Abstract
AIM
To isolate and identify the molds involved in mycotic keratitis; to isolate corresponding species from soil samples; to compare the extracellular enzyme activity indices of the molds isolated from keratitis cases and the corresponding soil isolates.
METHODS
The specimens were collected from the target patients attending the microbiology laboratory of tertiary eye hospital in Coimbatore, Tamilnadu state, India. The isolates were subjected for identification based on the growth on solid media, direct microscopy and lacto phenol cotton blue wet mount preparation. Extracellular enzymes such as lipase, deoxyribonuclease (DNase), α-amylase, protease, cellulase and pectinase produced by the fungal isolates were screened on solid media supplemented with the corresponding substrates. Based on growth and zone diameter, the enzyme activity indices were calculated and were compared with that of the soil fungal isolates.
RESULTS
A total of 108 clinical samples were collected from a tertiary eye care hospital and out of which 60 fungal isolates were obtained. Among these, Fusarium spp. (n=30), non sporulating molds (n=9), Aspergillus flavus (n=6), Bipolaris spp. (n=6), Exserohilum spp. (n=4), Curvularia spp. (n=3), Alternaria spp. (n=1) and Exophiala spp. (n=1) were identified and designated as FS1-30, NSM1-9, AF1-6, BS1-6, ES1-4, CS1-3, AS1 and EX1, respectively. For comparative analysis, soil samples were also collected from which, one isolate of each Fusarium spp., Aspergillus flavus, Bipolaris spp., Exserohilum spp., and Curvularia spp., respectively were selected. Highest lipase activity was seen in corneal isolate NSM2 (EAI= 2.14). The DNase activity was higher in NSM9 (EAI=1.88). In case of protease, Fusarium spp. (FS9) had prominent enzyme activity index of 1.38; α-amylase activity was also superior in corneal isolate FS13 with EAI of 1.63 when compared to other isolates. The enzyme activity index for cellulase was also noted to be higher in corneal isolates i.e. NSM7 with EAI of 1.98 when compared to other corneal and soil isolates. The pectinase activity index was also prominent for corneal isolate NSM5 versus the soil isolates, SAF1, SFS1, SES1, SBS1 and SCS 1 as 1.76 versus 1.47, 1.38, 1.16, 1.11 and 1.14, respectively.
CONCLUSION
The most common isolate was Fusarium spp. followed by Aspergillus, Curvularia, Exserohilum, Bipolaris, Exophiala and Alternaria species. Enzyme activity indices (EAI) of the enzymes analysed varied with the clinical and soil isolates with respect to protease and cellulase (P=0.01). Of all the strains compared it was noted that mean EAI was greater in many clinical fusarial isolates followed by non sporulating molds.
Keywords: mycotic keratitis, extracellular enzymes, enzyme activity index
INTRODUCTION
Corneal ulcer is defined as a loss of the corneal epithelium with underlying stromal infiltration and suppuration associated with signs of inflammation[1]. Several investigators[1]–[5] have reported the prevalence of bacterial and fungal pathogens isolated from ulcerated corneas, but still now there has not been a population based study demonstrating the true incidence of microbial keratitis in a developing country. Ocular fungal infections are being increasingly recognized as an important cause of ophthalmic mycoses and may be life threatening[6]. Keratitis due to filamentous fungi is believed to usually occur following trauma, the key predisposing factor, in healthy young males engaged in agricultural or other outdoor work[7].
Fungi gain access into the corneal stroma through a defect, usually through trauma, in the epithelium, then multiply and cause tissue necrosis and an inflammatory reaction. The organisms can penetrate an intact Descemet membrane (DM) and gain access into the anterior chamber or the posterior segment. Mycotoxins and proteolytic enzymes augment the tissue damage. Enzymes released from fungi are potential enhancers of both the pathogenicity and virulence of the pathogen. Presence of certain enzymes in corneal ulcers cases indicate that a correlation exist between the production of enzymes and inflammation of cornea. Since host cell membranes are composed of lipids and proteins, extracellular enzymes may play an important role in the invasion of the host tissues[8]. Because of all these, the study on the production and mode of action of extracellular enzymes is of high importance. However it remains to be studied whether fungal or tissue derived products mediate pathogenesis of fungal keratitis. Therefore only initial screening of extracellular enzymes produced in vitro by the corneal fungal isolates and their activity indices would be beneficial.
SUBJECTS AND METHODS
Collection and Processing of Samples
Clinical samples were collected after getting the informed consent from fungal keratitis patients attending Aravind Eye Hospital and Post Graduate Institute of Ophthalmology, Coimbatore, Tamilnadu state, India. A short consent form was prepared in the local language and after the approval from the Institutional Review Board (IRB), the patients were thoroughly informed about the study parameters. The procedural samples collected after the thorough examination by an ophthalmologist were included in the study. The specimen viz., corneal scrapings and corneal swabs were collected aseptically from a total of 108 fungal keratitis patients by using a sterile Kimura's spatula under slit lamp illumination after administering a local anesthetic[2]. The scrapped out materials were inoculated directly into solid media such as blood agar and Sabouraud's dextrose agar (SDA) in row of “C” shaped streaks. The scrapings were also subjected to Gram staining and 10% potassium hydroxide (KOH) mount to detect the presence of fungal hyphae[3]. The soil samples were collected aseptically from Coimbatore, Tamilnadu state, India and were immediately transported to the laboratory for processing on SDA plates using dilution plating technique.
Identification of Fungal Isolates
The fungal isolates obtained were point inoculated on SDA plates and incubated at room temperature for 3-4d. The fungal isolates were subjected to lacto phenol cotton blue (LCB) mount employing cello tape flag method[9]. A 1.5 inch (40 mm) strip of clear cellophane tape was taken. Tip of the tape was held securely with forceps and sticky side was gently pressed against the fungal mycelium. Then tape was placed on a small drop of lacto phenol cotton blue in a glass slide. The preparation was examined microscopically under 10× and 45× magnification for observing the details of spore body, spore morphology etc[10]. Identified fungal isolates were subjected to single spore culture method as described by Leslie et al[11]. All the isolates were maintained in 0.85% of saline solution in screw capped storage vials and stored at room temperature for further studies.
Screening for the Extracellular Enzyme Activity of the Fungal Isolates
The extracellular enzyme production was screened by using different solid media supplemented with corresponding substrates. For screening the extracellular enzymes such as α-amylase, DNase and protease, the isolates were inoculated on starch agar, DNase test agar and skim milk agar, respectively[12]–[14]. The extracellular enzymes like cellulase, pectinase and lipase were screened by inoculating the isolates on potato dextrose agar (PDA) media supplemented with 0.5% carboxymethyl cellulose, Czapek-Dox agar with 1.5% pectin and tributyrin agar base with 1% tributyrin oil, respectively[1],[15]–[17]. The plates were then incubated at 30°C for 72h. After incubation, the fungal mats were removed and observed for the zone formation employing corresponding solutions such as, iodine for α-amylase and pectinase, 0.03% Congo red and 1 mol/L NaCl (destaining agent) for cellulase, and 1 equivalents/L HCl for DNase.
Determination of Various Extracellular Enzyme Activity Indices of the Fungal Isolates
The enzyme activity index was calculated by measuring the diameter of growth and the diameter of zone[8].
The enzyme screenings were carried out in duplicate and mean EAI values (MEAI) were calculated. Statistical tools like percentage analysis for finding the percentage of the fungal isolates having the specific enzyme activity and unpaired t-test to find the statistical significance of various enzymes activity indices between the clinical and the corresponding environmental fungal isolates were employed for the interpretation of the results.
RESULTS
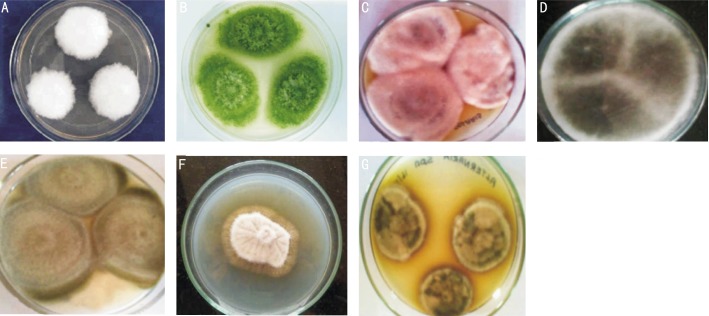
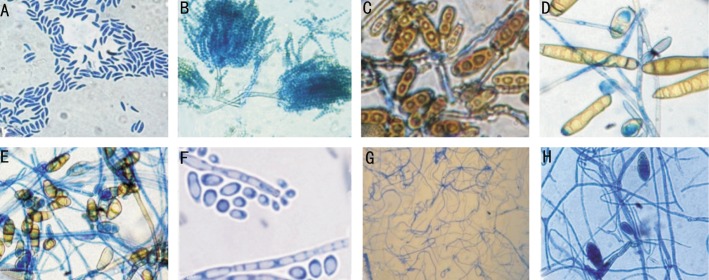
A total of 108 samples from the patients with infected corneal ulcers were obtained between October 2012 to August 2013. The patients attending microbiology laboratory of tertiary eye care hospital with suspected keratitis were included in the present study. Out of 108 corneal samples collected, a total of 48 isolates of bacteria and 60 isolates of fungi were obtained. Among the 60 isolates of fungi, Fusarium spp. (n=30), non sporulating molds (n=9), Aspergillus flavus (n=6), Bipolaris spp. (n=6), Exserohilum spp. (n=4), Curvularia spp. (n=3), Alternaria spp. (n=1), and Exophiala spp. (n=1) were identified (Figures 1, 2). The isolated Fusarium spp., non sporulating molds, Aspergillus flavus, Bipolaris spp., Exserohilum spp., Curvularia spp., Alternaria spp., and Exophiala spp. were designated as FS1-30, NSM1-9, AF1-6, BS1-6, ES1-4, CS1-3, AS1 and EX1, respectively. The soil isolates of Fusarium spp., Aspergillus flavus, Bipolaris spp., Exserohilum spp., and Curvularia spp., were designated as SFS1, SAF1, SBS1, SES1 and SCS1, respectively.
Figure 1. Colony morphology of molds isolated from keratomycosis and soil on SDA.
A: Fusarium spp; B: Aspergillus flavus; C: Bipolaris spp.; D: Exserohilum spp.; E: Curvularia spp.; F: Exophiala spp.; G: Alternaria spp.
Figure 2. Microscopic observation of molds isolated from keratomycosis and soil at (45×).
A: Fusarium spp.; B: Aspergillus flavus; C: Bipolaris spp.; D: Exserohilum spp.; E: Curvularia spp.; F: Exophiala spp.; G: Alternaria spp.; H: Non-sporulating molds.
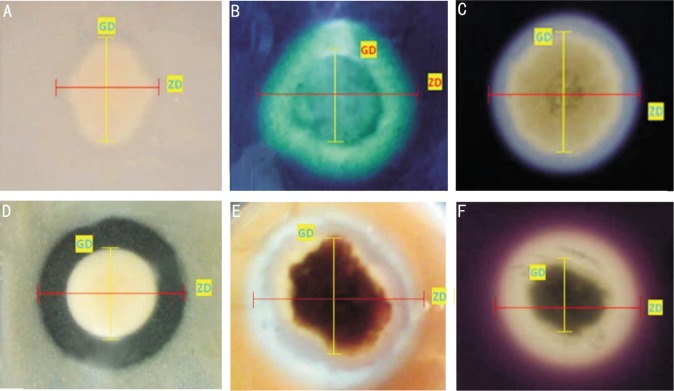
Out of the 60 isolates of molds, 58 (96.66%) isolates showed zone formation on tributyrin agar medium, which indicates the presence of lipase enzyme activity (Figure 3A) and only 2 (3.33%) isolates of Fusarium did not show lipase activity. Mean lipase activity index for Fusarium spp., non sporulating molds, Aspergillus flavus, Bipolaris spp., Exserohilum spp., Curvularia spp., Alternaria spp., and Exophiala spp. were 1.12, 1.37, 1.43, 1.27, 1.26, 1.21, 1.32 and 1.11, respectively. There was no significant difference in the lipase activity indices of the soil and clinical isolates (P=0.13). The MEAI for SAF1, SFS1, SES1, SBS1, and SCS1 was 1.47, 1.47, 1.13, 1.08, and 1.09, respectively (Table 1).
Figure 3. Extracellular enzyme activities of molds isolated from keratomycosis and soil.
A: Lipase on tributyrin agar; B: DNase on DNase test agar; C: α-Amylase on starch agar; D: Protease on skim milk agar; E: Cellulase on PDA with carboxymethyl cellulose; F: Pectinase on Czapek–Dox agar with pectin.
Table 1. Extracellular enzyme activity of the fungal isolates from keratomycosis and soil.
| S. No | Fungal Isolates | Isolates with lipase activity (%) | Lipase MEAI | Isolates with DNase activity (%) | DNase MEAI | Isolates with α-Amylase activity (%) | α-Amylase MEAI | Isolates with protease activity (%) | Protease MEAI | Isolates with pectinase activity (%) | Pectinase MEAI | Isolates with cellulase activity (%) | Cellulase MEAI |
| 1 | Fusarium spp. (n=30) | 93 | 1.12±0.38 | 93 | 1.21±0.36 | 96 | 1.18±0.26 | 73 | 0.82±0.56 | 93 | 1.07±0.30 | 83 | 0.97±0.45 |
| 2 | Non sporulating molds (n=9) | 100 | 1.37±0.30 | 66 | 0.92±0.73 | 66 | 0.77±0.59 | 66 | 0.74±0.56 | 100 | 1.28±0.12 | 77 | 0.99±0.63 |
| 3 | Aspergillus flavus (n=6) | 100 | 1.43 ± 0.22 | 83 | 1.16±0.57 | 100 | 1.21±0.10 | 50 | 0.56±0.62 | 100 | 1.26±0.22 | 50 | 0.6±0.66 |
| 4 | Bipolaris spp. (n=6) | 100 | 1.27± 0.16 | 50 | 0.67±0.75 | 100 | 1.18±0.10 | 83 | 0.88±0.43 | 100 | 1.31±0.13 | 66 | 0.75±0.60 |
| 5 | Exserohilum spp. (n=4) | 100 | 1.26± 0.13 | 25 | 0.30±0.62 | 100 | 1.14±0.18 | 100 | 1.08±0.03 | 75 | 0.82±0.55 | 25 | 0.26±0.53 |
| 5 | Curvularia spp. (n=3) | 100 | 1.21±0.11 | 100 | 1.12±0.04 | 100 | 1.15±0.16 | 66 | 0.69±0.60 | 66 | 0.85±0.75 | 66 | 0.78±0.68 |
| 6 | Alternaria spp. (n=1) | 100 | 1.32±0 | 100 | 1.28±0 | 100 | 1.12±0 | 0 | 0±0 | 100 | 1.15±0 | 0 | 0±0 |
| 7 | Exophiala spp. (n=1) | 100 | 1.11±0 | 100 | 1.45±0 | 100 | 1.39±0 | 0 | 0±0 | 100 | 1.15±0 | 100 | 1.20±0 |
| 8 | SAF1 (n=1) | 100 | 1.47±0 | 100 | 1.40±0 | 100 | 1.23±0 | 100 | 1.27±0 | 100 | 1.47±0 | 100 | 1.40±0 |
| 9 | SFS1 (n=1) | 100 | 1.47±0 | 100 | 1.37±0 | 100 | 1.40±0 | 100 | 1.57±0 | 100 | 1.38±0 | 100 | 1.31±0 |
| 10 | SES1(n=1) | 100 | 1.13±0 | 100 | 1.16±0 | 100 | 1.32±0 | 100 | 1.05±0 | 100 | 1.16±0 | 100 | 1.16±0 |
| 11 | SBS1 (n=1) | 100 | 1.08±0 | 100 | 1.16±0 | 100 | 1.14±0 | 100 | 1.16±0 | 100 | 1.11±0 | 100 | 1.18±0 |
| 12 | SCS1 (n=1) | 100 | 1.09±0 | 100 | 1.13±0 | 100 | 1.11±0 | 100 | 1.11±0 | 100 | 1.14±0 | 100 | 1.13±0 |
DNase enzyme activity was observed in 48 (80%) isolates (Figure 3B). Precisely, 12 (20%) of the isolates showed negative result. All the isolates of Curvularia spp., Alternaria spp., and Exophiala spp., showed DNase activity, whereas only 28 (93%) Fusarium spp., 6 (66%) non sporulating molds, 5 (83%) of Aspergillus flavus, 3 (50%) of Bipolaris spp. and 1 (25%) of Exserohilum spp. exhibited the DNase activity. Compared to the other fungal isolates, DNase activity was least in Exserohilum spp. ES1 alone had the DNase activity with EAI 1.23, whereas DNase activity was nil in all the other isolates (ES2-ES4). MEAI of DNase for Fusarium spp., non sporulating molds, Aspergillus flavus, Bipolaris spp., Exserohilum spp., Curvularia spp., Alternaria spp., Exophiala spp. and soil isolates are given in Table 1. There was no significant difference in the DNase activity indices of the soil and clinical isolates (P=0.21).
Out of 60 isolates of corneal molds screened, 56 (93%) isolates showed the α-amylase activity (Figure 3C). Only one isolate (2%) of Fusarium spp. and 3 (5%) isolates of non sporulating molds did not show enzyme activity on starch agar plates. FS14 had the highest α-amylase activity (MEAI 1.63); Curvularia spp. (CS3) had the lowest α-amylase activity (MEAI 1.03); The EAI for soil isolates SAF1, SFS1, SES1, SBS1, and SCS1 was 1.23, 1.40, 1.32, 1.14, and 1.11, respectively (Table 1). There was no significant difference in the α-amylase activity indices of the soil and clinical isolates (P=0.3).
Exactly, 42 (70%) isolates showed protease activity on skim milk agar (Figure 3D). Specifically, 22 (73%), 6 (66%), 3 (50%), 5 (83%), 4 (100%) and 2 (66%) of Fusarium spp., non sporulating molds, Aspergillus flavus, Bipolaris spp., Exserohilum spp. and Curvularia spp., respectively showed protease activity. The protease activity was absent in Alternaria spp. and Exophiala spp. Fusarium spp. (FS9) had the highest protease activity (MEAI 1.38); Curvularia spp. (CS1 and CS2) and Bipolaris spp. (BS2) had the lowest protease activity (MEAI 1.04). There was a statistically significant difference in the protease activity indices of the soil and clinical isolates (P=0.01). About, 43 (71%) isolates of fungi showed cellulase activity (Figure 3E). A total of 17 (28%) isolates did not produce zone and was considered negative for the cellulase production. Highest cellulase activity was observed in NSM7 with the enzyme activity index 1.98. FS29 and NSM3 showed lowest cellulase activity with MEAI 1.05. The mean cellulase activity index for the soil isolates were SAF1, SFS1, SES1, SBS1 and SCS1 was 1.40, 1.31, 1.16, 1.18, and 1.13, respectively. There was a statistically significant difference in the cellulase activity indices of the soil and clinical isolates (P=0.01).
The pectinolytic activity was determined in 56 (93%) isolates (Figure 3F). Only 2 (3%) isolates of Fusarium spp., 1 (2%) isolate each of Exserohilum spp. and Curvularia spp. did not show pectinase activity. The highest pectinase activity was observed in non sporulating mold (NSM5) (MEAI 1.76). Fusarium spp. (FS5) showed the lowest MEAI of 1.04 for pectinase. SAF1, SFS1, SES1, SBS1, and SCS1 had mean pectinase activity index of 1.47, 1.38, 1.16, 1.11 and 1.14, respectively (Table 1). There was no significant difference in the pectinase activity indices of the soil and clinical isolates (P=0.19).
DISCUSSION
The rate of positive culture in microbial keratitis ranges from 52% to 68% but depends on the severity of the ulcer and the criteria established for positive culture[1]. In India, nearly 30%-35% of all culture positive infectious keratitis are caused by fungi[1]. Laboratory diagnosis mainly depends upon proper collection and transport of clinical specimens. Corneal scraping is obtained usually by Kimura's spatula, under a slit lamp examination, after anaesthetizing the cornea with topical anesthetic like 0.4% proparcaine. Corneal biopsy is done by a minor trephining and anterior chamber (AC) aspirate using a sterile tuberculin syringe[18]. In the present study, among the 60 isolates, Fusarium spp. (50%) was the most common fungus isolated, followed by non sporulating molds (15%), Aspergillus flavus (10%), Bipolaris spp. (10%), Exserohilum spp. (6.6%), Curvularia spp. (5%), Alternaria spp. (1.6%), Exophiala spp. (10%). Srinivasan [19] reported that out of 155 fungal isolates cultured from 154 corneal ulcers 47.1% were Fusarium spp., 16.1% were Aspergillus spp, and the remaining organisms were a diverse mixture of unusual fungal pathogens including a large number of unidentified dematiacious fungal species (13.5%) and hyaline fungal species (9.6%). The pattern of fungal organisms, dominated by Fusarium spp., is similar to the spectrum of microbial keratitis reported from south Florida by Liesegang and Forster[4] and from Ghana by Hagan et al[5]. In south Florida 35% of the isolated organisms were fungi with Fusarium spp. accounting for 61%[4]. By contrast, in most of the world Aspergillus spp. or Candida spp. are the predominant fungal pathogens responsible for mycotic keratitis[20]. In the temperate climate of Nepal, Upadhyay et al[21] found that Aspergillus spp. accounted for 47% of all fungal pathogens followed by Candida spp. (13.2%) and Fusarium spp. (11.7%).
In the present study, extracellular lipase, α-amylase, DNase, protease, pectinase, and cellulase were screened; these enzymes may play an essential role in the tissue invasiveness[22]. Most of our isolates are plant pathogens and hence, to determine the relationship between the plant invading enzymes synthesized by the ocular isolates and the soil isolates, the extracellular enzyme activities were screened. Most of our isolates showed high EAI for these enzymes. The EAI for soil isolates are more are less similar with the clinical isolates. The significance of the EAI in environmental samples is still unknown. Link between strains with higher EAI and cases of invasive diseases, are yet to be determined.
However, the exact source and nature of proteolytic damage occurring to the cornea or the enzymology of tissue degrading following fungal infection has so far not been investigated. It remains to be studied whether fungal or tissues derived products mediate pathogenesis of fungal keratitis. Therefore only initial screening of extracellular enzymes produced in vitro by corneal isolates would be beneficial. In addition to rendering infected cornea free of fungal elements, inhibition of proteolytic activities of the cornea would be an attractive approach in the management of fungal keratitis[23]. Heat tolerance, growth rate, conidium size, adhesins, pigment production, toxic metabolites and extracellular enzymes are thought to be putative virulence factors of clinically important fungi[24]–[26]. Since host cell membranes are composed of lipids and proteins, extracellular enzymes of Fusarium species may play an important role in the invasion of the host tissues[27]. Gopinathan et al[23] have characterized the extracellular proteases produced in vitro by corneal fungal pathogens namely the Aspergillus favus and Fusarium solani when collagen was provided as the sole nitrogen source. Zhu et al[28] has reported the possible role of the extracellular proteases of a clinical isolate of Aspergillus flavus, from a severe case of keratitis, in the pathogenesis of fungal keratitis. Comparison of total phospholipase, phospholipase C and phospholipid acyl hydrolase activities between clinical and environmental isolates of A. fumigatus by Birch et al[29] revealed that, clinical A. fumigatus isolates produced more phospholipase C than environmental isolates. Alp and Arikan[8] detected phospholipase activity in all strains belonging to A. fumigatus and high phospholipase producer A. fumigatus isolates were found to be associated with development of invasive aspergillosis. Thus, the results of our study suggest that, the existence and degree of the activities of the extracellular enzymes in clinical isolates of fungal isolates tend to strain-based variations.
The molds isolated from mycotic keratitis appear to have the ability to produce many extracellular enzymes. Out of 60 fungal isolates from 108 corneal samples collected, it was noted that 58 (96.66%), 48 (80%), 56 (93%), 42 (70%), 43 (71%) and 56 (93%) molds were able to produce lipase, DNase, α-amylase, protease, cellulase and pectinase, respectively. Further, there was significant difference in the protease and cellulase activity (P=0.01) indices of the soil and clinical isolates. The impact of other factors on pathogenicity of fungal isolates and molecular determinants of virulence also remain to be further investigated.
Acknowledgments
Foundation: Partially supported by the University Grants Commission (UGC), Bahadur Shah Zafar Marg, New Delhi -110 002, India [F. No. 42–469/2013 (SR)].
Conflicts of interest: Mythili A, None; Babu Singh YR, None; Priya R, None; Shafeeq Hassan A, None; Manikandan P, None; Panneerselvam K, None; Narendran V, None; Shobana CS, None
REFERENCES
- 1.Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M. Etiology of suppurative corneal ulcers in Ghana and South India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86(11):1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacob P, Gopinathan U, Sharma S, Rao GN. Calcium alginate swab versus Bard Parker blade in the diagnosis of microbial keratiits. Cornea. 1995;14(4):360–364. doi: 10.1097/00003226-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Laila A, Salam MA, Hasan B, Begum N, Ahmed I. Etiological agents of suppurative corneal ulcer: Study of 56 cases. Bangladesh J Med Microbiol. 2009;3(1):33–36. [Google Scholar]
- 4.Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90(1):38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 5.Hagan M, Wright E, Newman M, Dolin P, Johnson G. Causes of suppurative keratitis in Ghana. Br J Ophthalmol. 1995;79(11):1024–1028. doi: 10.1136/bjo.79.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth C. Perfect states (telemorphs) of Fusarium species. In: Nelson PE, Toussoun TO, Cook R, editors. Fusarium: Disease, biology taxonomy. University Park. P: Pennsylvania state University press; 1981. pp. 446–452. [Google Scholar]
- 7.Tilak R, Singh A, Maurya OPS, Chandra A, Tilak V, Gulati AK. Mycotic keratitis in India: A five-year retrospective study. J Infect Dev Ctries. 2010;4(3):171–174. doi: 10.3855/jidc.309. [DOI] [PubMed] [Google Scholar]
- 8.Alp S, Arikan S. Investigation of extracellular elastase, acid proteinase & phospholipase activities as putative virulence factors in clinical isolates of Aspergillus species. J Basic Microbiol. 2008;48(5):1–7. doi: 10.1002/jobm.200700349. [DOI] [PubMed] [Google Scholar]
- 9.Harris JL. Safe, low-distortion tape touch method for fungal slide mounts. J Clin Microbiol. 2000;38(12):4683–4684. doi: 10.1128/jcm.38.12.4683-4684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larone DH. Medically Important Fungi: A guide to identification. 4th ed. Washington DC: American Society for Microbiology Press; 2002. [Google Scholar]
- 11.Leslie JF, Summerell BA, Bullock S. The Fusarium laboratory manual. Ames, Iowa, USA: Blackwell Publishers; 2006. [Google Scholar]
- 12.Prabakaran M, Thennarasu V, Mangala RA, Bharathidasan R, Chandrakala N, Mohan N. Comparative studies on the enzyme activities of wild and mutant fungal strains isolated from sugarcane field. Indian J Sci Technol. 2009;2(11):46–49. [Google Scholar]
- 13.Sanchez M, Colom F. Extracellular DNase activity of Cryptococcus neoformans and Cryptococcus gattii. Rev Iberoam Micol. 2010;27(1):10–13. doi: 10.1016/j.riam.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Sharma J, Singh A, Kumar R, Mittal A. Partial purification of an alkaline protease from a new strain of Aspergillus oryzae AWT 20 and its enhanced stabilization in entrapped Ca Alginate beads. Int J Microbiol. 2006;2(2) [Google Scholar]
- 15.Gautam SP, Bundela PS, Pandey AK, Awathi MK, Sarsaiya S. Screening of cellulolytic fungi for management of municipal solid waste. Journal of Applied Science in Environmental Sanitation. 2010;5(3):361–367. [Google Scholar]
- 16.Okafor UA, Okaochi VI, Chinedu SN, Ebuehi OAT, Onygeme-Okerenta BM. Pectinolytic activity of wild type filamentous fungi fermented on agro wastes. Afr J of Microbiol Research. 2010;4(24):2729–2734. [Google Scholar]
- 17.Maria GL, Sridhar KR, Raviraja NS. Antimicrobial and enzyme activity of mangrove endophytic fungi of southest coast of India. J Agric Technol. 2005;1(1):67–80. [Google Scholar]
- 18.Nayak N. Fungal infections of the eye-laboratory diagnosis and treatment. Nepal Med Coll J. 2008;10(1):48–63. [PubMed] [Google Scholar]
- 19.Srinivasan M. Use of traditional eyemedicines by corneal ulcer patients presenting to a hospital in South India. Ind J Ophthalmol. 2009;47(1):15–18. [PubMed] [Google Scholar]
- 20.Jones BR. Principles in the management of oculomycosis. Am J Ophthalmol. 1975;79(5):719–751. doi: 10.1016/0002-9394(75)90730-8. [DOI] [PubMed] [Google Scholar]
- 21.Upadhyay MP, Karmacharya PC, Koirala S, Tuladhar N, Bryan LE, Smolin G. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol. 1991;111(1):92–99. doi: 10.1016/s0002-9394(14)76903-x. [DOI] [PubMed] [Google Scholar]
- 22.Blanco J, Hontecillas R, Bouza E, Blanco I, Pelaez T, Munoz P, Molina JP, Garcia ME. Correlation between the Elastase Activity Index and Invasiveness of Clinical Isolates of Aspergillus fumigatus. J Clin Microbiol. 2002;40(5):1811–1813. doi: 10.1128/JCM.40.5.1811-1813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gopinathan U, Ramakrishna T, Wilcox M, Rao M, Balasubramanian S, Kulkarni A, Vemuganti GK, Rao GN. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Exp Eye Res. 2001;72(4):433–442. doi: 10.1006/exer.2000.0971. [DOI] [PubMed] [Google Scholar]
- 24.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12(2):310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouchara JP, Tronchin G, Larcher G, Chabasse D. The search for virulence determinants in Aspergillus fumigatus. Trends Microbiol. 1995;3(8):327–330. doi: 10.1016/s0966-842x(00)88965-9. [DOI] [PubMed] [Google Scholar]
- 26.Tomee JF, Kauffman HF. Putative virulence factors of Aspergillus fumigatus. Clin Exp Allergy. 2000;30(4):476–484. doi: 10.1046/j.1365-2222.2000.00796.x. [DOI] [PubMed] [Google Scholar]
- 27.Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol Rev. 2000;13(1):122–143. doi: 10.1128/cmr.13.1.122-143.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu WS, Wojdyla K, Donlon K, Thomas PA, Eberle HI. Extracellular proteases of Aspergillus favus. Fungal keratitis, proteases, and pathogenesis. Diagn Microbiol Infect Dis. 1990;13(6):491–497. doi: 10.1016/0732-8893(90)90081-6. [DOI] [PubMed] [Google Scholar]
- 29.Birch M, Denning DW, Robson GD. Comparison of extracellular phospholipase activities in clinical and environmental Aspergillus fumigatus isolates. Med Mycol. 2004;42(1):81–86. doi: 10.1080/13693780310001610029. [DOI] [PubMed] [Google Scholar]