Abstract
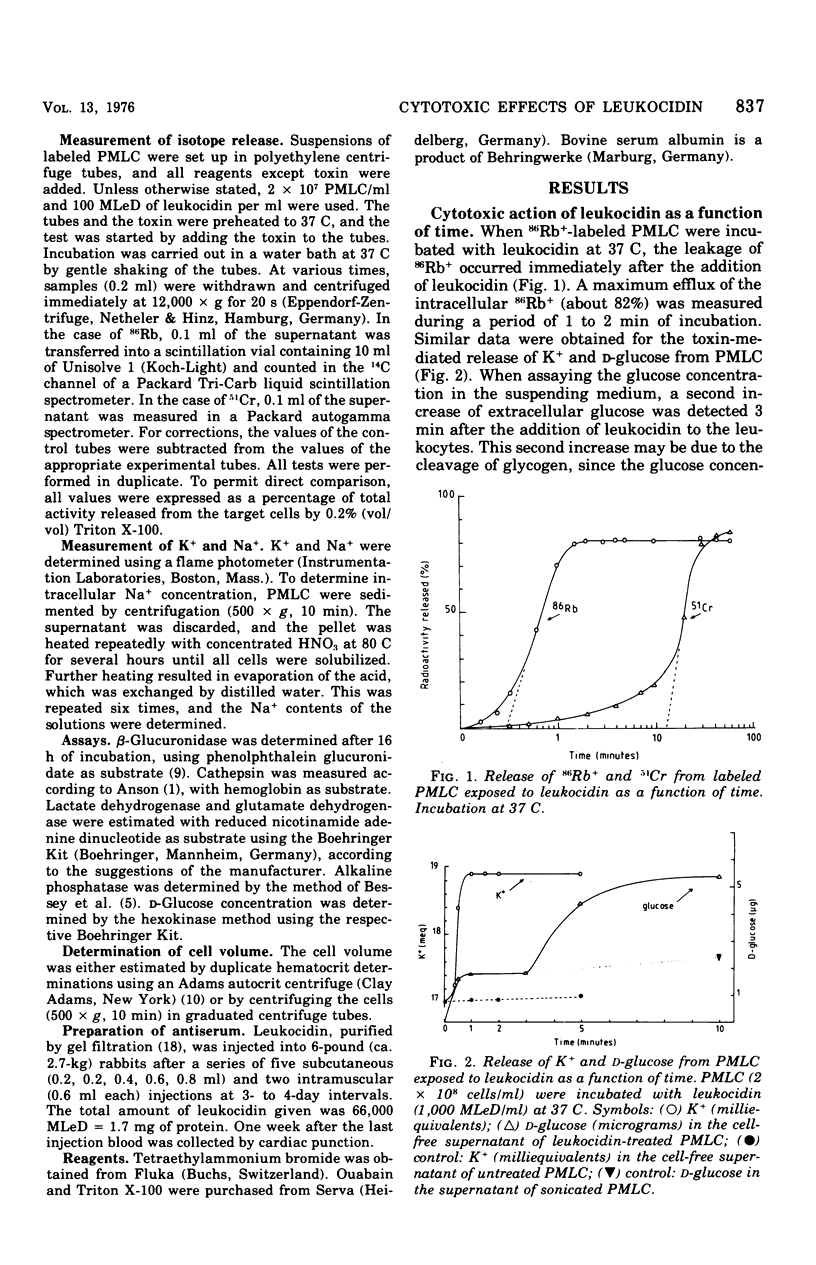
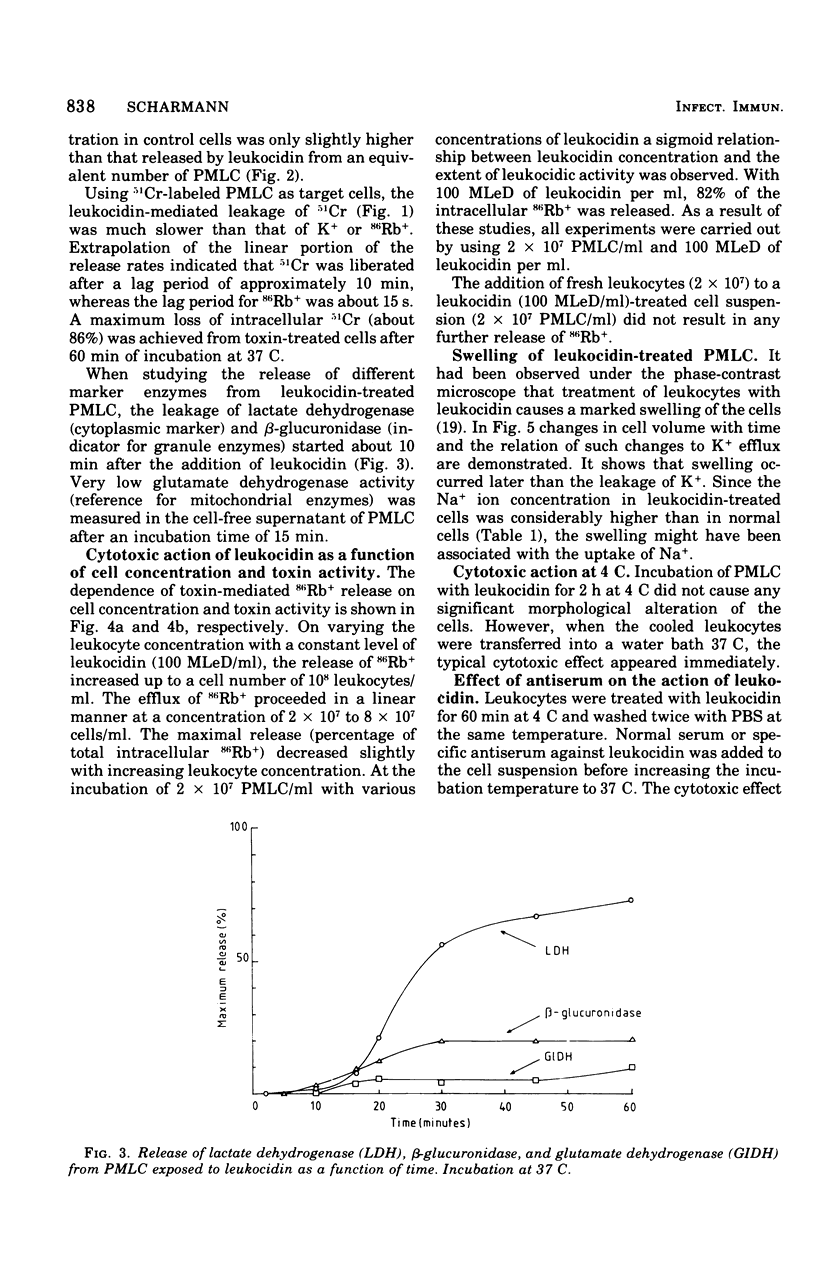
The cytotoxic action of leukocidin from Pseudomonas aeruginosa was studied in vitro by following the release of various intracellular markers from polymorphonuclear leukocytes from cattle (PMLC). Low-molecular markers (K+, 86Rb+, glucose) were lost from PMLC within 1 to 2 min after the addition of leukocytes. The release of high-molecular-weight indicators (51Cr, bound to intracellular protein; lactate dehydrogenase) occurred only after swelling of the cells, leading to an increased permeability of the plasma membrane. Calcium ions stimulated the leakage of granule enzymes but retarded or inhibited the release of cytoplasmic markers. At 4 C, leukocytes were unaffected by the toxin. Leukocidin, bound at 4 C to leukocytes and treated with antiserum against leukocidin, did not damage the cells upon increasing the incubation temperature to 37 C.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson M. L. THE ESTIMATION OF CATHEPSIN WITH HEMOGLOBIN AND THE PARTIAL PURIFICATION OF CATHEPSIN. J Gen Physiol. 1937 Mar 20;20(4):565–574. doi: 10.1085/jgp.20.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEHRENS M., ESCH F. GEWINNUNG VON LEUKOCYTEN AUS RINDERBLUT UNTER VERWENDUNG VON WASSER ALS HAEMOLYTIKUM. Experientia. 1963 Aug 15;19:406–407. doi: 10.1007/BF02171514. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Differences in enzyme content of azurophil and specific granules of polymorphonuclear leukocytes. I. Histochemical staining of bone marrow smears. J Cell Biol. 1968 Nov;39(2):286–298. doi: 10.1083/jcb.39.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. One-step growth curve of Western equine encephalomyelitis virus on chicken embryo cells grown in vitro and analysis of virus yields from single cells. J Exp Med. 1954 Feb;99(2):183–199. doi: 10.1084/jem.99.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHMAN W. H., SPRINGER B., BRUNETTEI R. Application of an improved glucuronidase assay method to the study of human blood beta-glucuronidase. J Biol Chem. 1948 Apr;173(2):449–456. [PubMed] [Google Scholar]
- Frimmer M., Kroker R. The role of extracellular K plus-concentration in phalloidin effects on isolated hepatocytes and perfused rat livers. Naunyn Schmiedebergs Arch Pharmacol. 1973;278(3):285–292. doi: 10.1007/BF00500289. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., VAN HEYNINGEN W. E. Staphylococcal leucocidins. Br J Exp Pathol. 1957 Apr;38(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegner D. Isolierung und Enzymbestand von Granula aus polymorphkernigen Leukozyten des peripheren Rinderblutes. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):544–554. [PubMed] [Google Scholar]
- Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. II. The use of various target cell markers to study cytolytic events. J Immunol. 1973 Jan;110(1):73–84. [PubMed] [Google Scholar]
- LOVE W. D., BURCH G. E. A comparison of potassium 42, rubidium 86, and cesium 134 as tracers of potassium in the study of cation metabolism of human erythrocytes in vitro. J Lab Clin Med. 1953 Mar;41(3):351–362. [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- WOODIN A. M., WIENEKE A. A. The accumulation of calcium by the polymorphonuclear leucocyte treated with staphylococcal leucocidin and its significance in the extrusion of protein. Biochem J. 1963 Jun;87:487–495. doi: 10.1042/bj0870487. [DOI] [PMC free article] [PubMed] [Google Scholar]



