Abstract
AIM
To investigate the role of tissue plasminogen activator (t-PA) and plasminogen activator inhibitor (PAI) in proliferative diabetic retinopathy (PDR) and to discuss the correlations among t-PA, PAI and vascular endothelial growth factor (VEGF) expressions.
METHODS
A total of 36 vitreous samples were collected from 36 patients with PDR (PDR group), and 17 vitreous samples from 17 patients with idiopathic macular hole were used as control. The concentrations of t-PA, PAI and VEGF in samples were determined by ELISA method. The correlations among t-PA, PAI and VEGF expressions were discussed.
RESULTS
The concentrations of t-PA, PAI and VEGF in the PDR group were significantly higher than those in the control group (P<0.001). The t-PA and PAI expressions were highly correlated with the VEGF expression (P<0.001).
CONCLUSION
In addition to VEGF, a variety of bioactive substances, such as t-PA and PAI, are involved in the pathogenesis involved in the angiogenesis of PDR. VEGF can activate t-PA expression, resulting in collagen tissue degradation and angiogenesis. VEGF may also activate the mechanism for endogenous anti-neovascularization.
Keywords: proliferative diabetic retinopathy, vascular endothelial growth factor, tissue plasminogen activator, plasminogen activator inhibitor, angiogenesis
INTRODUCTION
Retinal neovascularization can induce the transformation of diabetic retinopathy (DR) into proliferative diabetic retinopathy (PDR), including repeated vitreous hemorrhage and tractional retinal detachment, which is the leading cause of blindness among diabetic patients. Therefore, methods for the prevention and treatment of DR neovascularization are important to improve the life quality of patients. PDR is classified under retinal microangiopathy and is characterised by neovascularization. Many growth factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor, insulin-like Naga Innoko growth factor, hepatocyte growth factor and tumour necrosis factor, are involved in the pathological process of PDR[1]. Experiments show that the occurrence and development of retinal neovascularization is closely related to VEGF regulation[2],[3]. Some biologically active substances, such as tissue plasminogen activator (t-PA) and its inhibitor [i.e., plasminogen activator inhibitor (PAI)], participating in neovascularization, particularly in VEGF expression[4], are being investigated. High t-PA, PAI and VEGF expression levels are found in patients with retinal ischemic diseases, in which both t-PA and PAI expressions are significantly correlated with VEGF expression[2]. In retinal ischemic diseases, the retina produces a large amount of VEGF, which specifically acts with the VEGF receptor in endothelial cells and participates in each neovascularization step[1]. VEGF induces the synthesis and release of cell collagenase, endothelial cell migration and proliferation, as well as the change in the activation status of endothelial cells. VEGF also upregulates t-PA and PAI expressions, thereby promoting endothelial cell division, proliferation and migration, improving the vascular permeability and increasing related enzyme activities which change the extracellular matrix[5]. VEGF is a multifunctional cytokine that is important in retina and iris neovascularization[6]. VEGF activates the main process of vascular proliferation and is involved in the entire neovascularization process. Retinal neovascularization is a complex pathophysiological process accompanied with a series of changes in peripheral cells and basement membrane. Therefore, VEGF is not the only significant factor in retinal neovascularisation[7],[8]. PAI and t-PA are also important in neovascularization. A normal human eye has a certain level of PAI expression[3]. Some studies show relatively low t-PA activity and high PAI activity in diabetic patients with PDR. The severity of PDR and duration of diabetes are negatively correlated with t-PA activity but positively correlated with PAI activity[9],[10].
In this study, the roles of t-PA and PAI in PDR were investigated. The correlations between t-PA, PAI and VEGF expressions were discussed. This report provides information on the pathophysiological process of retinal neovascularization in PDR and reference indexes for DR prevention and control.
SUBJECTS AND METHODS
Reagents and Apparatus
VEGF ELISA kit (Sigma), t-PA ELISA kit (Sweden), PAI ELISA kit (Sweden) and Metretec 960 enzyme mark instrument (450 nm, USA) were used in this study.
Sample Preparation
The study was conducted in agreement with the guidelines of the Declaration of Helsinki. All specimens were collected at the General Hospital of Ningxia Medical University and after obtaining informed written consent under a General Hospital of Ningxia Medical University Institutional Review Board approved protocol. Thirty-six vitreous samples were collected from 36 patients with type 2 diabetes mellitus complicated with PDR (PDR group). Other 17 vitreous samples from 17 patients with idiopathic macular hole were used as control. Conventional three-port pars plana vitrectomy was performed. After closing the perfusion, the vitrectomy probe was inserted into the central vitreous cavity to incise and draw 0.3 mL of vitreous. The vitrectomy probe was then extracted. Patients with previous intraocular surgery were excluded, and no pre-treatment with anti-VEGFs was performed. Some patients received combined phaco-surgery simultaneously after obtaining vitreous body specimen. The extracted vitreous was transferred into a 0.5 mL sterile Eppendorff centrifuge tube and preserved in a refrigerator at -20°C for further use.
Determination of t-PA, PAI and VEGF Concentrations
The vitreous samples were centrifugated. VEGF, t-PA and PAI concentrations were determined by ELISA method. Results were presented with the D-value of sample absorbance (A) to blank. The standard curve (x, concentration of standard; y, A) was drawn, and the t-PA, PAI, and VEGF concentrations in samples were calculated. Data were expressed as mean±SD. Correlation and simple regression analysis and t-test were performed for comparisons between two groups.
RESULTS
Expression Levels of t-PA, PAI and VEGF
A total of 36 patients (including 16 males and 20 females, average age of 63.3±10.6y) were collected in the study. The patients were selected in accordance with the International Clinical Diabetic Retinopathy Disease Severity Scale (2002). Simultaneously, other 17 patients (including 6 males and 11 females, average age of 61.4±8.7y) with idiopathic macular hole were identified as control. Results demonstrated that t-PA was absent in two cases from the control group. Table 1 shows that the t-PA and PAI expressions in the PDR group are significantly higher than that in the control group (P<0.001).
Table 1. Expressions of t-PA, PAI and VEGF in two groups.
| Cytokine | PDR group (µg/L) | Control group (µg/L) |
| t-PA | 1.01±0.8b | 0.2±0.4 |
| PAI | 6.8±6.1b | 1.7±1.5 |
| VEGF | 16.4±12.3 | 2.5±1.8 |
bP<0.001 vs the control group.
Correlations Among t-PA, PAI and VEGF Expressions
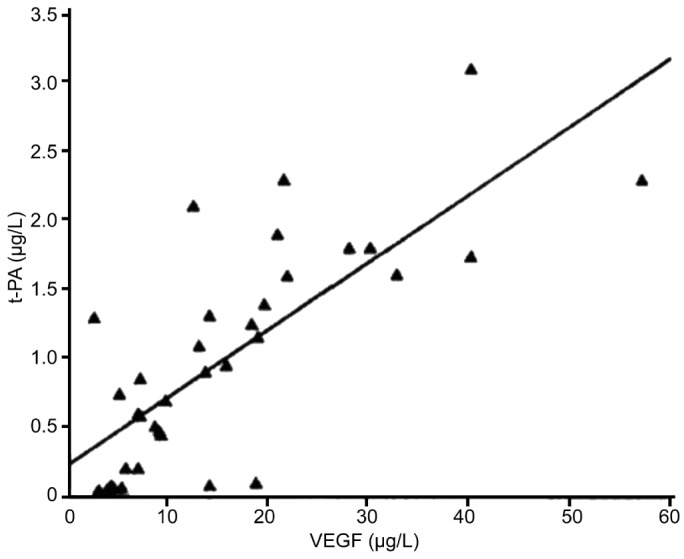
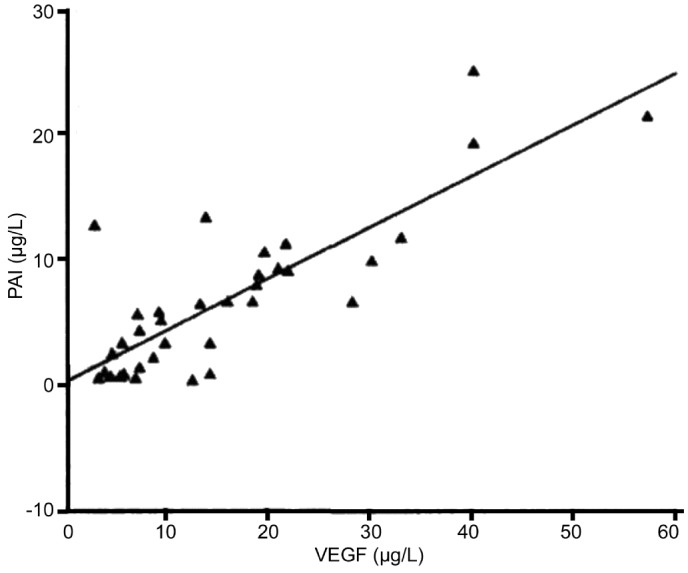
Figures 1 and 2 show that t-PA and PAI expressions were significantly correlated with VEGF expression (x, VEGF concentration; y, t-PA concentration; P<0.001). PAI expression was also significantly correlated with VEGF expression (x, VEGF concentration; y, PAI concentration; P<0.001).
Figure 1. The relationship between the expression of t-PA and VEGF in PDR.
Figure 2. The correlation between the expression of PAI and VEGF in PDR.
DISCUSSION
Recent studies show that the occurrence and development of retinal neovascularization are closely related with VEGF regulation[8]. VEGF is the most specific mitogen to mitogen most specific to vascular endothelial cells, causing endothelial cell division and proliferation and increasing vascular permeability. VEGF is highly expressed in various proliferative lesions of retinal vessels[11]–[13]. Studying these bioactive substances provides thorough understanding of the pathophysiological process of retinal neovascularization, which can be used as basis for developing new PDR treatment strategies.
With regard to morphology, vascular proliferation is a series of biological processes, which are derived from original vascular tissue structure and terminated with new vascular tissue structure. In this process, vascular tissue formation and new matrix component production always exist. Microvascular permeability is initially changed. Subsequently, the intravascular plasma protein components enter the extravascular space. The extravascular fibrin components then evolve into a hotbed for proliferation and growth of new vascular tissues. Cell matrix degradation is an important precondition for endothelial cell proliferation and migration. After activation of related enzymes, the basement membrane is degraded for mitosis and migration of vascular endothelial cells on microvascular inner wall[10],[14]. Studies on tumors vascular tissues show that t-PA plays a major role in endothelial cell matrix degradation. In addition, the expression levels of bioactive substances, such as t-PA and VEGF, are important indicators of recurrence and prognosis of some tumors.
In this study, the t-PA concentration in PDR group is significantly higher than that in the control group. Thus, t-PA is highly expressed in PDR, and t-PA may play an important role in PDR occurrence and development[4],[14],[15]. Results also show that most of the patients in this study are in the active or advanced stage of lesion. In addition, t-PA expression in the PDR group is significantly correlated with the VEGF expression[16]. In PDR, when the VEGF expression increases the retinal capillary permeability and exosmosis of intravascular fibrin components, t-PA is activated, which then induces the extracellular matrix and vascular endothelial cell basement membrane degradation. Cell matrix degradation process is an important precondition for endothelial cell proliferation and migration. Specifically, after activation of related enzymes, the basement membrane is degraded for the mitosis and migration of vascular endothelial cells on the microvascular inner wall. Thus, t-PA participates in vascular proliferation and accelerates neovascularization. However, this mechanism needs further confirmation.
Result of this study shows that PAI is also significantly expressed in the PDR group, and the PAI expression is significantly correlated with the VEGF expression. Although a certain level of PAI expression is found in normal human eyes, high PAI expression shows significant connection with PDR[9],[10]. Thus, during activation of retinal neovascularization, VEGF may activate the endogenous anti-neovascularization. Further studies on this mechanism provide deeper understanding of the pathophysiological process of retinal neovascularization and serve as a basis for the development of therapeutic strategies for retinal neovascularization. High t-PA, PAI and VEGF expression levels are found in PDR patients. PAI and t-PA expression are significantly correlated with VEGF expression. Therefore, VEGF and a variety of bioactive substances, such as t-PA and PAI, are involved in retinal neovascularization in patients with PDR. During this process, VEGF induces the division, proliferation and migration of vascular endothelial cells and simultaneously activates related enzymes (e.g. t-PA), which degrade the basement membrane and promote neovascularization. In addition, VEGF may activate the mechanism for endogenous anti-neovascularization.
Acknowledgments
Foundation: Supported by Natural Science Foundation of Ningxia (No.NZ10129)
Conflicts of Interest: Wu SL, None; Zhan DM, None; Xi SH, None; He XL, None.
REFERENCES
- 1.Mitamura Y, Harada C, Harada T. Role of cytokines and trophic factors in the pathogenesis of diabetic retinopathy. Curr Diabetes Rev. 2005;1(1):73–81. doi: 10.2174/1573399052952596. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz de Almodovar C, Luttun A, Carmeliet P. An SDF-1trap for myeloid cells stimulates angiogenesis. Cell. 2006;124(1):18–21. doi: 10.1016/j.cell.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Shams N, Ianchulev T. Role of vascular endothelial growth factor in ocular angiogenesis. Ophthalmol Clin North Am. 2006;19(3):335–344. doi: 10.1016/j.ohc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Ramasamy K, Abraham C, Gupta V, Gupta A. Diabetic retinopathy: an update. Indian J Ophthalmol. 2008;56(3):178–188. [PMC free article] [PubMed] [Google Scholar]
- 5.Funatsu H, Yamashita H, Noma H, Mimura T, Nakamura S, Sakata K, Hori S. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243(1):3–8. doi: 10.1007/s00417-004-0950-7. [DOI] [PubMed] [Google Scholar]
- 6.Read O, Cook C. Retinopathy in diabetic patients evaluatedat a primary care clinic in Cape Town. S Afr Med J. 2007;97941-942(10):944. [PubMed] [Google Scholar]
- 7.Wagner J, Jan Danster AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, Schalekamp MA, Ganten D. Demonstratic renin mRNA, angiotensin mRNA, and angiotensin coverting enzyme expression in thehuman eye: evidence for an intraocular angiotensin system. Br J Ophthalmol. 2006;80(2):159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li CR, Sun SG, Jiang DY, Hong W. The role of ginkgo biloba extract in treating diabetic retinopathy. Guoji Yanke Zazhi. 2006;6(1):78–81. [Google Scholar]
- 9.Izuta H, Matsunaga N, Shimazawa M, Avery RL. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol Vis. 2010;16(16):130–136. [PMC free article] [PubMed] [Google Scholar]
- 10.PetrovIcm G, Korosec P, Kosnik M, Miller W. Localand genetic determinants of vascular endothelial growthfactor expression in advanced proliferative diabeticretinopathy. Mol Vis. 2008;30(14):1382–1387. [PMC free article] [PubMed] [Google Scholar]
- 11.Katano H, Kamiya K, Mase M, Tanikawa M, Yamada K. Tissue plasminogen activator in chronic subdural hematomas as a predictor of recurrence. J Neurosurg. 2006;104(1):79–84. doi: 10.3171/jns.2006.104.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Basu P, Som S, Choudhuri N, Das H. Contribution of the blood glucoselevel in perinatal asphyxia. Eur J Pediatr. 2009;168(7):833–838. doi: 10.1007/s00431-008-0844-5. [DOI] [PubMed] [Google Scholar]
- 13.Dorotheo EU, Tang RA, Bahrani HM, Shing Y. Her visionwas tied down. Surv Ophthalmol. 2005;50(6):588–597. doi: 10.1016/j.survophthal.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell. 2005;17(4):491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, Ferrannini E. Clustering of insulin resis-tancewith vascular dysfunction and low-grade inflammation in type2 diabetes. Diabetes. 2006;55(2):1133–1140. doi: 10.2337/diabetes.55.04.06.db05-1076. [DOI] [PubMed] [Google Scholar]
- 16.Izuta H, Chikaraishi Y, Adachi T, Shimazawa M, Sugiyama T, Ikeda T, Hara H. Extracellular SOD and VEGF are increased in vitreous bodies from proliferative diabetic retinopathy patients. Mol Vis. 2009;15:2663–2672. [PMC free article] [PubMed] [Google Scholar]