Abstract
AIM
To determine the safety of prophylactic intracameral moxifloxacin after cataract surgery in patients with penetrating keratoplasty (PKP).
METHODS
In this retrospective study of consecutive patients who had phacoemulsification cataract surgery after PKP, were treated with intracameral moxifloxacin 0.5% ophthalmic solution (0.5 mg/0.1 mL). The main outcome measures were anterior chamber reaction, best corrected visual acuity (BCVA), corneal endothelial cell count (ECC), and central corneal thickness (CCT).
RESULTS
Fifty-five patients were recruited (26 males, 29 females). The mean age was 54.36±4.97y (range 45-64y). All eyes had improved postoperative BCVA. The mean BCVA was 0.25 preoperatively and 0.57 postoperatively, which was statistically significant (P<0.001). One eye had 3+, 7 eyes had 2+, 12 eyes had 1+ and 8 eyes had trace amount of aqueous cells on the first day after surgery. All eyes had no anterior chamber cells at subsequent follow up examinations. Effective phacoemulsification time was 4.33±1.01s. The mean ECC was 2340.20 cells/mm2 preoperatively and 1948.75 cells/mm2 1mo postoperatively (P<0.001). The increase of 21.09 µm in postoperative pachymetry 1mo after surgery was statistically significant (P<0.001).
CONCLUSION
No untoward effects were observed after intracameral injection of moxifloxacin (0.5 mg/0.1 mL) in terms of anterior chamber reaction, CCT, ECC, and visual rehabilitation at the conclusion of cataract surgery in patients with PKP.
Keywords: cataract surgery, intracameral injection, moxifloxacin, penetrating keratoplasty, phacoemulsification
INTRODUCTION
Cataract surgery is one of the most common surgical procedures performed in the world. Although the complications of this surgery are relatively rare, endophthalmitis is a serious and potentially vision-threatening complication, with only 35% of patients achieving visual acuity better than ≥20/200[1].
Several approaches have been advocated to decrease the rate of postoperative endophthalmitis. Preoperative povidone–iodine antisepsis combined with preoperative and postoperative topical antibiotic therapy is considered the standard of care[2]–[4]. Increasing attention is drawn to the value of an intracameral injection of antibiotic at the end of cataract surgery to provide immediate, high antibiotic levels that are sustained for a period of time. Among the antibiotics given intracamerally, cefuroxime, vancomycin and moxifloxacin are the most commonly used[5],[6].
Moxifloxacin is a fourth-generation fluoroquinolone antibacterial agent that is active against many gram-positive, gram-negative, atypical, and anaerobic ocular pathogens. Considering the possible complications with vancomycin and cefuroxime, moxifloxacin seems to be the better choice of antibiotic for endophthalmitis prophylaxis because of its broad-spectrum coverage and mode of action[7],[8].
To our knowledge, this is the first report of a topical ophthalmic preparation applied as a prophylactic agent intracamerally after cataract surgery in patients with penetrating keratoplasty (PKP). Our study evaluated the safety of injecting prophylactic intracameral moxifloxacin in human eyes having cataract surgery in patients with PKP.
SUBJECTS AND METHODS
This retrospective study included eyes of consecutive patients who had phacoemulsification cataract surgery after PKP for corneal pathology at the Department of Ophthalmology, Istanbul University Cerrahpasa Medical Faculty, between May 2008 and February 2012. All patients had a follow-up of 1mo and signed an informed consent document. The study was in accordance with the tenets of the Helsinki Declaration.
Selection Criteria
Patients with previous PKP due to corneal pathology (herpes stromal scar (n=19), stromal dystrophy (n=17), keratokonus (n=10), fuchs dystrophy (n=9) requiring cataract surgery were included. Grade of nuclear sclerosis was N2 and N3. Cataract surgery was performed at least 3mo after PKP in all patients. Patients were over 18y of age. Patients with glaucoma, uveitis, media opacities other than cataract (cornea or vitreous) and visual pathway problems and patients taking prostaglandin analogue agents, systemic immunosuppressants or anticoagulants and cataract surgery cases with intraperative complications including posterior capsule rupture, vitreous loss and/or prolonged surgery time were excluded from the study. Any patients who had a prior eye operation except keratoplasty were also excluded.
Surgical Technique
All surgeries (penetrating keratoplasty and cataract) were performed by the same surgeon (O.S.A.). Penetrating keratoplasty surgeries were performed under local or general anesthesia. All recipient and donor corneas were trephined with hand-held trephines. Graft diameter ranged from 7.5-7.75 mm, and the host diameter was 0.25 mm smaller, except in cases of keratoconus (same size). Donor cornea was sutured with 12-16 interrupted and a single continuous sutures using 10-0 nylon.
Cataract surgery was performed under topical anesthesia. Pupils were dilated with 1% cyclopentolate, 1% tropicamide and 2.5% phenylephrine drops. All surgeries were performed using topical anesthesia of proparacaine (Alcaine). Uneventful phacoemulsification was performed in a standardized fashion through a 2.75 mm clear corneal incision. Sodium hyaluronate 3.0%-chondroitin sulfate 4.0% (Viscoat) and sodium hyaluronate 1.0% (Provisc) were used for endothelial protection, anterior chamber stabilization for continuous curvilinear capsulorhexis and intraocular lens (IOL) implantation respectively. AcrySof IQ SA60AT intraocular lenses (Alcon Laboratories Inc., Fort Worth, Texas) were implanted.
All patients underwent periorbital antisepsis with gauze soaked povidone-iodine 10% application on the closed lids, eyelashes, brow and the adjacent forehead, nose, cheeks, and temporal orbital area for 3min. The conjunctival sac was vigorously irrigated with 5 mL of povidone iodine 5% solution just prior to surgery after 3min, conjunctival sac was irrigated with 30 mL balanced salt solution.
Towards the end of the operation, the contents of a newly opened bottle of moxifloxacin ophthalmic solution 0.5% (Vigamox) was aspirated by a nurse into a sterile tuberculin syringe, a volume slightly in excess of 0.1 mL (0.3-0.4 mL) of the pure moxifloxacin 0.5% ophthalmic solution. No solution, including saline, was added to dilute the commercial preparation. The excess amount was discarded, leaving 0.5 mg/0.1 mL of the nonpreserved moxifloxacin in the tuberculin syringe ready for injection into the anterior chamber. The solution prepared in the syringe was injected using a 27-gauge cannula through the side port into the capsular bag after hydration of the side ports and water tightness of corneal incisions controlled. Moxifloxacin ophthalmic solution (Vigamox 0.5%; Alcon Laboratories Inc., Fort Worth, Texas) supplied as a sterile isotonic solution, with pH at 6.8 and osmolality of 290 mOsm/kg, making it compatible with the humans anterior chamber fluid (pH 7.4; osmolality 305 mOsm/kg).
Postoperative topical Vigamox 0.5% was instilled every 2h while awake on the day of the surgery and then tapered to 6 times a day for 1wk and 4 times a day for subsequent 2wk. Topical prednisolone acetate 1% (Pred Forte) was also given postoperatively using the same dosage schedule used for Vigamox.
Examinations
The patients were scheduled for follow-up 1d, 1wk, 1mo after surgery. All patients had a complete eye examination including best corrected visual acuity (BCVA) (LogMar as a decimal), slitlamp examination, anterior chamber reaction (cells) and central corneal thickness (CCT) measurement with ultrasound pachymetry on the day of surgery and at postoperative follow-up. Specular microscopy was performed using a Noncon Robo (Konan Medical) noncontact specular microscope. The image of the endothelium was obtained on an incorporated screen. After a clear image of the central endothelium was captured, the centers of at least 100 contiguous endothelial cells were marked. The endothelial cell count (ECC) and other cell parameters were then displayed on the computer screen. The microscopy was repeated 3 times for each measurement, and the mean value was used for analysis. Specular microscopy of the donor corneal endothelium and CCT was performed preoperatively and repeated postoperatively at 1mo.
Anterior chamber cells were measured using a 2 mm long and 1 mm wide slit beam with maximal light intensity and magnification before mydriasis and scored as follows: trace=1 to 5 cells; +1=6 to 15 cells; +2=16 to 25 cells; +3=26 to 50 cells; +4=more than 50 cells.
Statistical Analysis
Data analysis was performed using SPSS software (version 15.0, SPSS, Inc.). Preoperative and postoperative comparisons of CCT and ECC were performed with the paired samples test. BCVA was studied with Wilcoxon signed-rank test. A P value less than 0.05 was considered statistically significant.
RESULTS
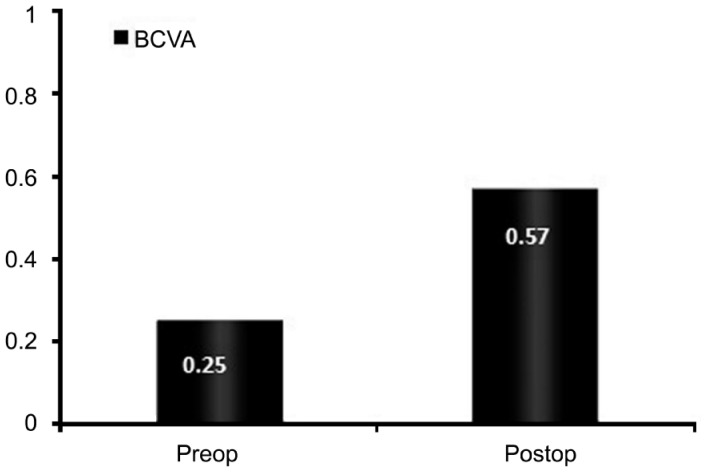
Fifty-five eyes of 55 patients (26 males, 29 females) were included in the study. The mean age was 54.36±4.97y (range 45-64y). All eyes had improved postoperative BCVA. The mean BCVA was 0.25±0.11 preoperatively and 0.57±0.24 postoperatively, which was statistically significant (P<0.001) (Figure 1). No postoperative epithelial defects was observed. No eye had corneal edema on the first day postoperatively, except for 3 eyes, which had mild corneal stromal edema. No eye had corneal edema at subsequent follow-up examinations. One day after cataract surgery, 1 eye had 3+, 7 eyes had 2+, 12 eyes had 1+ and 8 eyes had trace amount of aqueous cells. All eyes had no anterior chamber cells at subsequent follow up examinations. Effective phacoemulsification time (EPT) was 4.33±1.01s (range 2.5-5.9s).
Figure 1. Mean preoperative and 1mo postoperative best corrected visual acuity (BCVA).
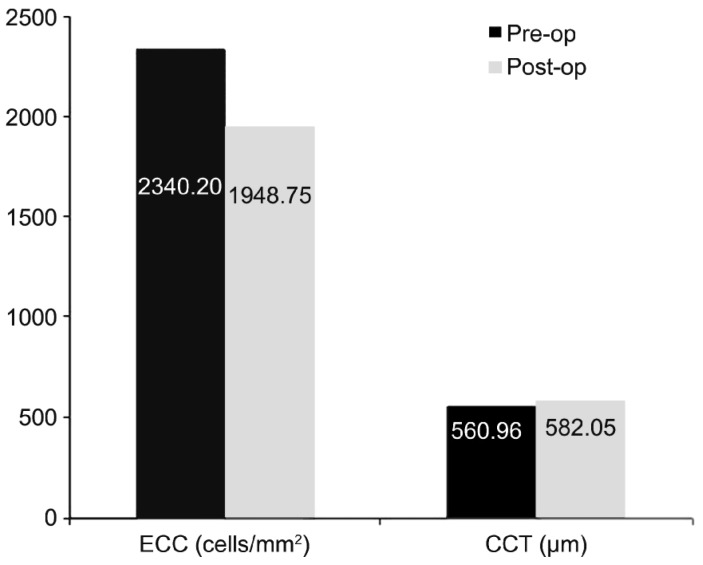
The mean ECC was 2340.20±187.21 cells/mm2 preoperatively and 1948.75±246.76 cells/mm2 1mo postoperatively. The ECC was statistically significantly lower than preoperatively (P<0.001). The mean CCT was 560.96±13.22 µm preoperatively and 582.05±17.21 µm 1mo postoperatively; the difference was statistically significant (P<0.001) (Figure 2).
Figure 2. Mean preoperative and 1mo postoperative endothelial cell count (ECC) (cells/mm2) and central corneal thickness (CCT) (µm).
DISCUSSION
Age-related cataract is one of the leading causes of visual impairment worldwide and cataract extraction is one of the most frequently performed surgical procedures in the world. Endophthalmitis is an uncommon but serious intraocular infection that occurs most commonly as a complication of intraocular surgery and often causes severe visual impairment or even the loss of an eye[9].
In spite of improvements in microsurgical and aseptic techniques, a study of Medicare patients who underwent cataract extraction from 1994 to 2001 reported a an increased rate of postoperative endophthalmitis in the study period[10]. A systematic review also reported evidence of an increasing rate[11]. Several studies suggest that the route of antibiotic administration may be among the most important factors in preventing postoperative endophthalmitis[3],[5],[6].
Antibiotic penetration into the anterior chamber after topical drops is relatively low compared with intracameral doses, restricted by an intact corneal epithelium and dilution and removal by tears[12]. Direct intracameral injection of antibiotic has several advantages over topical drop regimens. This route of injection delivers very high concentrations of an antibiotic agent to the anterior chamber at the close of surgery with the presumed effect of eradicating bacteria before wound closure and in the immediate postoperative period. Commonly used dosage schedules such as 1 drop 4 times daily, even with an additional drop, produced therapeutic moxifloxacin levels up to 1.9 µg/mL in the aqueous humor[13]. Preoperative dosing schedules with more frequent drop instillation, ranging from 1 drop every 10min for one hour before surgery and every 2h for 3d before surgery produced aqueous humor levels 1.58 µg/mL and 2.3 µg/mL respectively[14],[15].
In our study, the 500 µg dose of moxifloxacin used is expected to produce an aqueous humor concentration of approximately 500 µg/0.525 mL (the approximate fluid capacity of the combined anterior and posterior chambers after crystalline lens extraction with an IOL implanted in the capsular bag is 0.525 mL) or comparative concentrations of 952 µg/mL[16].
These peak aqueous humor levels in the anterior chamber after injection was approximately more than 3000-fold above the tested MIC50 (minimum inhibitory concentration) of moxifloxacin against common ocular pathogens such as Staphylococcus aureus (0.03 µg/mL), Staphylococcus epidermidis (0.06 µg/mL), Propionibacterium acnes (0.25 µg/mL), and Bacillus cereus (0.13 µg/mL)[8].
Moxifloxacin is a fourth-generation fluoroquinolone antibiotic agent. Compared with earlier generations of fluoroquinolones such as ciprofloxacin, ofloxacin, and levofloxacin, moxifloxacin offers broader spectrum and more potent activity against gram-positive bacteria. it is also rapidly bactericidal against many ocular isolates resistant to older fluoroquinolones[8],[17]. Moxifloxacin is commercially available as a self-preserved ophthalmic formulation, containing no benzalkonium chloride (BAK) or other preservatives known to have toxic effects on ocular tissues[18]. As a self preserved solution, Vigamox ophthalmic solution requires no special preparation for intracameral injection, reducing the risk for toxic anterior segment syndrome (TASS). In addition, some studies of human beings did not show intraocular toxicity after injection of intracameral moxifloxacin[16],[19],[20]. Vancomycin and cefuroxime is available in a systemic preparation that must be reconstituted using saline solution before it can safely be delivered to the eye. Reconstituting the drug for intracameral use may increase the risk for TASS because an undesired concentration of the drug may be inadvertently injected if a mistake occurs during the preparation or dilution process[21].
In our study, statistically significant evidence of reduced endothelial cells, increased corneal thickness and increased BCVA was found 1mo postoperatively compared to preoperatively.
We excluded patients if they had complications that could be the possible causes of endothelial cell loss after keratoplasty or cataract surgery including graft rejection, glaucoma, uveitis, and corneal ulceration.
In healthy eyes that have not undergone operation, endothelial cell density declines at about 0.6% per year[22]. After cataract extraction, the rate increases to 2.5% per year from 1-10y after operation, whether or not an intraocular lens was implanted[23].
Shimmura et al[24] reported the average loss of endothelial cells before and 3mo after cataract surgery in PKP was 15.7%±17.3% (phacoemulsification and IOL implantation were scheduled for approximately 3mo after PKP (average 4.2mo), and in the study by Kim EC and Kim MS[25], endothelial cell loss rate was reported 19.03% in eyes with previous PKP and 7.91% in eyes with normal corneas 1mo after cataract surgery. In the same study, the period between keratoplasty and cataract surgery was 15.72±5.96mo with a range from 9 to 23mo. In the present study, percentage of corneal endothelial cell loss after phacoemulsification in transplanted cornea was 16.7%±8.7% at 1mo. So, we believe that similar results show intracameral Vigamox 0.5 mg/0.1 mL appeared to be nontoxic in terms of endothelial cell density.
No cells and flare were detected in all eyes at the 1wk postoperatively similar to the study of Espiritu et al[16], showed that Vigamox causes no inflammation in the anterior chamber and vitreus.
Espiritu et al[16] and Lane et al[19] injected an intracameral dose of 0.5 mg/0.1 mL and 0.25 mg/0.05 mL of the commercially available undiluted moxifloxacin 0.5% ophthalmic solutions, respectively, whereas Arbisser[6] used the moxifloxacin 0.5% drop solution diluted with balanced salt solution to a 0.1 mg/0.1 mL dose for intracameral injection. All of these studies showed no increased safety risk associated with prepared doses of intracameral injection of moxifloxacin, which appears to be safe in the prophylaxis of endophthalmitis after cataract surgery. In the present study, we used a solution of Vigamox drops, injecting a dose of 0.5 mg/0.1 mL after cataract surgery in patients with PKP. The results in our study support to the safety profile of an intracameral injection of moxifloxacin for the prophylaxis of endophthalmitis.
In conclusion, the findings in our study support the safety of an intracameral injection of Vigamox (0.5 mg/0.1 mL) in terms of anterior chamber reaction, CCT, ECC and visual rehabilitation. This study established that Vigamox can safely be given intracamerally after cataract surgery in patients with PKP. However, follow-up was for only 1mo. Further studies to prove its effectiveness in preventing endophthalmitis and longer follow-up are required.
Acknowledgments
Conflicts of Interest: Arslan OS, None; Arici C, None; Unal M, None; Cicik E, None; Mangan MS, None; Atalay E, None
REFERENCES
- 1.Al-Mezaine HS, Kangave D, Al-Assiri A, Al-Rajhi AA. Acute-onset nosocomial endophthalmitis after cataract surgery: incidence, clinical features, causative organisms, and visual outcomes. J Cataract Refract Surg. 2009;35(4):643–649. doi: 10.1016/j.jcrs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Carrim ZI, Mackie G, Gallacher G, Wykes WN. The efficacy of 5% povidone-iodine for 3 minutes prior to cataract surgery. Eur J Ophthalmol. 2009;19(4):560–564. doi: 10.1177/112067210901900407. [DOI] [PubMed] [Google Scholar]
- 3.Vazirani J, Basu S. Role of topical, subconjunctival, intracameral, and irrigative antibiotics in cataract surgery. Curr Opin Ophthalmol. 2013;24(1):60–65. doi: 10.1097/ICU.0b013e32835a93be. [DOI] [PubMed] [Google Scholar]
- 4.Gordon-Bennett P, Karas A, Flanagan D, Stephenson C, Hingorani M. A survey of measures used for the prevention of postoperative endophthalmitis after cataract surgery in the United Kingdom. Eye (Lond) 2008;22(5):620–627. doi: 10.1038/sj.eye.6702675. [DOI] [PubMed] [Google Scholar]
- 5.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39(1):8–14. doi: 10.1016/j.jcrs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 6.Arbisser LB. Safety of intracameral moxifloxacin for prophylaxis of endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34(7):1114–1120. doi: 10.1016/j.jcrs.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Yoeruek E, Spitzer MS, Saygili O, Tatar O, Biedermann T, Yoeruek E, Bartz-Schmidt KU, Szurman P. Comparison of in vitro safety profiles of vancomycin and cefuroxime on human corneal endothelial cells for intracameral use. J Cataract Refract Surg. 2008;34(12):2139–2145. doi: 10.1016/j.jcrs.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Stroman DW, Dajcs JJ, Cupp GA, Schlech BA. In vitro and in vivo potency of moxifloxacin and moxifloxacin ophthalmic solution 0.5%, a new topical fluoroquinolone. Surv Ophthalmol. 2005;50(Suppl 1):S16–S31. doi: 10.1016/j.survophthal.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Durand ML. Endophthalmitis. Clin Microbiol Infect. 2013;19(3):227–234. doi: 10.1111/1469-0691.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology. 2005;112(8):1388–1394. doi: 10.1016/j.ophtha.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, McDonnell PJ. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Arch Ophthalmol. 2005;123(5):613–620. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda M, Yamada M, Kinoshita S, Inatomi T, Ohashi Y, Uno T, Shimazaki J, Satake Y, Maeda N, Hori Y, Nishida K, Kubota A, Nakazawa T, Shimomura Y. Comparison of corneal and aqueous humor penetration of moxifloxacin, gatifloxacin and levofloxacin during keratoplasty. Adv Ther. 2012;29(4):339–349. doi: 10.1007/s12325-012-0016-x. [DOI] [PubMed] [Google Scholar]
- 13.McCulley JP, Caudle D, Aronowicz JD, Shine WE. Fourth-generation fluoroquinolone penetration into the aqueous humor in humans. Ophthalmology. 2006;113(6):955–959. doi: 10.1016/j.ophtha.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 14.Lai WW, Chu KO, Chan KP, Choy KW, Wang CC, Tsang CW, Pang CP. Differential aqueous and vitreous concentrations of moxifloxacin and ofloxacin after topical administration one hour before vitrectomy. Am J Ophthalmol. 2007;144(2):315–318. doi: 10.1016/j.ajo.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 15.Hariprasad SM, Blinder KJ, Shah GK, Apte RS, Rosenblatt B, Holekamp NM, Thomas MA, Mieler WF, Chi J, Prince RA. Penetration pharmacokinetics of topically administered 0.5% moxifloxacin ophthalmic solution in human aqueous and vitreous. Arch Ophthalmol. 2005;123(1):39–44. doi: 10.1001/archopht.123.1.39. [DOI] [PubMed] [Google Scholar]
- 16.Espiritu CR, Caparas VL, Bolinao JG. Safety of prophylactic intracameral moxifloxacin 0.5% ophthalmic solution in cataract surgery patients. J Cataract Refract Surg. 2007;33(1):63–68. doi: 10.1016/j.jcrs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski RP, Dhaliwal DK, Karenchak LM, Romanowski EG, Mah FS, Ritterband DC, Gordon YJ. Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates. Am J Ophthalmol. 2003;136(3):500–505. doi: 10.1016/s0002-9394(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 18.Ayaki M, Yaguchi S, Iwasawa A, Koide R. Cytotoxicity of ophthalmic solutions with and without preservatives to human corneal endothelial cells, epithelial cells and conjunctival epithelial cells. Clin Experiment Ophthalmol. 2008;36(6):553–559. doi: 10.1111/j.1442-9071.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 19.Lane SS, Osher RH, Masket S, Belani S. Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg. 2008;34(9):1451–1459. doi: 10.1016/j.jcrs.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Kernt M, Neubauer AS, Liegl RG, Lackerbauer CA, Eibl KH, Alge CS, Ulbig MW, A AK. Intracameral moxifloxacin: in vitro safety on human ocular cells. Cornea. 2009;28(5):553–561. doi: 10.1097/ICO.0b013e318191447b. [DOI] [PubMed] [Google Scholar]
- 21.Mamalis N, Edelhauser HF, Dawson DG, Chew J, LeBoyer RM, Werner L. Toxic anterior segment syndrome. J Cataract Refract Surg. 2006;32(2):324–333. doi: 10.1016/j.jcrs.2006.01.065. [DOI] [PubMed] [Google Scholar]
- 22.Yunliang S, Yuqiang H, Ying-Peng L, Ming-Zhi Z, Lam DS, Rao SK. Corneal endothelial cell density and morphology in healthy Chinese eyes. Cornea. 2007;26(2):130–132. doi: 10.1097/ICO.0b013e31802be63e. [DOI] [PubMed] [Google Scholar]
- 23.Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology. 1994;101(6):1014–1023. doi: 10.1016/s0161-6420(94)31224-3. [DOI] [PubMed] [Google Scholar]
- 24.Shimmura S, Ohashi Y, Shiroma H, Shimazaki J, Tsubota K. Corneal opacity and cataract: triple procedure versus secondary approach. Cornea. 2003;22(3):234–238. doi: 10.1097/00003226-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Kim EC, Kim MS. A comparison of endothelial cell loss after phacoemulsification in penetrating keratoplasty patients and normal patients. Cornea. 2010;29(5):510–515. doi: 10.1097/ICO.0b013e3181c11e0e. [DOI] [PubMed] [Google Scholar]