Abstract
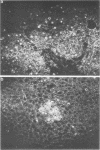
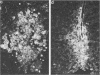
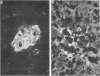
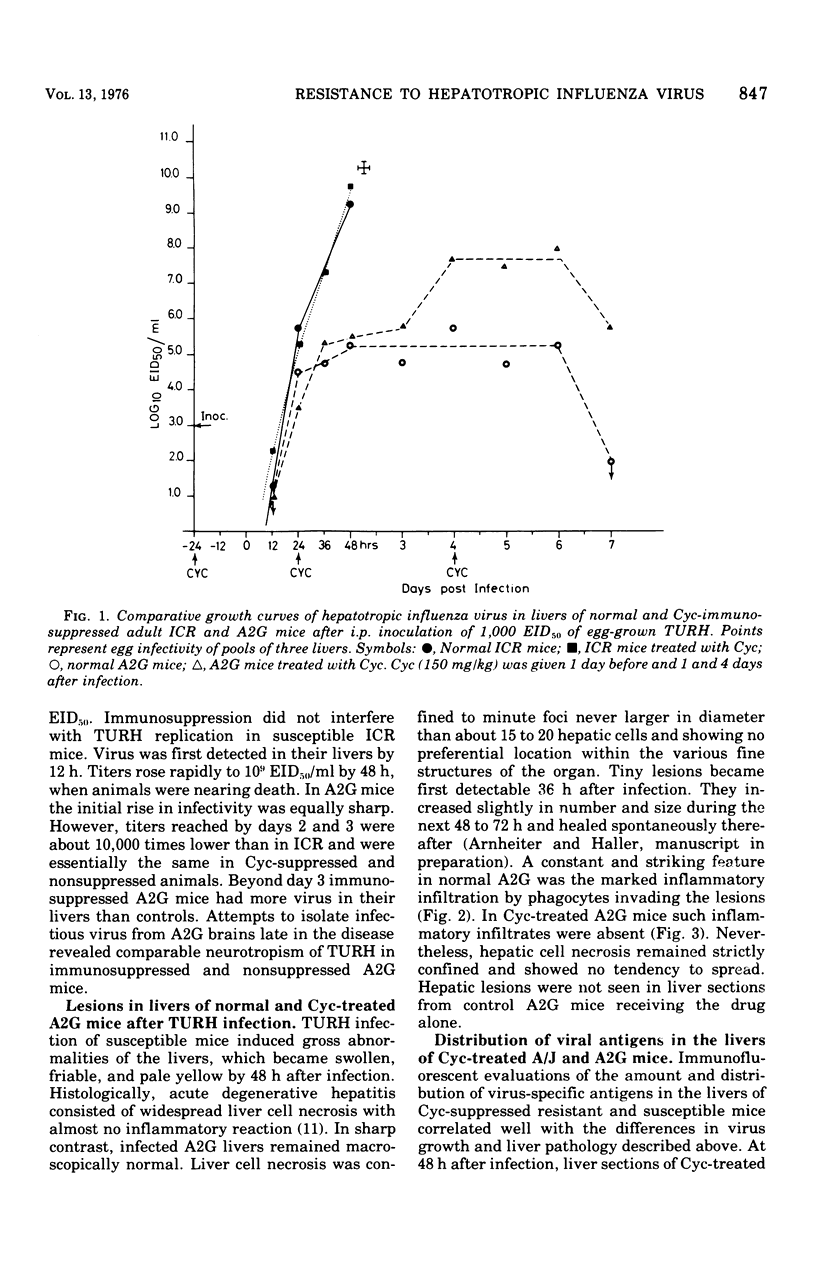
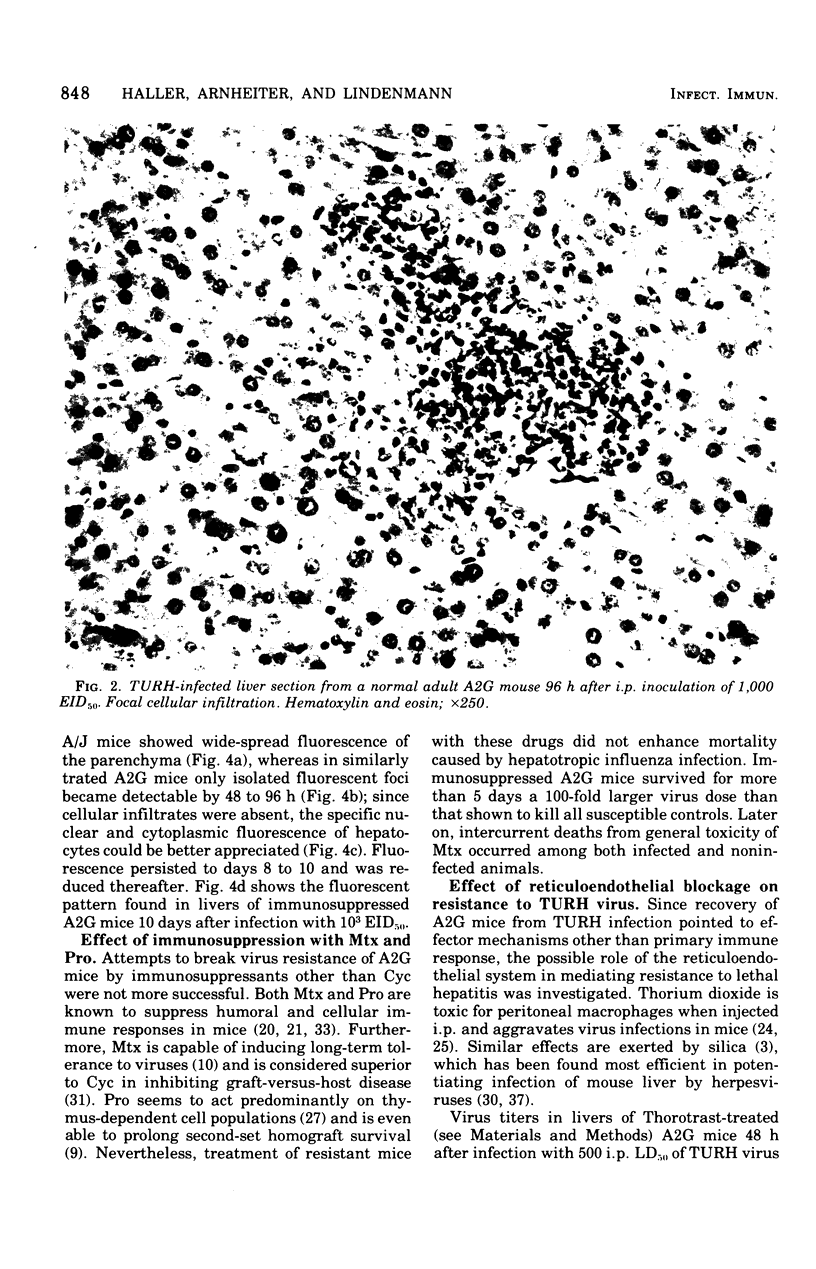
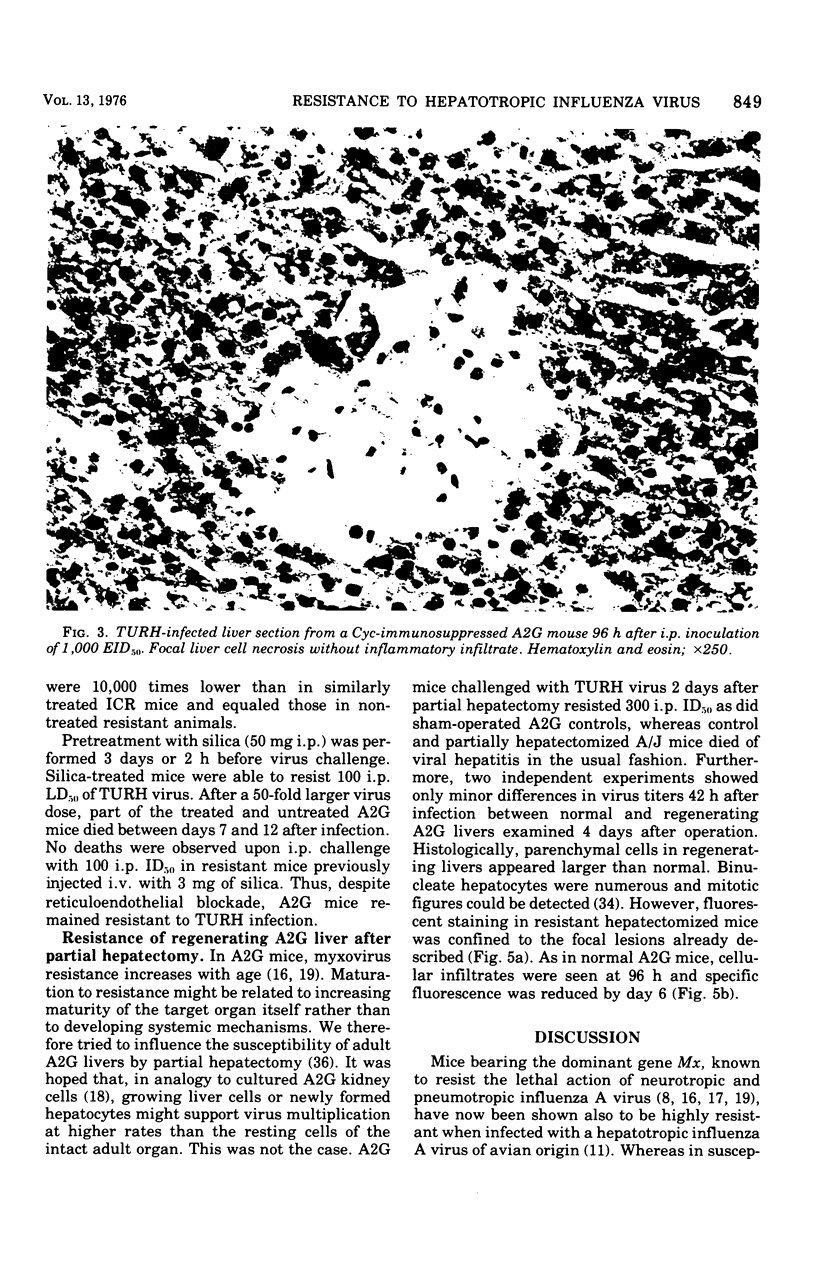
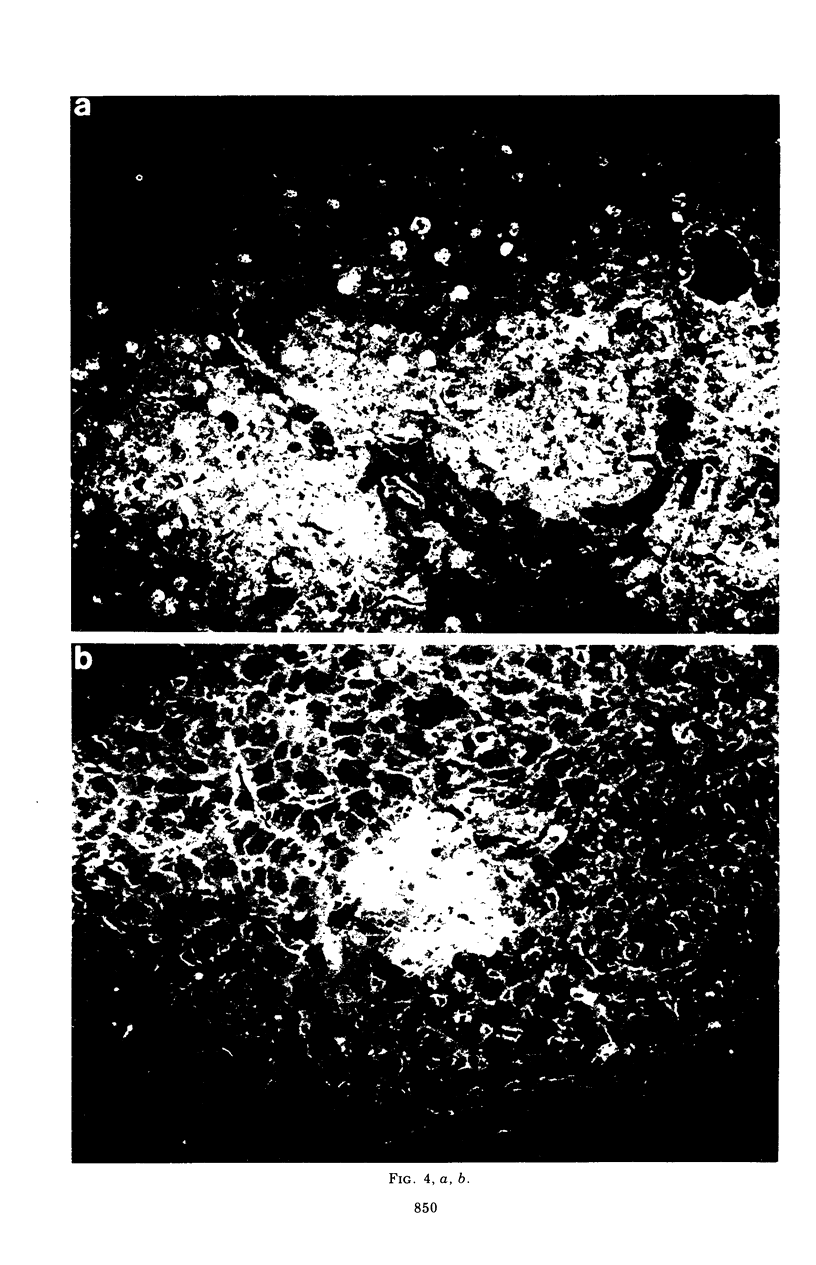
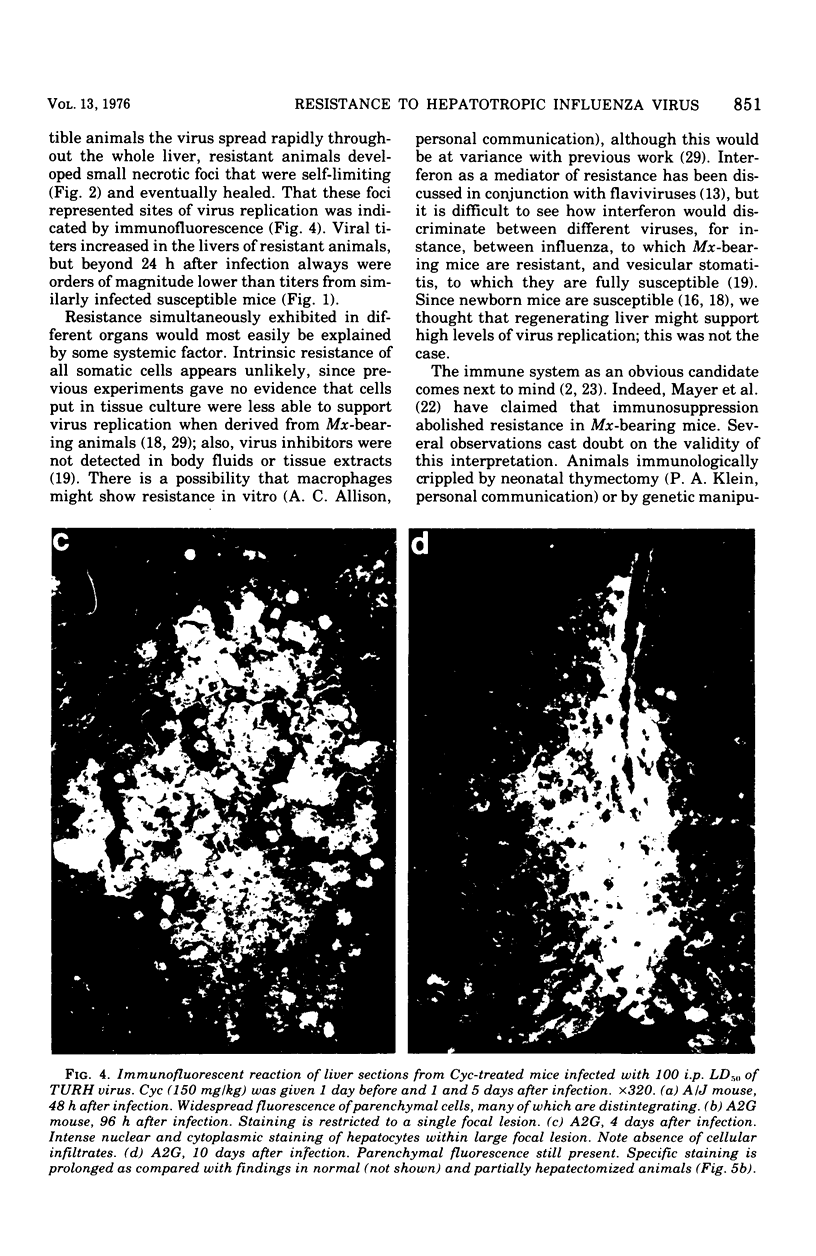
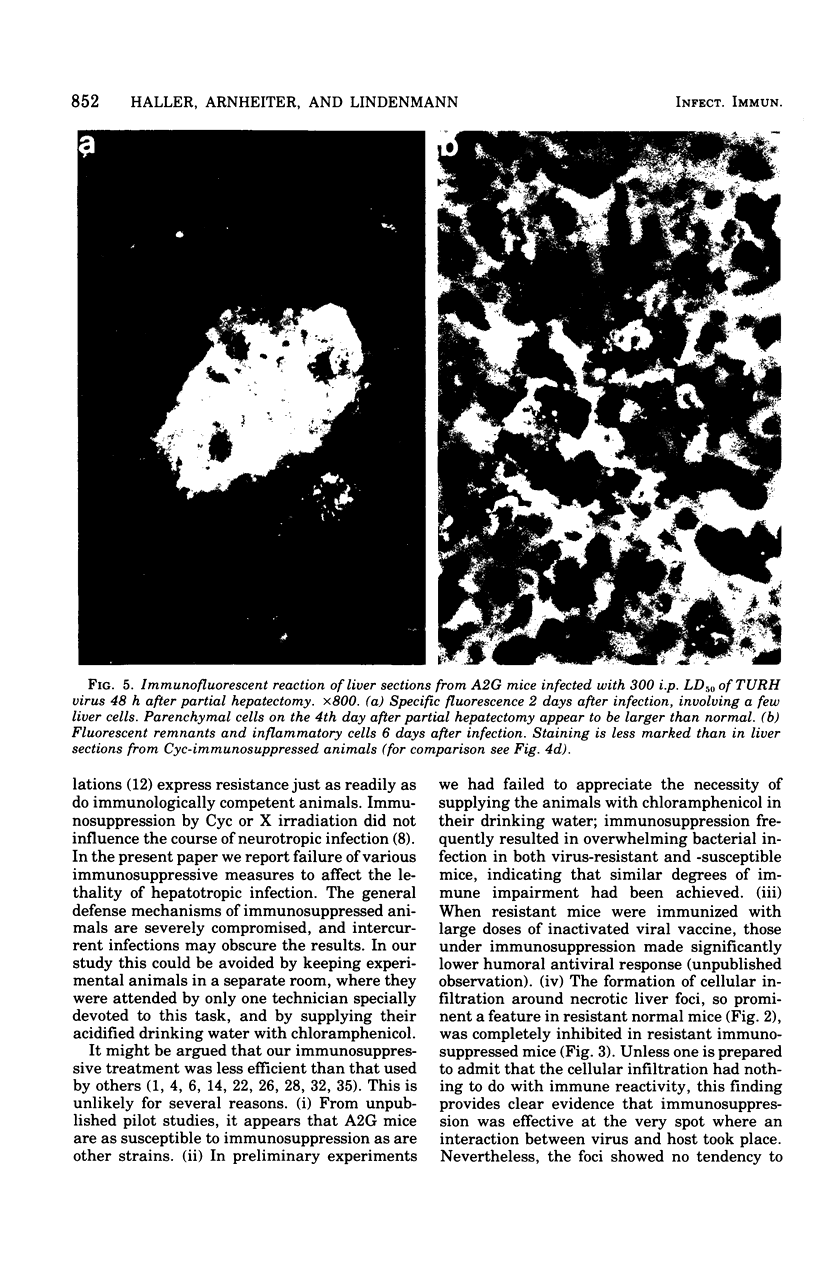
Mice carrying the gene Mx were resistant to the lethal action of a hepatotropic line of avian influenza A virus. In resistant animals, foci of liver necrosis were self-limiting, and maximal virus titers reached were much below those in susceptible animals. Resistance could not be abrogated by immunosuppressive treatment with cyclophosphamide, methotrexate, or procarbazine, although such treatment prevented cellular infiltration at sites of virus replication and appeared to delay virus clearance. Silica and thorium dioxide, thought to inhibit macrophage function, likewise failed to abolish resistance. Regenerating liver tissue did not support more extensive virus replication than did intact adult liver.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C. Immunosuppression by alkylating agents--tolerance induction. Transplant Proc. 1973 Sep;5(3):1221–1226. [PubMed] [Google Scholar]
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Camenga D. L., Nathanson N., Cole G. A. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J Infect Dis. 1974 Dec;130(6):634–641. doi: 10.1093/infdis/130.6.634. [DOI] [PubMed] [Google Scholar]
- Carter W. A., De Clercq E. Viral infection and host defense. Science. 1974 Dec 27;186(4170):1172–1178. doi: 10.1126/science.186.4170.1172. [DOI] [PubMed] [Google Scholar]
- Cole G. A., Nathanson N. Potentiation of experimental arbovirus encephalitis by immunosuppressive doses of cyclophosphamide. Nature. 1968 Oct 26;220(5165):399–401. doi: 10.1038/220399a0. [DOI] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H. Genetically determined resistance to infection with group B arboviruses. II. Increased production of interfering particles in cell cultures from resistant mice. J Infect Dis. 1974 Mar;129(3):248–256. doi: 10.1093/infdis/129.3.248. [DOI] [PubMed] [Google Scholar]
- Fiske R. A., Klein P. A. Effect of immunosuppression on the genetic resistance of A2G mice to neurovirulent influenza virus. Infect Immun. 1975 Mar;11(3):576–587. doi: 10.1128/iai.11.3.576-587.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floersheim G. L. Effect of methylhydrazine derivatives on the survival of second-set skin homografts. Nature. 1966 Aug 6;211(5049):638–639. doi: 10.1038/211638a0. [DOI] [PubMed] [Google Scholar]
- HAAS V. H., STEWART S. E. Sparing effect of A-methopterin and guanazolo in mice infected with virus of lymphocytic choriomeningitis. Virology. 1956 Aug;2(4):511–516. doi: 10.1016/0042-6822(56)90007-1. [DOI] [PubMed] [Google Scholar]
- Haller O., Lindenmann J. Athymic (nude) mice express gene for myxovirus resistance. Nature. 1974 Aug 23;250(5468):679–680. doi: 10.1038/250679a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Okada Y., Tajiri T., Amano H., Aoki N. Intestinal resistance in the experimental enteric infection of mice with a mouse adenovirus. 3. Suppressive effect of cyclophosphamide on the establishment and duration of the intestinal resistance. Jpn J Microbiol. 1973 Nov;17(6):503–511. doi: 10.1111/j.1348-0421.1973.tb00936.x. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J. INHERITANCE OF RESISTANCE TO INFLUENZA VIRUS IN MICE. Proc Soc Exp Biol Med. 1964 Jun;116:506–509. doi: 10.3181/00379727-116-29292. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., LANE C. A., HOBSON D. THE RESISTANCE OF A2G MICE TO MYXOVIRUSES. J Immunol. 1963 Jun;90:942–951. [PubMed] [Google Scholar]
- Liske R. A comparative study of the action of cyclophosphamide and procarbazine on the antibody production in mice. Clin Exp Immunol. 1973 Oct;15(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan T., Santos G. W., Quinn R. P. Immunosuppressive drugs. Pharmacol Rev. 1970 Jun;22(2):189–247. [PubMed] [Google Scholar]
- Mayer V., Schulman J. L., Kilbourne E. D. Nonlinkage of neurovirulence exclusively to viral hemagglutinin or neuraminidase in genetic recombinants of A-NWS (HON1) influenza virus. J Virol. 1973 Feb;11(2):272–278. doi: 10.1128/jvi.11.2.272-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C. Host defenses against viral disease. N Engl J Med. 1974 Feb 7;290(6):323–329. doi: 10.1056/NEJM197402072900608. [DOI] [PubMed] [Google Scholar]
- Monath T. P., Borden E. C. Effects of thorotrast on humoral antibody, viral multiplication, and interferon during infection with St. Louis encephalitis virus in mice. J Infect Dis. 1971 Mar;123(3):297–300. doi: 10.1093/infdis/123.3.297. [DOI] [PubMed] [Google Scholar]
- Quagliata F., Phillips-Quagliata J. M., Floersheim G. L. Immunosuppression by procarbazine. I. Sites of action of the drug and effect on adjuvant arthritis and circulating antibody responses. Cell Immunol. 1972 Feb;3(2):198–212. doi: 10.1016/0008-8749(72)90160-8. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Effects of immunosuppression on coxsackie B-3 virus infection in mice, and passive protection by circulating antibody. J Gen Virol. 1973 Jun;19(3):339–351. doi: 10.1099/0022-1317-19-3-339. [DOI] [PubMed] [Google Scholar]
- Rusanova N. A., Solov'ev V. D. K probleme estestvennogo protivovirusanogo immuniteta. Nasledstvennyi kharakter i mekhanizm rezistentnosti myshei linii A2G k virusu grippa. Vopr Virusol. 1966 Jul-Aug;11(4):398–402. [PubMed] [Google Scholar]
- Storb R., Graham T. C., Shiurba R., Thomas E. D. Treatment of canine graft-versus-host disease with methotrexate and cyclo-phosphamide following bone marrow transplantation from histoincompatible donors. Transplantation. 1970 Aug;10(2):165–172. doi: 10.1097/00007890-197008000-00003. [DOI] [PubMed] [Google Scholar]
- Thind I. S., Price W. H. The effect of cyclophosphamide treatment on experimental arbovirus infections. Am J Epidemiol. 1969 Jul;90(1):62–68. doi: 10.1093/oxfordjournals.aje.a121050. [DOI] [PubMed] [Google Scholar]
- Thomas E. D., Storb R. The effect of amethopterin on the immune response. Ann N Y Acad Sci. 1971 Nov 30;186:467–474. doi: 10.1111/j.1749-6632.1971.tb47002.x. [DOI] [PubMed] [Google Scholar]
- WILSON M. E., STOWELL R. E., YOKOYAMA H. O., TSUBOI K. K. Cytological changes in regenerating mouse liver. Cancer Res. 1953 Jan;13(1):86–92. [PubMed] [Google Scholar]
- Worthington M., Rabson A. S., Baron S. Mechanism of recovery from systemic vaccinia virus infection. I. The effects of cyclophosphamide. J Exp Med. 1972 Aug 1;136(2):277–290. doi: 10.1084/jem.136.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOYAMA H. O., WILSON M. E., TSUBOI K. K., STOWELL R. E. Regeneration of mouse liver after partial hepatectomy. Cancer Res. 1953 Jan;13(1):80–85. [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]