Abstract
AIM
To investigate whether photoreceptor necroptosis induced by z-VAD-FMK (pan caspase inhibitor) was involved the activation of autophagy and whether Necrostatin-1, a specific necroptosis inhibitor, could inhibit this induction of autophagy after experimental retinal detachment.
METHODS
Experimental retinal detachment models were created in Sprague-Dawley rats by subretinal injection of sodium hyaluronate and subretinal injections of z-VAD-FMK, vehicle or z-VAD-FMK plus Necrostatin-1. Three days after retinal detachment, morphologic changes were observed by transmission electron microscopy. In other animals, retinas were subjected to immunoprecipitation and Western Blotting, then probed with anti-RIP1, phosphoserine, LC-3II or caspase 8 antibody.
RESULTS
It was proved by immunoprecipitation and western blotting, that photoreceptor necroptosis was mediated by caspase-8 inhibition and receptor interacting protein kinase (RIP1) phosphorylation activation. Transmission electron microscope and western blotting results indicated that photoreceptor necroptosis was involved the LC-3II and autophagosomes induction. We also discovered Necrostatin-1 could inhibit RIP1 phosphorylation and LC-3II induction.
CONCLUSION
These data firstly indicate photoreceptor necroptosis is associated with the activation of autophagy. Necrostatin-1 protects photoreceptors from necroptosis and autophagy by down-regulation of RIP1 phosphorylation and LC-3II.
Keywords: retinal detachment, autophagy, necroptosis
INTRODUCTION
Retinal detachment (RD), defined as the separation of the neurosensory retina from subjacent retina pigment epithelium, is a common cause of visual impairment[1]–[3]. Photoreceptor cell death is an important mechanism of vision loss after RD. Death receptor-induced apoptosis plays a critical role in photoreceptor cell death, which is associated with caspase activation. Evidences also showed that caspase inhibitor like z-VAD-FMK (a pan caspase inhibitor) failed to prevent cell death or neuronal functional damage[4]–[6]. Necroptosis is a recently discovered, caspase-independent, regulated cell death[4]–[7]. Receptor interacting protein kinase (RIP1), a death-domain containing kinase, is specifically involved in regulating necroptosis[5],[8]. Autophagy, another caspase-independent process, is considered as a clean-up mechanism for cell death[5]. Autophagy can also be activated during necroptosis, and is directly associated with the conversion of the microtubule associated protein, LC-3I to LC-3II (a bio-marker of autophagy)[5],[9]–[12]. Trichonas et al[13] reported for the first time that RIP1 mediated RD-induced necroptosis and z-VAD-FMK would shift photoreceptor death from apoptosis to necroptosis. Nonetheless, it remains unknown, whether autophagy can be activated in photoreceptor necroptosis, and whether Necrostatin-1, a specific inhibitor of necroptosis, can also inhibit autophagy induction after experimental RD.
Here we hypothesized that photoreceptor necroptosis induced by z-VAD-FMK was involved the activation of autophagy and Necrostatin-1 protected photoreceptors from necroptosis and autophagy, which was mediated by down-regulation of RIP1 phosphorylation and LC-3II[14]. We first tested the hypothesis by transmission electron microscope (TEM), which was performed to investigate whether necroptosis induced by z-VAD-FMK was involved the activation of autophagy. We then addressed whether z-VAD-FMK induced RIP1 phosphorylation (a bio-marker of necroptosis) and LC-3II induction by Western Blotting, which triggered activation of necroptosis and autophagy[8]. Finally, it was investigated whether Necrostatin-1 treatment inhibited RIP1 phosphorylation and LC-3II induction. The results of this study would firstly provide new evidence that autophagy was activated in z-VAD-FMK-induced photoreceptor necroptosis and Necrostatin-1 not only inhibited photoreceptor necroptosis but autophagy, which may be a promising combined therapeutic direction against neuronal damage in RD.
MATERIALS AND METHODS
Surgical Induction of Retinal Detachment
All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the guidelines established by the University Committee on Use and Care of Animals of the Shanghai JiaoTong University and Anhui Medical University. All animal experiments were conducted with the approval of the Animal Research Committee, School of Medicine, Shanghai Jiaotong University and Anhui Medical University. Male Sprague-Dawley rats (n=120) weighing 260-280 g were provided by the Laboratory Animal Center of the Shanghai First People's Hospital, School of Medicine, Shanghai JiaoTong University. Experimental RD was induced as described previously[2],[15],[16]. Briefly, the rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate and their pupils were dilated with 0.5% tropicamide and 0.5% phenylephrine hydrochloride eye drop (China Santen Pharmaceutical, Jiangsu province, China). A subretinal injector with 30-gauge needle was inserted into the subretinal space via an external trans-scleral trans-choroidal approach. The subretinal injector was connected to a syringe and 1% sodium hyaluronate (Bausch & Lomb Freda, Jinan, Shandong province, China) was gently injected into the subretinal space to enlarge RD (50 µL each)[17]. In addition, vehicle (dimethyl sulfoxide, DMSO), a 300 µmol/L solution of z-VAD-FMK (available from Enzo, PA, USA), or z-VAD-FMK combined with Necrostatin-1 (400 µmol/L, available from Merck, Darmstadt, Germany) was gently injected into the subretinal space to enlarge RD (5 µL each). The dose of compound was selected based on previous studies[5],[13],[18],[19]. RD was created only in the right eye of each animal, with the left eye serving as a control. This was confirmed by surgical microscope in every animal.
Transmission Electron Microscope Photomicrographs in the Outer Nuclear Layer (ONL)
As described previously[2], three days after RD, TEM was performed. Briefly, the eyes remained immersed in 4% glutaraldehyde (0.1 mol/L phosphate buffer, pH 7.4) for 24h at 4°C. The detached retinas were removed and post-fixed in 1% osmium tetroxide (0.1 mol/L sodium phosphate buffer solution, pH 7.2), dehydrated in ethanol and water, and embedded in Eponate. The retina was photographed by a JEM-1200EX electron microscope (JEM, Tokyo, Japan). The apoptotic and necrotic cell numbers in ONL were calculated from 6 eyes. Five sections were randomly selected in each eye. For each sample, about 200 photoreceptors were photographed and subjected to quantification of cell death modes in a masked fashion. Then, the percentage of apoptotic and necrotic cells was calculated. Photoreceptors showing cellular shrinkage and nuclear conde nsation were defined as apoptotic cells, whereas photoreceptors associated with cellular and organelle swelling and discontinuities in plasma and nuclear membrane were defined as necrotic cells. Electrondense granular materials were labeled simply as end-stage cell death/unclassified[13],[20],[21].
Immunoprecipitation and Western Blotting
As previous study described[1],[13],[22], the neural retina was collected on three days after RD. Equal amount of retinal lysates (1 mg) were incubated with 1 µL (1 mg/mL) anti-RIP1 antibody (Cell Signaling Technology, Boston, MA, USA) and 40 µL of protein A/G agarose beads (Beyotime Institute of Biotechnology, Jiangsu province, China), according to the manufacturer's instructions, at 4°C overnight. Beads were washed 5 times with Tris-buffered saline solution and the immunopellets were then subjected to Western blotting.
Samples were run on 8% sodium dodecylsulfate-polyacrylamide gel electrophoresis. After electrophoretic separation, the proteins were transferred to nitrocellulose membranes (Whatman, Maidstone, UK). The nitrocellulose membrane was blocked by incubation with 5% bovine serum albumin in tris-buffered saline and tween (TBST) (0.02% Tween-20 in tris-buffered saline, pH 7.4) for 2h at room temperature. After blocking, the membrane was reacted with RIP1 (1:1000, Cell Signaling Technology), phosphoserine (1:100, Enzo), LC-3 (1:500, Santa Cruz Biotechnology, CA, USA) antibody or caspase-8 (1:1000, Prosci Incorporated, USA), β-actin (1:1000, Beyotime Institute of Biotechnology, Jiangsu Province, China) antibody. Membranes were then washed thrice and incubated with horseradish-peroxidase-labelled secondary antibody (diluted 1:5000 in TBST, Santa Cruz Biotechnology) for 2h at room temperature. Bands were visualized by chemiluminescence (ECL, Amersham Pharmacia Biotech, Piscataway, NJ, USA), according to the manufacturer's instructions and were exposed to X-ray film. The density of the signal was quantified using Bandscan software (Version 4.3, Glyko, Inc., Novato, CA).
Statistical Analysis
Data are expressed as mean±SD. Assuming that the data meet normal distribution and variances are equal, the one-way analysis of variance (ANOVA) followed by validation using Student-Newman-Keuls tests was used to analyze the statistical differences in western blotting densitometric data and cell number among TEM assays. Analyses were performed by computer (SPSS 17.0 for windows, SPSS Inc., Chicago, IL, USA). For all comparisons, a P value less than 0.05 was considered statistically significant.
RESULTS
z-VAD-FMK Induced Necroptosis in Photoreceptors After Experimental Retinal Detachment
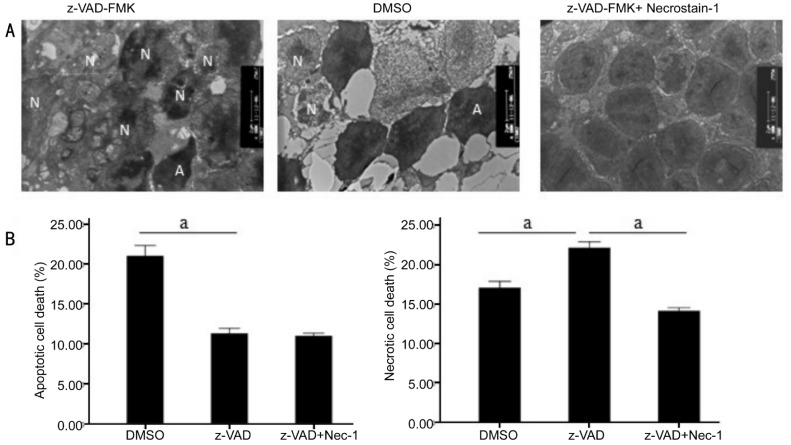
z-VAD-FMK induced necroptosis in photoreceptors were observed from morphological change by transmission electron microscopy. The morphology of photoreceptors, induced by caspase inhibitors was more in line with characteristics of necroptosis, which characterized by chromatin condensation, loss of plasma membrane integrity and many autophagosomes (Figure 1A). On the third day after RD, the transmission electron microscopy showed that percentage of necrotic cells (22.10%±0.78%) increased in z-VAD-FMK treated retina compared with the vehicle treated retina (17.04%±0.81%), but percentage of apoptotic photoreceptor death (11.28%±0.66%) decreased in z-VAD-FMK group compared with the vehicle group (20.98%±1.33%) after RD (n=6, per group, P<0.01) (Figure 1B). TEM results confirmed z-VAD-FMK induced necroptosis in photoreceptors after experimental RD.
Figure 1. Involvement of autophagosomes formationin necroptosis during RD-induced photoreceptor necroptosis were observed by TEM in three groups (DMSO, z-VAD-FMK and z-VAD-FMK combined with Necrostatin-1 groups).
A: Representative photomicrographs of TEM showed autophagy in the ONL on the third day after RD in the retina (A: Apoptotic cell; N: Necrotic cell). Autophagy formation could be visualized in z-VAD-FMK-treated group, which was by far the most confirmative analysis for autophagy. B: Quantification of necrotic and apoptotic photoreceptor death after RD. z-VAD-FMK treatment increased the percentage of necrotic cells and decreased apoptotic photoreceptor death after RD compared with DMSO treated control groups. z-VAD-FMK combined with Necrostatin-1 substantially led to a decrease in both forms of cell loss. At the same time, autophagy formation was significantly inhibited (n=6, per group, asterisks indicated aP<0.01; Scale bar, 2 µm).
LC-3 (II) and Autophagosomes Induction was Involved in z-VAD-FMK-induced Photoreceptor Necroptosis After Experimental Retinal Detachment
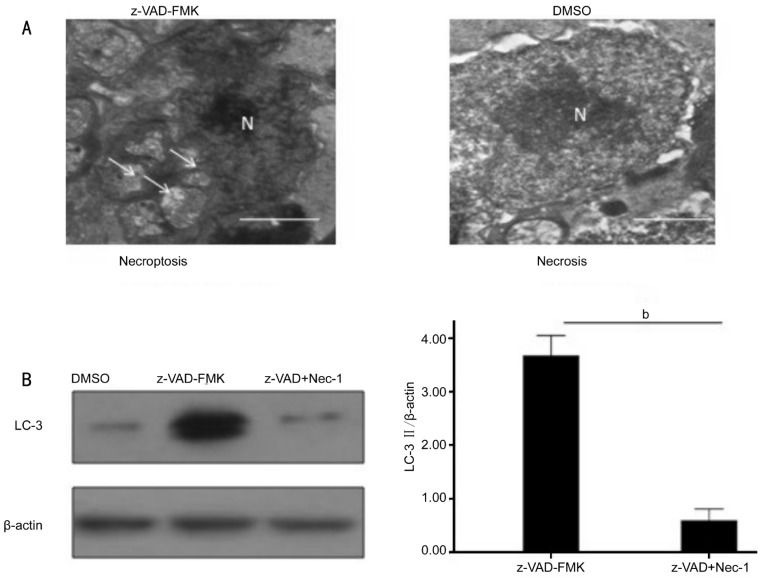
Recently studies discovered necroptosis signaling could activate autophagy, considered as a secondary marker of necroptosis[11]. Besides the consistent results of necroptosis activation after interference of z-VAD-FMK, the transmission electron microscopy showed z-VAD-FMK treatment induced necroptosis with evocative of autophagy, which was characterized by extensive vacuolization (Figure 2A). The autophagic activation biomarker LC-3II, analyzed by western blotting, increased accordingly three days after RD (Figure 2B).
Figure 2. Necrostatin-1 inhibited autophagosomes formation and the activation of autophagy marker LC-3II in photoreceptor after experimental RD.
A: z-VAD-FMK treatment induced necroptosis cell death with evocative of autophagy, and was characterized by extensive vacuolization (white arrowhead); B: Necrostatin-1 combined with z-VAD-FMK treatment inhibited the induction of LC-3II compared with z-VAD-FMK -treated groups (asterisks indicated bP<0.01; Scale bar=1 µm).
These data showed that induction of LC-3II and autophagosomes was involved in z-VAD-FMK-induced photoreceptor necroptosis after experimental RD, which indicated autophagy activation.
Up-regulation of RIP1 Phosphorylation was Mediated by Caspase-8 Inhibition in z-VAD-FMK-induced Photoreceptor Necroptosis
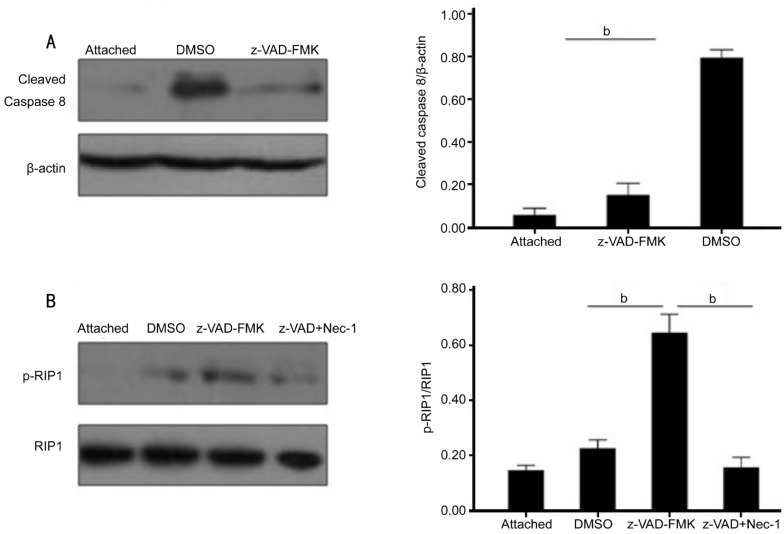
RIP1 phosphorylation is a key early signaling event in necroptosis[9],[23]. We tested whether z-VAD-FMK inhibited caspase-8 activation. Western blot densitometric analysis demonstrated that caspase-8 activation was significantly inhibited in z-VAD-FMK-treated retina compared with DMSO-treated retina on three days after RD induction (n=6, per group, P<0.01) (Figure 3A). Next, to further explore the necroptosis induction mechanism of action of z-VAD-FMK in experimental RD, we tested whether z-VAD-FMK promoted RIP1 phosphorylation by immunoprecipitation. Three days after RD, RIP1 phosphorylation was elevated in z-VAD-FMK treated retina compared with DMSO-treated retina (Figure 3B).
Figure 3. z-VAD-FMK promoted RIP1 phosphorylation by inhibiting caspase-8 activation.
A: Increases in caspase-8 activation after RD and the inhibition of this induction by z-VAD-FMK. Quantification analysis of caspase-8 activation demonstrated a significantly decrease in the z-VAD-FMK-treated retina (0.15±0.02) compared with DMSO-treated retina (0.79±0.02) (n=6, per group, asterisks indicated aP<0.01). B: Immunoprecipitation and Western blotting expression for RIP1 phosphorylation from control group, z-VAD-FMK and DMSO-treated groups three days after RD. Quantification analysis of RIP1 phosphorylation demonstrated a significantly increase in the z-VAD-FMK treated retina (0.64±0.03) compared with DMSO-treated retina (0.22±0.01). Necrostatin-1 combined with z-VAD-FMK treatment substantially inhibited this elevated RIP1 phosphorylation (n=6, per group, asterisks indicated aP<0.01).
These results showed that up-regulation of RIP1 phosphorylation was mediated by caspase-8 inhibition in z-VAD-FMK-induced photoreceptor necroptosis after experimental RD, which indicated necroptosis activation.
Necrostatin-1 Inhibited Photoreceptor Necroptosis by RIP1 Phosphorylation and LC-3II Down-regulation
Necrostatin-1, an RIPK1 allosteric inhibitor, has been proved as a potent and selective inhibitor of necroptosis[9]. We then assessed the inhibition effect of cell death using Necrostatin-1 after RD. Necrostatin-1 combined with z-VAD-FMK substantially led to a decrease both in apoptosis and necroptosis forms of cell loss (14.10%±0.41% necrotic cells, 10.98%±0.35% apoptotic cells) compared with z-VAD-FMK and DMSO treated group (n=6, per group, P<0.01; Figure 1B). At the same time, autophagy formation induced by z-VAD-FMK was significantly inhibited by Necrostatin-1 (Figure 1A). It was further discovered, by analysis of RIP1 and LC-3II expression, Necrostatin-1 combined with z-VAD-FMK treatment substantially inhibited RIP1 phosphorylation (Figure 3B) and LC-3 (II) induction (Figure 2B) compared with z-VAD-FMK treated group. These results demonstrated Necrostatin-1 could down-regulate RIP1 phosphorylation and LC-3II in z-VAD-FMK-incuded photoreceptor necroptosis after experimental RD, which suggested necroptosis and autophagy inhibition.
DISCUSSION
In current study, TEM and western blotting results firstly indicated that necroptosis induced by z-VAD-FMK was involved the LC-3II and autophagosomes induction, which indicated autophagy activation. It was also proved by immunoprecipitation and western blotting, that up-regulation of RIP1 phosphorylation in z-VAD-FMK-induced photoreceptor necroptosis was mediated by caspase-8 inhibition, which triggered activation of necroptosis. We also discovered Necrostatin-1 could inhibit RIP1 phosphorylation and LC-3II induction. The results of this study for the first time provided evidence that photoreceptor necroptosis induced by z-VAD-FMK was associated with the activation of autophagy and Necrostatin-1 could inhibit photoreceptor necroptosis and autophagy by down-regulation of RIP1 phosphorylation and LC-3II.
Caspases activation-induced apoptosis previously have been shown to play a critical role in RD, but caspases inhibition by z-VAD-FMK failed to prevent photoreceptor death[17], [24]–[26]. In this study, the morphologic change induced by z-VAD-FMK was assessed by TEM, which could provide important results not obtainable at the light microscopic level. Besides the classic characteristics of necroptosis: chromatin condensation, loss of plasma membrane integrity and many autophagosomes, the ultrastructure change of autophagy was also observed in our study[4],[5],[8],[27]. Altogether, these results confirmed that z-VAD-FMK treatment could induced necroptosis in photoreceptors, which explained why z-VAD-FMK treatment provided partial protection to avoid retinal cells death.
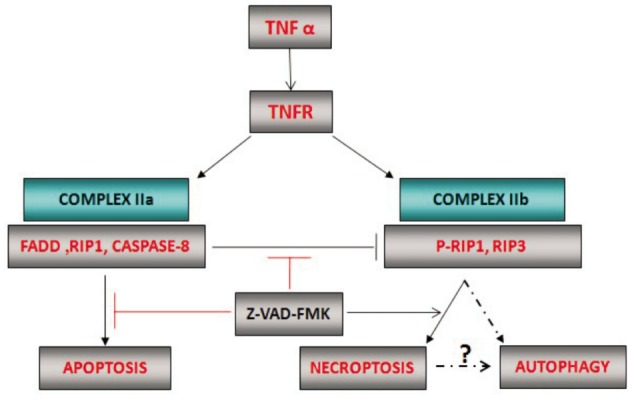
The work of Trichonas et al[13] has highlighted the importance of necroptosis after RD. Similar to their work we also showed z-VAD-FMK treatment induced RIP1 phosphorylation, which indicated necroptosis activation. Previous studies discovered RIP1 phosphorylation is a key early signaling event in necroptosis which can be inactivated by caspase-8[23]. As a pan-caspase inhibitor, z-VAD-FMK surely can inhibit caspase-8 activation. So we explored further the effect of z-VAD-FMK on RIP1 phosphorylation in this RD model. Our results demonstrated z-VAD-FMK not only inhibited caspase-8 activation but also promoted RIP1 phosphorylation compared with DMSO-treated retina (P<0.01). These results first indicated that z-VAD-FMK led to promote RIP1 phosphorylation by inhibiting caspase-8 activation (Figure 4).
Figure 4. The mechanism of up-regulation of RIP1 phosphorylation and LC-3II in z-VAD-FMK-induced photoreceptor necroptosis. When cells undergo tumor necrosis factor (TNF)-induced apoptosis, RIP1 phosphorylation is inactivated by caspase-8. Caspase inhibitor, z-VAD-FMK, can switch the death receptor induced apoptotic response to necroptosis by inhibition caspases-8 activation and up-regulation RIP1 phosphorylation. Then RIP1 phosphorylation can induce the activation of autophagy marker LC-3II by a currently unknown pathway.
Autophagy, a large-scale protein degradation and catabolic mechanism, has been implicated in caspase-independent cell death[5], [28],[29]. Recently studies show necroptosis signaling can activate autophagy[5],[11]. So it remained unknown whether necroptosis induced by z-VAD-FMK involved the activation of autophagy. Therefore, we assessed the autophagy activation by TEM. Since the autophagy is directly associated with the conversion of the microtubule associated protein, LC-3I to LC-3II, and the inhibition of LC-3II has been shown to cause reduction in autophagy, we also further tested autophagy marker LC-3II expression by western blotting[5],[18]. Our data showed that, on three day after RD, z-VAD-FMK treatment induced cell death with evocative of autophagy, which was characterized by extensive vacuolization. Along with necrotic photoreceptor increasing, there was an elevation of LC-3II expression accordingly. These data all first suggested the activation of autophagy marker LC-3II was involved in z-VAD-FMK induced necroptosis after RD.
Necrostatin-1, an RIPK1 allosteric inhibitor, has proven to be able lead to a decrease in both apoptosis and necroptosis forms of cell loss if combined with z-VAD-FMK[5],[13]. More important, our results, for the first time, further confirmed, in addition to the inhibition of necroptosis by Necrostatin-1, at the same time, autophagy formation was also significantly inhibited. Rosenbaum et al[18] reported similar findings in a model of retinal ischemia. They found that, pretreatment with z-VAD-FMK, Necrostatin-1 inhibited not only necroptosis but autophagy and improved functional outcome. Our data further explained this cell death and protection process: Necrostatin-1 treatment provided significant protection of photoreceptors by simultaneous inhibition of RIP phosphorylation and LC-3II induction, which indicated simultaneous inhibition of necroptosis and autophagy.
However, the functional role of autophagy still remains a subject of debate. Besirli et al[24] discovered autophagy activation was controlled, in part, by Fas-receptor activation and prevented photoreceptors from apoptosis. Cai et al[30] demonstrated the activated autophagy played a protective role against palmitate-induced hepatocytes apoptosis. On the contrary, it was also reported that the involvement of autophagy in necroptosis further reinforce the process of cell death, which indicates the possibility that autophagy may be a clean-up mechanism for cell death[31]–[33]. Owen et al[34] found that inhibition of autophagy resulted in increased bacterial survival. Degterev et al[5] further showed autophagy was a downstream of necroptosis signaling pathway. The results in our observation suggested that the activation of autophagy marker LC-3II was controlled by necroptosis signaling, which indicated autophagy involved in necroptosis also contributed to cell damage. Although the autophagy is directly associated with the conversion of LC-3I to LC-3II, autophagy is a very complicated and fine regulated process, therefore, we will further look at additional markers such as autophagy associated gene (Atg) 7, 5, 12, beclin-1 etc. in z-VAD-FMK-induced photoreceptor necroptosis in the future.
There are some limitations to this study. The sample number of the experimental RD models was not large. This study was designed to only investigate three days changes after RD. Further research is needed to elucidate the long-term effect. The evidence that whether Necrostatin-1 could down-regulate RIP1 phosphorylation and LC-3II when given after RD was not provided.
In summary, we discovered necroptosis induced by z-VAD-FMK is associated with the autophagosomes formation and activation of autophagy marker LC-3II. Necrostatin-1 reduced photoreceptors from necroptosis and autophagy by inhibition of RIP1 phosphorylation (necroptosis marker) and LC-3II induction (autophagy activation marker). Autophagy cooperated with necroptosis, exacerbate the cell damage after RD, which may be a promising combined therapeutic direction against neuronal damage in RD. But, it still remains unknown how autophagy is activated in necroptosis pathway, thus, future studies, exploring the relationship between autophagy and necroptosis, are necessary.
Acknowledgments
The authors thank Wen-Qiu Wang, Qing Gu, Yue Liu, Yue-Qin Tang, Yuan-Yuan Gong (Shanghai First People's Hospital, School of Medicine, Shanghai Jiaozong University) for their invaluable assistance in this study. We thank Dr. Yang Qin (Menlo Park, California, USA) for help in improving the presentation of this paper. Part of this article's abstract has been presented on ARVO meeting 2013.
Foundations: Supported by National Basic Research Program of China “973 Program” (No.2011CB707506); Natural Science Foundation of Anhui Province (No.1408085QH159); National Natural Science Foundation of China (No.81170861, 81400407 and 30973259); Shanghai Key Basic Research Foundation (No.11JC141601); Shanghai Scholar Leadship Foundation (No.12XD1404100).
Conflicts of Interest: Dong K, None; Zhu ZC, None; Wang FH, None; Ke GJ, None; Yu Z, None; Xu X, None.
REFERENCES
- 1.Liu H, Qian J, Wang F, Sun X, Xu X, Xu W, Zhang X, Zhang X. Expression of two endoplasmic reticulum stress markers, GRP78 and GADD153, in rat retinal detachment model and its implication. Eye. 2010;24(1):137–144. doi: 10.1038/eye.2009.20. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Xu X, Wang F, Zhang X, Yu Z, Lu H, Ho PC. Effects of nerve growth factor for retinal cell survival in experimental retinal detachment. Curr Eye Res. 2007;32(9):765–772. doi: 10.1080/02713680701531082. [DOI] [PubMed] [Google Scholar]
- 3.Kayama M, Nakazawa T, Thanos A, Morizane Y, Murakami Y, Theodoropoulou S, Abe T, Vawas D, Miller JW. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti-apoptotic Akt kinase. Am J Pathol. 2011;178(3):1080–1091. doi: 10.1016/j.ajpath.2010.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han W, Xie J, Li L, Liu Z, Hu X. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis. 2009;14(5):674–686. doi: 10.1007/s10495-009-0334-x. [DOI] [PubMed] [Google Scholar]
- 5.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1(2):112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 6.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325(5938):332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 7.Feoktistova M, Geserick P, Panayotova-Dimitrova D, Leverkus M. Pick your poison: the Ripoptosome, a cell death platform regulating apoptosis and necroptosis. Cell Cycle. 2012;11(3):460–467. doi: 10.4161/cc.11.3.19060. [DOI] [PubMed] [Google Scholar]
- 8.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135(7):1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4(5):313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137(6):1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 11.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22(2):263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenabeele P, Declercq W, Van Herreweghe F, Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signal. 2010;3(115):re4. doi: 10.1126/scisignal.3115re4. [DOI] [PubMed] [Google Scholar]
- 13.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW, Vawas DG. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010;107(50):21695–21700. doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong K, Sun X. Targeting death receptor induced apoptosis and necroptosis: a novel therapeutic strategy to prevent neuronal damage in retinal detachment. Med Hypotheses. 2011;77(1):144–146. doi: 10.1016/j.mehy.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Xu X, Wang F, Zhang X, Ho PC, Liu H, Qian J, Yu Z, Lu H, Xu W. Nerve growth factor helps protect retina in experimental retinal detachment. Ophthalmologica. 2008;222(1):58–61. doi: 10.1159/000109281. [DOI] [PubMed] [Google Scholar]
- 16.Dong K, Zhu H, Song Z, Gong Y, Wang F, Wang W, Zheng Z, Yu Z, Gu Q, Xu X, Sun X. Necrostatin-1 protects photoreceptors from cell death and improves functional outcome after experimental retinal detachment. Am J Pathol. 2012;181(5):1634–1641. doi: 10.1016/j.ajpath.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Hisatomi T, Sakamoto T, Murata T, Yamanaka I, Oshima Y, Hata Y, Ishibashi T, Inomata H, Susin SA, Kroemer G. Relocalization of apoptosis-inducing factor in photoreceptor apoptosis induced by retinal detachment in vivo. Am J Pathol. 2001;158(4):1271–1278. doi: 10.1016/S0002-9440(10)64078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbaum DM, Degterev A, David J, Rosenbaum PS, Roth S, Grotta JC, Cuny GD, Yuan J, Savitz SI. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010;88(7):1569–1576. doi: 10.1002/jnr.22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You Z, Savitz SI, Yang J, Degterev A, Yuan J, Cuny GD, Moskowitz MA, Whalen MJ. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab. 2008;28(9):1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hisatomi T, Sakamoto T, Sonoda KH, Tsutsumi C, Qiao H, Enaida H, Yamanaka I, Kubota T, Ishibashi T, Kura S, Susin SA, Kroemer G. Clearance of apoptotic photoreceptors: elimination of apoptotic debris into the subretinal space and macrophage-mediated phagocytosis via phosphatidylserine receptor and integrin alphavbeta3. Am J Pathol. 2003;162(6):1869–1879. doi: 10.1016/s0002-9440(10)64321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Nomenclature Committee on Cell Death 2009 Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16(1):3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Wang F, Lu F, Xu S, Hu W, Huang J, Gu Q, Sun X. The antiangiogenic effects of integrin alpha5beta1 inhibitor (ATN-161) in vitro and in vivo. Invest Ophthalmol Vis Sci. 2011;52(10):7213–7220. doi: 10.1167/iovs.10-7097. [DOI] [PubMed] [Google Scholar]
- 23.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13(19):2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besirli CG, Chinskey ND, Zheng QD, Zacks DN. Autophagy activation in the injured photoreceptor inhibits fas-mediated apoptosis. Invest Ophthalmol Vis Sci. 2011;52(7):4193–4199. doi: 10.1167/iovs.10-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo AC, Woo TT, Wong RL, Wong D. Apoptosis and other cell death mechanisms after retinal detachment: implications for photoreceptor rescue. Ophthalmologica. 2011;226(Suppl 1):10–17. doi: 10.1159/000328206. [DOI] [PubMed] [Google Scholar]
- 26.Hisatomi T, Sakamoto T, Goto Y, Yamanaka I, Oshima Y, Hata Y, Ishibashi T, Inomata H, Susin SA, Kroemer G. Critical role of photoreceptor apoptosis in functional damage after retinal detachment. Curr Eye Res. 2002;24(3):161–172. doi: 10.1076/ceyr.24.3.161.8305. [DOI] [PubMed] [Google Scholar]
- 27.Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, Luo J, Hu X. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther. 2007;6(5):1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 28.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 30.Cai N, Zhao X, Jing Y, Sun K, Jiao S, Chen X, Yang H, Zhou Y, Wei L. Autophagy protects against palmitate-induced apoptosis in hepatocytes. Cell Biosci. 2014;4:28. doi: 10.1186/2045-3701-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, Morrissette NS, Walsh CM. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105(43):16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Y, Kim SO, Li Y, Han J. Autophagy contributes to caspase-independent macrophage cell death. J Biol Chem. 2006;281(28):19179–19187. doi: 10.1074/jbc.M513377200. [DOI] [PubMed] [Google Scholar]
- 33.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304(5676):1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 34.Owen KA, Meyer CB, Bouton AH, Casanova JE. Activation of focal adhesion kinase by Salmonella Suppresses Autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog. 2014;10(6):e1004159. doi: 10.1371/journal.ppat.1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]