from $3,000 - $8,000 per cycle, which is proportionately more expensive based upon GDP values in these countries.40
Despite evidence supporting a decreased risk of HIV transmission in serodiscordant partnerships with the use of safer conception techniques, there are few prospective studies evaluating the acceptability and feasibility of these methods amongst HIV-serodiscordant couples who desire children, especially in SSA. Although SW-IUI represents one of the lower cost options, the CDC has not changed its recommendation since 1990 against SW-IUI. The CDC’s position is based on a single reported case of HIV seroconversion in a woman using improper techniques.41, 42 As a result, the use of approved assisted reproductive technology for male HIV-infected serodiscordant couples has been limited to SW-ICSI in the USA, which has also limited the availability of these services due to high costs and restricted access particularly in public health settings. The National Perinatal HIV Hotline and Clinicians Network (“The Perinatal HIV Hotline”) as of March 2013 reported only 17 clinics offering IVF and seven offering SW-IUI to HIV-serodiscordant couples.43 The number of service providers may be limited as a result of the existing CDC recommendations. As of 2003, the CDC outlined three components that must be fulfilled before it would change its policy statement and consider the endorsement of insemination for HIV-affected couples: expansion of the follow-up of European women inseminated with processed semen, evaluation of the effectiveness of laboratory techniques for removing HIV from semen, and evaluation of the transfer of technology of semen processing to non-research settings.42 We believe that the evidence required to support new policy guidelines endorsing the use of SW-IUI by the CDC as a safer method of conception for HIV-affected couples already exists; however, the decision has not been overturned. Global leaders who shape public policy agendas should reconsider the scientific evidence that could enhance the provision of safer conception options as MPTs to HIV-affected couples desiring children.
Reproductive Services in Sub-Saharan Africa
The pronatalist nature of many low-resource countries defines individuals through parenthood and children are highly valued by cultural norms.44, 45 In SSA, many HIV-infected women and men express a desire for children either immediately or in the near future and being without a child attracts significant stigma.11 HIV-infected women report that pregnancy and childbirth are ways for them to regain their sense of womanhood and sexuality, often making childbearing a high personal priority.46 To these women, “family planning” is not just prevention and management of unwanted pregnancy but also planning for their family with the assistance of their healthcare provider and the provision of clinical services.45 Therefore, in cultures where self-worth and identity are inextricably linked to childbearing, encouraging HIV-affected couples to abstain from reproduction or to consistently use condoms while not providing any support and information on options to safely conceive are unrealistic. Therefore, the incorporation of safer conception strategies with condoms as an MPT is critical to addressing reproductive desires, HIV/STI prevention, and contraception depending on the circumstances and reproductive desires of the HIV-infected individual.
In low resource-environments, the cost, availability, and knowledge of assisted reproductive services may limit their accessibility to HIV-affected couples.38 To date, three assisted reproductive clinics in Nairobi, Kenya provide safer conception interventions for HIV-affected couples desiring children (Personal communication – Dr. Alfred Murage). Although timed unprotected intercourse is theoretically an acceptable intervention because it can be easily adapted and accepted by healthcare providers and HIV-affected couples in low-resource environments, there are inherent challenges to its use and reliability as the only safer conception option for HIV-affected couples. It may be unethical to recommend timed unprotected intercourse as a means of safer conception for high risk HIV-serodiscordant couples despite the lack of costs associated with this method in low-resource environments where adherence to antiretroviral therapy and HIV RNA viral load assessments are not readily available. Furthermore, in HIV-serodisordant couples in SSA with underlying infertility (Figure 1), conception may not occur thus unprotected sexual encounters aimed at achieving conception may be futile with continued risk of HIV transmission. Other safer conception interventions require evaluation to expand the repertoire of services and options available to HIV-affected couples desiring children. Redefining reproductive health strategies to include access to fertility services and innovative uses for MPT beyond contraception has the potential to improve the nature and quality of reproductive services for women and men worldwide.
Expansion of the Reproductive Health Paradigm for HIV-Affected Couples
Closing the reproductive services gap will require an acknowledgement and support of the reproductive intentions and fertility desires of HIV-affected couples.47 Therefore, public health agencies, ministries of health, policy makers, healthcare providers, researchers and donors must first acknowledge the reproductive intentions as well as the associated challenges of preventing HIV transmission in HIV-serodiscordant couples who desire a biological family.13 Closing the gap in clinical services will require integration of reproductive healthcare services into HIV prevention interventions along with the development of evidence-based clinical guidelines for healthcare providers. These guidelines will expose the research and product gaps creating a need for MPTs that address multiple reproductive health needs.
Implementation studies need to be conducted to ensure that the successful outcomes reported in high-resource settings can be replicated in low-resource environments with a high HIV prevalence. These studies should evaluate whether interventions such as consistent condom use with TVI during the periovulatory period and SW-IUI are feasible and acceptable to healthcare providers and HIV-affected couples as components of HIV prevention interventions. The findings of these studies will help define the cadre of best practices for reproductive fertility services and product demands that will meet the satisfaction of healthcare providers and HIV-affected couples. Enhancing the understanding of healthcare providers around HIV related stigma may require adoption of new skills that will create an engaging environment for reproductive discussions and preconception counseling with HIV-affected couples. These discussions will help facilitate the continuum of care from preconception to the postpartum period once pregnancy is achieved. Furthermore, reproductive healthcare training programs are needed in order to effectively enhance the availability and quality of information provided to HIV-affected individuals.48 Pre-service training for nurses, clinical officers, medical officers, and community health workers has been critical to the successful scale up of HIV prevention, care and treatment services in low-resource environments.49 Expanding the perspective of healthcare providers in training on “family planning” will have a great impact on changing the mindset of future generations to come to improve the lives of those they serve. In low-resource environments, public-private partnerships may help bring technical expertise, research and equipment, which may improve the provision of affordable assisted reproductive services50 and interventions that include innovative MPTs.
Conclusion
An expanded reproductive health paradigm requires redefining the “family planning” vision with the embracement of the fertility intentions of all women and men, including those with HIV, who maintain a desire to have children. In an expanded reproductive health paradigm, fertility evaluations and assisted reproductive services should not only be considered for the infertile but also for those seeking safer conception and for HIV prevention. MPT product development should focus on the multipurpose nature and characteristics inherent to the condom. Like the condom, new MPTs should offer: short term or single use, prevention of HIV/STIs and subsequent infertility, and unintended pregnancy. Providing fertile HIV-infected women with the possibility of preserving their fertility and a safer option for conception is empowering given the stigma and isolation they may already encounter as a result of their HIV status, particularly in cultures where reproduction defines one’s value in society. Despite some of the promising evidence supporting the use of MPTs, their availability, accessibility and acceptability are still in question. The prospect of an HIV-uninfected partner and child may be a strong motivator for uptake of old and new MPTs in the future.
Acknowledgments
At the time of manuscript preparation, Dr. Mmeje was at the University of California, San Francisco and was supported by a National Institute of Health (NIH) training grant (T32AI065388) from the National Institutes of Allergy and Infectious Diseases (NIAID). The content is solely the responsibility of the authors and does not represent the official views or opinions of the NIAID, NIH or the World Health Organization.
Funding
Not applicable.
Footnotes
Contribution to authorship
The manuscript was conceptualized by OM and SVP while OM was an intern at the WHO. CRC, AM, JO, and JK all contributed to the development, writing and review of the manuscript with contributions drawn from their research and clinical experiences with HIV-affected couples. OM and SVP led the writing process.
Disclosure of interests
The authors have no competing interests.
References
- 1.Bishop M, Foreit K. Seodiscordant Couples Sub-Saharan Africa: What Do Survey Data Tell Us? Washington, DC: Futures Group, Health Policy Initiative, U.S. Agency for International Development Task Order 1; 2010. [Google Scholar]
- 2.Dunkle K, Stephenson R, Karita E, Chomba E, Kayitenkore K, Vwalika C, et al. New Heterosexually Transmitted HIV Infections in Married or Cohabiting Couples in Urban Zambia and Rwanda: an Analysis of Survey and Clinical Data. Lancet. 2008;371:22183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 3.UN Joint Programme on HIV/AIDS (UNAIDS), World AIDS Day report: Results. Nov 20, 2012. [9 February 2014]. available at: http://www.refworld.org/docid/50eeba942.html.
- 4.Kenya AIDS Indicator Survey (KAIS) 2012. U.S. President’s Emergency Plan for AIDS Relief (PEPFAR); 2013. [9 February 2014]. available at: http://nascop.or.ke/library/3d/Preliminary%20Report%20for%20Kenya%20AIDS%20indicator%20survey%202012.pdf. [Google Scholar]
- 5.Brubaker S, Bukusi E, Odoyo J, Achando J, Okumu A, Cohen CR. Pregnancy and HIV transmission among HIV-discordant couples in a clinical trial in Kisumu, Kenya. HIV Medicine. 2011;12(5):316–21. doi: 10.1111/j.1468-1293.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 6.Snow R, Winter R, Harlow S. Gender Attitudes and Fertility Aspirations among Young Men in Five High Fertility East African Countries. Studies in Family Planning. 2013;44(1):1–24. doi: 10.1111/j.1728-4465.2013.00341.x. [DOI] [PubMed] [Google Scholar]
- 7.de Walque D, Kline R. The Association between Remarriage and HIV Infection in 13 Sub-Saharan African Countries. Studies in Family Planning. 2012;43(1):1–10. doi: 10.1111/j.1728-4465.2012.00297.x. [DOI] [PubMed] [Google Scholar]
- 8.Sunderam S, Hollander L, Macaluso M, Vucetich A, Jamieson DJ, Osimo F, et al. Safe Conception for HIV Discordant Couples through Sperm-Washing: Experience and Perceptions of Patients in Milan, Italy. Reproductive Health Matters. 2008;16(31):211–219. doi: 10.1016/S0968-8080(08)31342-1. [DOI] [PubMed] [Google Scholar]
- 9.Hayford S, Agadjanian V, Luz L. Now or Never: Perceived HIV Status and Fertility Intentions in Rural Mozambique. Studies in Family Planning. 2012;43(3):191–99. doi: 10.1111/j.1728-4465.2012.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delvaux T, Nostlinger C. Reproductive Choice for Women and Men Living with HIV: Contraception, Abortion and Fertility. Reproductive Health Matters. 2007;15(29 Supplement):46–66. doi: 10.1016/S0968-8080(07)29031-7. [DOI] [PubMed] [Google Scholar]
- 11.Dyer S, Pennings G. Consideration Regarding Government Funding of Assisted Reproductive Techniques in Low Resource Settings. Facts, Visions and View in Obstetrics and Gynecology Monograph. 2010:17–21. [Google Scholar]
- 12.Friend DR, Clark MR. Product Development Workshop 2013: HIV and Multipurpose Prevention Technologies. Antiviral Research. 2013;100:S1–S2. doi: 10.1016/j.antiviral.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Harrison PF, Hemmerling A, Romano J, Whaley KJ, Young Holt B. Developing Multipurpose Reproductive Health Strategies: An Integrated Strategy. AIDS Research and Treatment. 2013:790154. doi: 10.1155/2013/790154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Report of the International Conference on Population and Development; Cairo. 1994. At: http://www.un.org/popin/icpd/conference/offeng/poa.html. [Google Scholar]
- 15.van Der Poel S. Historical Walk: The HRP Special Programme and Infertility. Gynecological Obstetrics Investigations. 2012;74:218–227. doi: 10.1159/000343058. [DOI] [PubMed] [Google Scholar]
- 16.Sexual and reproductive health of women living with HIV/AIDS: Guidelines on care, treatment and support for women living with HIV/AIDS and their children in resource-constrained settings. At: http://www.who.int/hiv/pub/guidelines/sexualreproductivehealth.pdf.
- 17.Cohen M, Chen Y, McCauley M, Gambie T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual Transmission of HIV According to Viral Load and Antiretroviral Therapy: Systematic Review and Meta-analysis. AIDS. 2009;13:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 19.Matthews L, Smit J, Cu-Uvin S, Cohan D. Antiretrovirals and Safer Conception for HIV-Serodiscordant Couples. Current Opinion HIV and AIDS. 2012;7(6):569–578. doi: 10.1097/COH.0b013e328358bac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whetham J, Taylor S, Charlwood L, Keith T, Howell R, McInnes C, et al. Pre-exposure Prophylaxis for Conception (PrEP-C) as a Risk Reduction Strategy in HIV-Positive Men and HIV-Negative Women in the UK. AIDS Care. 2014;26(3):332–6. doi: 10.1080/09540121.2013.819406. [DOI] [PubMed] [Google Scholar]
- 21.Preconception counseling and care for HIV-infected women of childbearing age: Reproductive Options for HIV-Concordant and Serodiscordant Couples. Department of Health and Human Services; Jul, 2012. At: http://www.aidsinfo.nih.gov/guidelines/html/3/perinatal-guidelines/153/reproductive-options-for-hiv-concordant-and-serodiscordant-couples. [Google Scholar]
- 22.Committees on Practice Bulletins. Practice Bulletin. American College of Obstetrics and Gynecology; 2010. Number December. Gynecologic Care for Women with Human Immunodeficiency Virus; p. 177. [DOI] [PubMed] [Google Scholar]
- 23.The Ethics Committee of the American Society for Reproductive Medicine. Human Immunodeficiency Virus and Infertility Treatment. Fertility and Sterility. 2010;94(1):11–15. doi: 10.1016/j.fertnstert.2010.01.077. [DOI] [PubMed] [Google Scholar]
- 24.Mmeje O, Cohen C, Cohan D. Evaluating Safer Conception Options for HIV-Serodiscordant Couples (HIV-infected female/HIV-uninfected male): A Closer Look at Vaginal Insemination. Infectious Diseases in Obstetrics and Gynecology. 2012:1–7. doi: 10.1155/2012/587651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekker LG, Black V, Myer L, Rees H, Cooper D, Mall S, et al. Guideline on Safer Conception in Fertile HIV-Infected Individuals and Couples. The Southern African Journal of HIV Medicine. 2011:32–44. [Google Scholar]
- 26.Loutfy M, Margolese S, Money D, Gysler M, Hamilton S, Yudin MH, et al. Canadian HIV Pregnancy Planning Guidelines. Journal of Obstetrics and Gynaecology Canada. 2012;34(6):575–590. doi: 10.1016/S1701-2163(16)35274-4. [DOI] [PubMed] [Google Scholar]
- 27.The ESHRE Ethics and Law Task Force. Taskforce 8: Ethics of Medically Assisted Fertility Treatment for HIV Positive Men and Women. Human Reproduction. 2004;19(11):2454–56. doi: 10.1093/humrep/deh467. [DOI] [PubMed] [Google Scholar]
- 28.Barriero P, Romero J, Leal M, Hernando V, Asencio R, de Mendoza C, et al. Natural Pregnancies in HIV-Serodiscordant Couples Receiving Successful Antiretroviral Therapy. Journal of Acquired Deficiency Syndrome. 2006;43(3):324–26. doi: 10.1097/01.qai.0000243091.40490.fd. [DOI] [PubMed] [Google Scholar]
- 29.Barriero P, Castilla J, Labargaa P, Soriano V. Is Natural Conception a Valid Option for HIV-Serodiscordant Couples? Human Reproduction. 2007;22(9):2352–58. doi: 10.1093/humrep/dem226. [DOI] [PubMed] [Google Scholar]
- 30.Nosarka S, Hoogendijk C, Siebert S, Kruger TF. Assisted Reproduction in the HIV-Serodiscordant Couple. South African Medical Journal. 2007;97(1):24–26. [PubMed] [Google Scholar]
- 31.Mandelbrot L. Reproduction and HIV: Has the Condom Become Irrelevant? Gynécologie, Obstétrique and Fertilité. 2011;40(1):58–61. doi: 10.1016/j.gyobfe.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Van Blerkom J, Ombelet W, Klerkx E, Janssen M, Dhont N, Nargund G, et al. First Births with Simplified Culture System for Clinical IVF and Embryo Transfer. Reproductive BioMedicine Online. 2013 doi: 10.1016/j.rbmo.2013.11.012. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Sauer Mark, Wang J, Douglas N, Nakhuda GS, Vardhana P, Jovanovic V, et al. Providing Fertility Care to Men Seropositive for Human Immunodeficiency Virus: Reviewing 10 Years of Experience and 420 Consecutive Cycles of In Vitro Fertilization and Intracytoplasmic Sperm Injection. Fertility and Sterility. 2009;91(6):2455–60. doi: 10.1016/j.fertnstert.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Sevasi V, Mandia L, Laoreti A, Detin I. Reproductive Assistance in HIV Serodiscordant Couples. Human Reproduction Update. 2013;19(2):136–50. doi: 10.1093/humupd/dms046. [DOI] [PubMed] [Google Scholar]
- 35.Semprini A, Macaluso M, Hollander L, Vucetich A, Duerr A, Mor G, et al. Safe Conception for HIV-Discordant Couples: Insemination with Processed Semen from the HIV-infected Partner. American Journal of Obstetrics and Gynecology. 2013;208(5):402.e1–402.e9. doi: 10.1016/j.ajog.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Nicopoullos J, Almeida P, Vourliotis M, Gouolding R, Gilling-Smith C. A Decade of Sperm Washing: Clinical Correlates of Successful Insemination Outcome. Human Reproduction. 2010;25(8):1869–1876. doi: 10.1093/humrep/deq134. [DOI] [PubMed] [Google Scholar]
- 37.van Balen F, Gerrits T. Quality of Infertility Care in Poor-Resource Areas and the Introduction of New Reproductive Technologies. Human Reproduction. 2011;16(2):215–219. doi: 10.1093/humrep/16.2.215. [DOI] [PubMed] [Google Scholar]
- 38.Murage A, Muteshi M, Githae F. Assisted Reproduction Services Provision in a Developing Country: Time to Act? Fertility and Sterility. 2011;96(4):966–968. doi: 10.1016/j.fertnstert.2011.07.1109. [DOI] [PubMed] [Google Scholar]
- 39.Lampe MA, Smith DK, Anderson GJ, Edwards AE, Nesheim SR. Achieving Safe Conception in HIV-Discordant Couples: The Potential Role of Oral Pre-Exposure Prophylaxis (PrEP) in the United States. American Journal of Obstetrics and Gynecology. 2011;204(6):488.e1–488.e8. doi: 10.1016/j.ajog.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Jones H, Cooke I, Kempers R, Brinsden P, Saunders D. International Federation of Fertility Societies Surveillance 2010. Fertility and Sterility. 2010;95(2):491. doi: 10.1016/j.fertnstert.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Epidemiologic Notes and Reports: HIV-1 infection and artificial insemination with processed semen. 15. Vol. 39. Morbidity and Mortality Weekly Report; 1990. Apr, pp. 249pp. 255–256. At: http://www.cdc.gov/mmwr/preview/mmwrhtml/00001604.htm. [PubMed] [Google Scholar]
- 42.Duerr A, Jamieson D. Assisted Reproductive Technologies for HIV-Discordant Couples. American Journal of Bioethics. 2003;3(1):45–7. doi: 10.1162/152651603321611971. [DOI] [PubMed] [Google Scholar]
- 43.Cohan D, Weber S, Aaron E. CDC Should Reverse Its Recommendation Against Semen Washing-Intrauterine Insemination for HIV-Serodifferent Couples. American Journal of Obstetrics and Gynecology. 2013;209(3):284. doi: 10.1016/j.ajog.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Pennings G. Ethical Issues of Infertility Treatment in Developing Countries. Facts, Views and Vision in Obstetrics and Gynecology Monograph. 2012:17–23. [Google Scholar]
- 45.Inhorn M. Right to Assisted Reproductive Technology: Overcoming Infertility in Low-Resource Countries. International Journal of Gynecology and Obstetrics. 2009;106(2):172–4. doi: 10.1016/j.ijgo.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 46.Thornton A, Romanelli F, Collins J. Reproduction Decision Making for Couples Affected by HIV: A Review of the Literature. Topics in HIV Medicine. 2004;12(2):61–67. [PubMed] [Google Scholar]
- 47.Crankshaw T, Matthews L, Giddy J, et al. A Conceptual Framework for Understanding HIV Risk Behavior in the Context of Supporting Fertility Goals among HIV-Serodiscordant Couples. Reproductive Health Matters. 2012;20(39S):50–60. doi: 10.1016/S0968-8080(12)39639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaye P, Nelson D. Effective Scale-Up: Avoiding the Same Old Traps. Human Resources for Health. 2009;14:7, 2. doi: 10.1186/1478-4491-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renaggli V, De Ryck I, Jacob S, Yeneneh H, Sirgu S, Sebuyira LM, et al. HIV Education for Health-Care Professionals in High Prevalence Countries: Time to Integrate a Pre-Service Approach into Training. Lancet. 2008;372:341–343. doi: 10.1016/S0140-6736(08)61119-8. [DOI] [PubMed] [Google Scholar]
- 50.Akande EO. Affordable Assisted Reproductive Technologies in Developing Countries: Pros and Cons. Human Reproduction. 2008;(1):12–4. [Google Scholar]


