Abstract
AIM
To compare and evaluate the phacoemulsification parameters and postoperative endothelial cell changes of two different phacoemulsification machines, each with different modes, but also to assess the relationship between postoperative endothelial cell loss and the phacoemulsification parameters, as well as the other factors in both groups.
METHODS
This prospective observational study was comprised of consecutive eligible cataract patients operated with phacoemulsification technique performed by the same surgeon using either a WHITESTAR Signature Ellips FX (transversal, group 1) or Infiniti OZil IP (torsional, group 2) machine.
RESULTS
The study included 86 patients. Baseline characteristics in the groups were similar. The median nuclear sclerosis grade was 3 (2-4) in the first group and 2 (2-4) in the second group (P=0.265). Both groups had similar phacoemulsification needle times (group 1: 60.63±36 s; group 2: 55.98±30 s; P=0.789). The percentage of endothelial cell loss 30d after surgery ranged from 3% to 15% with a median of 7% in group 1, and from 2% to 13% with a median of 6% in group 2; however, there was no statistically significant difference between the groups (P=0.407). Hexagonality (P=0.794) and the coefficient of variation (CV; P=0.142) did not differ significantly between the groups before and 30d after surgery. A significant positive correlation was found between the endothelial cell loss and nuclear sclerosis grade (group 1: P<0.001; group 2: P<0.001) and between the endothelial cell loss and average phacoemulsification power (group 1: P=0.007; group 2: P=0.008).
CONCLUSION
Both of these machines were efficient, with similar endothelial cell loss. This endothelial cell loss was related to the increased nuclear sclerosis grade and increased phacoemulsification power.
Keywords: endothelial cell changes, transversal, torsional, phacoemulsification
INTRODUCTION
Today, cataract is still one of the most important treatable causes of blindness worldwide. Phacoemulsification is currently the most commonly used procedure for cataract extraction[1]–[3]. Ultrasound (US) power required for traditional or longitudinal phacoemulsification (phaco) continues to be a risk factor for endothelial cell loss and tissue damage. Modern phaco technology aimed to reduce US power and improve efficiency[4]. Over the last few years, the main purpose of this surgery has been reduction of ultrasound energy to protect the endothelial cells of cornea. In order to reduce the amount of ultrasound energy needed, several techniques such as power modulations (pulse, burst, multiburse), improved pump systems, different disassembling techniques like quick chop, and also several infusion systems have been developed[5],[6].
Conventional phaco surgery, in which the phaco tip vibrates back and forth movement to emulsify and aspirate the lens material, uses longitudinal technology, in which the movement of the phaco tip pushes nuclear fragments away with each forward stroke, and so can result in decreased cutting efficiency.
In an attempt to increase US efficiency during phaco cataract surgery, transversal and torsional phaco technologies were introduced[7]–[10]. The main difference between these machines and the conventional phaco systems is the mechanism of energy delivery driving the movement profile of the needle tip. WhiteStar Signature Elips-FX (Abbott Medical Optics, Inc., USA) uses transversal technology, in which a straight or curved needle tip vibrating at 38 kHz can be used to generate side-to-side as well as longitudinal movement. Infiniti-OZil-IP (Alcon Laboratories, Inc., Fort Worth, TX, USA) uses torsional tecnology, in which a curved needle tip vibrating at 32 kHz generates oscilalating rotary and side-to-side movement. The lateral tip movement of torsional phaco, while moving in both directions without a repellent force, shears the lens material. This technology represents a significant improvement in the emulsifying efficiency of US during phaco [11]–[14].
Existing studies are known to have experimentally compared torsional and transversal phaco systems in terms of vacuum, surge, and thermal effect. There are limited studies, in terms of corneal endothelial changes, to compare torsional with transversal phaco.
Our study, by evaluating their intraoperative parameters, compared postoperative corneal changes of these 2 different phacoemulsification machines.
SUBJECTS AND METHODS
This prospective observational study included 86 consecutive cataractous eyes of 86 patients undergoing cataract surgery through a 2.8 mm corneal incision. Initially, we included 60 patients for each group; however, because of the fact that the patients who did not obey the regular follow up were excluded, only 35 patients in group 1 (transversal, group 1) and 51 in group 2 (torsional, group 2) were included the study. All surgeries were performed by the same surgeon (SD) at Kayseri Training and Research Hospital between October 2012 and December 2012. Our study was conducted with the approval of the ethical committee of Erciyes University Faculty of Medicine. All study proceedings were in accordance with the principles of Helsinki. Informed consent was obtained from all patients enrolled in the study.
The nuclear or corticonuclear sclerosis grade was determined I to IV according to the Lens Opacities Classification System II (LOCS). Inclusion criteria were patients 50y of age or older and the presence of a senile nuclear cataract. Preoperative exclusion criteria included: coexisting ocular disease (ocular surface, corneal, or retinal diseases) or history of trauma/surgery/inflammation, pseudoexfoliation, poor papillary dilatation, endothelial cell count (ECC) less than 1500 cells/mm, and corneal dystrophies. Intraoperative exclusion criteria included: complicated surgeries; for example, posterior capsule tear and zonular dialysis.
Preoperatively, a complete ocular examination and noncontact intraocular lens (IOL) power calculation (IOL master) were performed. Pachymetry measurements [central corneal thickness (CCT)] with a corneal topography machine (Pentacam, OCULUS Optikgerate GmbH, Wetzlar, Germany) and ECC with noncontact specular microscopy (SP 3000P, Topcon) were performed preoperatively (CCT-pre and ECC-pre) and postoperatively on the first day (CCT-post-1 and ECC-post-1) and one month (CCT-post-30 and ECC-post-30) after surgery. Corneal endothelial analysis included the measurements of the endothelial cell density (ECD), the coefficient of variation (CV), hexagonality (HEX), and endothelial cell loss (ECLoss%) [ECLoss%=(ECC-pre-ECC-post)/ECC-pre X 100].
The patients were randomly assigned to either of the machines, WhiteStar Signature® Ellips™ FX (transversal, group 1) or Infiniti OZil IP (torsional, group 2), for the surgery. For the transversal machine, group 1, chop parameters were set as follows: aspiration, 30 cc/min linear; vacuum, 400 mm Hg linear with non-zero start of 250 mm Hg; the chamber stabilization environment (CASE) vacuum at 320 mm Hg; CASE time at 500 ms; the power, continuous FX 40% linear; bottle height, 100 cm above eye level. A Signature FX phaco handpiece and 0.9 mm flared curved tip were chosen. As for the torsional machine, group 2, chop parameters were set as follows: aspiration, 32 cc/min linear; vacuum, 400 mm Hg fixed; phaco power, longitudinal 0% and torsional amplitude, 15 to 80 continuous with a dynamic rise of 3; Ozil IP power delivery option was activated to produce a 10 ms longitudinal phaco pulse when 90% vacuum was reached; bottle height, 100 cm. Ozil torsional handpiece and Kelman style curved 45° phacoemulsification tip are used. As infusion, balanced salt solution (BSS, Alcon Laboratories, Inc.) was used.
All surgeries were performed by the same surgeon (SD) under topical anesthesia, proparacaine eye drops (Alcaine 0.5%, Alcon). A standard clear corneal (at the steep meridian) incision was performed using a 2.8 mm ClearCut blade (Alcon Laboratories). One or two side ports 180° apart were created with a 1.0 mm ClearCut blade. A dispersive ophthalmic viscosurgical device (OVD), sodium hyaluronate 3.0%:chondroitin sulfate 4.0% (Viscoat ®, Alcon Laboratories) was injected into the anterior chamber and then a curvilinear rhexis of 5–5.5 mm was performed. After hydrodissection, nucleus disassembly was started using the quick-chop technique, impaling the curved phaco-tip in the central part of the nucleus (1–1.5 mm). This was followed by cortical clean-up using either coaxial or bimanual I/A handpieces and the capsular bag was expanded with cohesive OVD sodium hyaluronate 1.0% (Provisc®, Alcon Laboratories, Inc.) followed by Acrysof (Acrysof, SN60AT, Alcon Laboratories, Inc.) posterior chamber intraocular lens insertion using a Monarch III injector and C-cartridge system (Alcon Laboratories). This was followed by subsequent removal of OVD and hydration of the wound and side ports.
Intracameral moxifloxacin was given at the end of each surgery. All the patients were administered the routine postoperative treatment as follows: Postoperative regimen included corticosteroid drops (prednisolone acetate 1.5%, Pred Forte; Allergan) and antibiotics 8 times a day (moxifloxacin 0.5%, Vigamox; Alcon), which were gradually tapered and discontinued after 1mo. Patients were also evaluated during the follow-up at 1d, 1wk and 1mo after surgery. Not only did we perform the routine examination, but we evaluated the corneal endothelium and pachymetry data at 1d and one month after surgery, as well. The parameters on the screens of machines calculated automatically at the end of the surgery were recorded.
Statistical Analysis
Shapiro-Wilk's test was used. Histogram and q-q plots were examined to assess the data normality. To compare the differences between devices, independent samples t-test was used for normal distributed variables and Mann Whitney U tests was used for non-normal distributed data. For comparison of continuous variables in each group over time, Friedman analysis was used followed by a Siegel-Castellan multiple comparison test. Spearman's test was used for correlation analysis. Values are expressed as n (%), mean±SD or median (25th-75th percentiles). Analyses were conducted using R 2.15.3 (www.r-project.org ) software with P<0.05 being statistically significant.
RESULTS
The study included 86 patients, with 35 eyes in group 1 (transversal, group 1) and 51 eyes in group 2 (torsional, group 2). The baseline characteristics of the groups were similar, and the mean ages of the patients were 66.11±8.91y and 68.65±9.67y (P=0.493) in groups 1 and 2, respectively. The mean nuclear sclerosis grades were 2.7±1.43 in the first group and 2.61±1.20 in the second group (P=0.265). The preoperative mean best corrected visual acuities were 0.16±0.14 in the first group and 0.15±0.12 in the second group (P=0.367), and postoperative mean best corrected visual acuities were 0.89±0.11 in the first group and 0.91±0.09 in the second group (P=0.295). Preoperative intraocular pressures were 16.74±2.95 in the first group and 15.85±3.15 in the second group (P=0.483), and the postoperative intraocular pressures were 15.86±3.45 mm Hg in the first group and 14.92±2.78 mm Hg in the second group (P=0.497). Both of the groups had similar phacoemulsification needle times (group 1: 60.63±36.04 s; group 2: 55.98±29.78s; P=0.789).
In group 1, the average ultrasound (U/S) power, the total phacoemulsification time, and the effective phacoemulsification time (EPT) using EFX, denoting the amount of ultrasound delivered not only by transversal motion but also by longitudinal motion, were analyzed. The EFX was roughly the EPT with a specific coefficient for the transversal movement expressed in seconds.
In group 2, the U/S total equivalent power in position 3, cumulative dissipated energy (CDE), U/S total time, average phacoemulsification power, torsional time, average torsional amplitude, average torsional amplitude in position 3, equivalent average in position 3, aspiration time, and estimated fluid used (BSS volume) were analyzed.
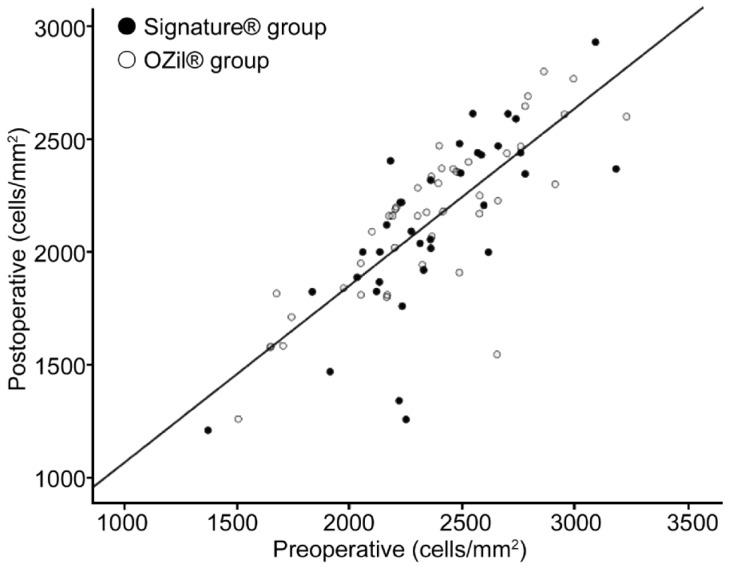
The percentage of endothelial cell loss 30d after surgery ranged from 3% to 15%, with a median of 7% in group 1 and from 2% to 13% with a median of 6% in group 2; however, there was no statistically significant difference between the two groups (P=0.407) (Figure 1). Likewise, hexagonality (P=0.794) and the coefficient of variation (CV; P=0.142) did not differ significantly between the groups before surgery and at 30d after surgery. However, we found less changes in the CCT measured before surgery and at 30d after surgery in the torsional group (P=0.022).
Figure 1. Scatter diagram of postoperative endothelial cells in relation to preoperative values in the two groups.
A significant positive correlation was found between the endothelial cell loss and nuclear sclerosis grade (group 1: P<0.001; group 2: P<0.001), and the endothelial cell loss associated with the phacoemulsification parameters in group 1 and group 2. A negative correlation between the endothelial cell loss and EFX (rho=-0.385; P=0.022) and average phacoemulsification power (rho=-0.446; P=0.007) was found in group 1, and negative correlations between the endothelial cell loss and CDE (rho=-0.331; P=0.028), U/S total time (rho=-0.307; P=0.043), average phacoemulsification power (rho=-0.396; P=0.008), torsional time (rho=-0.473; P=0.001), average torsional amplitude (rho=-0.319; P=0.035), and BSS volume (rho=-0.446; P=0.002) were found in group 2.
In this paper, a significant difference was observed in the mean of the central corneal thickness at 1d and at 0-30d post-operation (Table 1). Additionally, differences were observed in the HEX (%) at 1d post operation.
Table 1. Variables preoperatively, and at 1 and 30d postoperatively. Changes before and after 1d (0-1) and before and after 30d (0-30) are shown.
| Variability | Group1 (n=35) | Group 2 (n=51) | P |
| CCT (mm) | |||
| pre | 544.5 (512.5-553.5) | 524 (494-557) | 0.336 |
| post1 | 587 (564-712) | 563 (534-627) | 0.019 |
| post30 | 540 (530.5-560.5) | 522 (515-549) | 0.221 |
| 0-1 | 0.1 (0.03-0.26) | 0.07 (0.04-0.13) | 0.263 |
| 0-30 | 0.01 (0-0.04) | -0.01 [(-0.02)- 0.01)] | 0.022 |
| ECD (cell/mm2) | |||
| pre | 2327 (2163-2594) | 2392 (2173-2575) | 0.847 |
| post1 | 2211 (1880-2369) | 2203 (2056-2509) | 0.408 |
| post30 | 2120 (1888-2430) | 2178 (1857-2370) | 0.925 |
| 0-1 | -0.05 [(-0.10)-(-0.01)] | -0.04 [(-0.13)-(-0.01)] | 0.704 |
| 0-30 | -0.07 [(-0.15)-(-0.03)] | -0.06 [(-0.13)-(-0.02)] | 0.407 |
| CV | |||
| pre | 0.37 (0.34-0.43) | 0.40 (0.36-0.44) | 0.069 |
| post1 | 0.38 (0.35-0.43) | 0.43 (0.37-0.45) | 0.164 |
| post30 | 0.41 (0.34-0.42) | 0.36 (0.34-0.38) | 0.134 |
| 0-1 | 0.00 [(-0.07)- 0.08)] | 0.00 [(-0.11)-0.19)] | 0.706 |
| 0-30 | 0.00 [(-0.08)- 0.09)] | - 0.09 [(-0.20)-0.06)] | 0.142 |
| HEX (%) | |||
| pre | 51 (41-55) | 46.5 (39-54) | 0.241 |
| post1 | 49.5 (36-56.5) | 40 (37-46) | 0.079 |
| post30 | 54 (39-60) | 50 (40-54) | 0.258 |
| 0-1 | 0.03 [(-0.22)- 0.26)] | - 0.19 [(-0.33)-0.04)] | 0.044 |
| 0-30 | 0.07 [(-0.09)- 0.11)] | 0.13 [(-0.20)-0.54)] | 0.794 |
| ECLoss (%) | |||
| 0-1 | 5% (1%-10%) | 4% (1%-13%) | 0.704 |
| 0-30 | 7% (3%-15%) | 6% (2%-13%) | 0.407 |
Values are expressed as median (25th-75th percentiles); CCT: Central corneal thickness (mm); ECD: Endothelial cell density (cell/mm2); CV: Coefficient of variation; HEX: Hexagonality (%); ECLoss: Endothelial cell loss (%); pre: Preoperative measurement; post1: Measurement 1d after surgery; post30: Measurement 30d after surgery; 0-1: The difference between preoperative and postoperative day 1 measurements; 0-30: The difference between preoperative and postoperative days 30 measurements.
DISCUSSION
Transversal and torsional technologies were purported to be more effective and safer surgeries than the longitudinal systems. Whitestar tecnology shortened the pulse's duration to significantly increase the frequency of ultrasound pulses. This allowed us to decrease the duty cycle produced; thus, the cumulative ultrasound time was significantly reduced. These changes affected the production of heat and total ultrasound energy delivered by the phacoemulsification tip, and they practically eliminated the risk of wound burn. The OZil handpiece replaced the axial movements of a traditional phacoemulsification needle with the sideways oscillation of a bent Kelman tip, which showed that eliminating longitudinal repelling forces at the phacoemulsification tip dramatically, improved the followability and reduced the chatter of the fragments[7]–[10].
We have already evaluated both of these phacoemulsification machines to be effective and safe. Having precise fluidic control systems, the Whitestar Signature Ellips FX, more actively monitoring the pressure in the aspiration line, and its motor responding more rapidly to react to any changes in that pressure, like system's CASE software, can automatically adjust the fluid for the best balance of efficacy and safety, which does not require post occlusion surge.
The Infiniti-Ozil IP has Fluidic Management System, which controls the fluidic environment better because of the tubing's reduced compliance, so we can have finer control of the fluidic parameters and less efficient lower ultrasound settings and lower vacuum parameters. Because these two machines have advanced-fluid control system, less fluid turbulence and surge will emerge, which will result in less endothelial cell loss.
However, because of the different algorithms of the two machines, they could not be compared in terms of the intraoperative phacoemulsification parameters, such as the effective phacoemulsification time, cumulative dissipated energy, and the amount of BSS utilized. The only comparable feature of the machines was the phacoemulsification needle time. Thus, in comparing the efficacy and safety of the two machines, instead of the intraoperative parameters, we examined corneal and endothelial cells, which were clinically considered to be more important, and compared these measurements.
In both of the groups it was found that there was a similar phacoemulsification needle time on the same nuclear sclerosis grades by the same surgeon. The mean endothelial cell losses were 7% and 6% in groups 1 and 2, respectively. The endothelial cell loss after torsional phacoemulsification was found to be 5.12%±4.48% in moderate cataracts and 24.02%±20.04% in hard cataracts by Kim et al[15] and 7.2%±4.6% by Reuschel et al[8]. This difference in measurements varied between studies, depending on the hardness of the cataract.
The aim of this study was to examine two different machines using two different techniques, and demonstrate their possible effects on postoperative endothelial cell loss in cataract cases with similar nuclear sclerosis. Consequently, we determined that the two machines showed similar effects on endothelial cell loss, and the distinction between the machines made no difference for the cataract cases having similar nuclear sclerosis.
Postoperative corneal thickness and edema were suggested to be effective measurements of surgically induced endothelial damage, but these are indirect methods to predict endothelial changes[16]. The central corneal thickness (CCT) is considered to be a reliable measure of a transient corneal edema. In this paper, a significant difference was observed in the mean of the central corneal thickness at 1d post-surgery and the results 0-30d later. We observed less changes in corneal thickness measured before surgery and at 30d after surgery in the torsional group. This result suggested that a transient corneal edema may be more prominent in the transversal group than in the torsional group, or it could be related to the preoperative differences in CCT values, and not be of clinical importance.
Ventura et al[16] compared the corneal thickness and endothelial density before and after cataract surgery, and showed that the endothelial cell numerical density within the physiological range was not correlated with the central corneal thickness. According to this study, corneal cell depletion is not reflected in corneal thickness measurements until there has been a substantial loss of corneal endothelial cells.
Christakis and Braga-Mele[7] found results similar to ours in the central corneal thickness changes. They compared three phacoemulsification mechanisms (longitudinal, transversal, and torsional) and the torsional group in this study had the smallest increase in corneal thickness.
In addition to the measurement of corneal thickness, we evaluated the corneal endothelium by specular microscopy, which, compared to other studies, can be considered to be the most distinctive feature of our study. It is clear that evaluating endothelial cells with a direct method (like specular microscopy) rather than an indirect measurement of corneal thickness is more valuable.
Some specular microscopy parameters are endothelial cell density, hexagonality, and the coefficient of variation. Hexagonality is the percentage of regular hexagonal cells to the total number of cells with different morphologies, and it should be 100% for an ideal corneal endothelium. However, for a healthy cornea, hexagonality is generally best measured at 60%-70%. In the early postoperative period, while hexagonality increases, ECC, the number of cells per mm, begins to decrease. And endothelial pump functions in the damaged regions generally improve within a period of 14d.
The coefficient of variation showed an objective parameter to evaluate the variability between cell areas. The normal value must be under 30%[17]. The endothelial cells are nonreplicating, which is why the enlargement and migration of residual cells compensate in areas of endothelial cell loss[18]–[20].
An interesting point of our study was that the endothelial cell changes were not different between the groups. The percentages of postoperative endothelial cell loss, hexagonality, and the coefficient of variation did not differ significantly between the groups before and 30d after surgery. Only in hexagonality was there a significant difference between before and 1d after surgery. We assumed that the difference came from the local post-surgical inflammatory corneal changes resulting in tissue stress; however, after the period of recovery, the difference disappeared between the groups.
A significant positive correlation was found between the endothelial cell loss and nuclear sclerosis grade, and between the endothelial cell loss and phacoemulsification power and time. Similar results were obtained in the studies of Mahdy et al[21] and Assaf and Roshdy[22]. Mahdy et al's[21] study revealed that there was a relatively wide range of endothelial cell loss that increased with the increasing grade of nuclear hardness, and it revealed that the high cumulative dissipated energy and large volume of BSS used during phacoemulsification was associated with a high endothelial cell loss.
In summary, our study compared the reliability of two phacoemulsification machines, both of which were found to be safe to perform cataract surgery since they both caused minimal corneal and endothelial changes. There was a statistically significant difference in the central corneal thickness between these machines, but the direct method, namely specular microscopy, was more meaningful for evaluating the damage, and we found no difference in the parameters of the endothelial cells between these machines. Both of these machines were efficient, with similar endothelial cell loss. This endothelial cell loss, on which the phacoemulsification machine type had no effect*, was related to the increased nuclear sclerosis grade and increased phacoemulsification power.
Acknowledgments
We thank Dr Faruk Balkaya (English Department of Kayseri Erciyes University) for his contribution to English terms and language.
Conflicts of Interest: Ataş M, None; Demircan S, None; Karatepe Haşhaş AS, None; Gülhan A, None; Zararsız G, None.
REFERENCES
- 1.Kelman CD. Phaco-emulsification and aspiration: a new technique of cataract removal. Am J Ophthalmol. 1967;64(1):23–35. [PubMed] [Google Scholar]
- 2.Linebarger EJ, Hardten DR, Shah GK, Lindstrom RL. Phacoemulsification and modern cataract sugery. Surv Ophthalmol. 1999;44(2):123–147. doi: 10.1016/s0039-6257(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 3.Minassian DC, Rosen P, Dart JK, Reidy A, Desai P, Sidhu M, Kaushal S, Wingate N. Extracapsular cataract extraction compared with small incision surgery by phacoemulsification: a randomized trial. Br J Ophthalmol. 2001;85(7):822–829. doi: 10.1136/bjo.85.7.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi K, Hayashi H, Nakao F, Hayashi F. Risk factors for corneal endothelial injury during phacoemulsification. J Cataract Refract Surg. 1996;22(8):1079–1084. doi: 10.1016/s0886-3350(96)80121-0. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman RS, Fine IH, Packer M. New phacoemulsification technology. Curr Opin Ophthalmol. 2005;16(1):38–43. doi: 10.1097/00055735-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Fishkind WJ. The phaco machine: analyzing new technology. Curr Opin Ophthalmol. 2013;24(1):41–46. doi: 10.1097/ICU.0b013e32835b0770. [DOI] [PubMed] [Google Scholar]
- 7.Christakis PG, Braga-Mele RM. Intraoperative performance and postoperative outcome comparison of longitudinal, torsional and transversal phacoemulsification machines. J Cataract Refract Surg. 2012;38(2):234–241. doi: 10.1016/j.jcrs.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 8.Reuschel A, Bogatsch H, Barth T, Wiedemann R. Comparison of endothelial changes and power settings between torsional and longitudinal phacoemulsification. J Cataract Refract Surg. 2010;36(11):1855–1861. doi: 10.1016/j.jcrs.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 9.Jun B, Berdahl JP, Kim T. Thermal study of longitudinal and torsional ultrasound phacoemulsification: tracking the temperature of the corneal surface, incision, and handpiece. J Cataract Refract Surg. 2010;36(5):832–837. doi: 10.1016/j.jcrs.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Rekas M, Montes-Mico R, Krix-Jachym K, Klus A, Stankiewicz A, Ferrer-Blasco T. Comparison of torsional and longitudinal modes using phacoemulsification parameters. J Cataract Refract Surg. 2009;35(10):1719–1724. doi: 10.1016/j.jcrs.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 11.Tognetto D, Cecchini P, Leon P, Di Nicola M, Ravalico G. Stroke dynamics and frequency of three phacoemulsification machines. J Cataract Refract Surg. 2012;38(2):333–342. doi: 10.1016/j.jcrs.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 12.Schmutz JS, Olson RJ. Thermal comparison of Infiniti OZil and Signa-ture Ellips phacoemulsification systems. Am J Ophthalmol. 2010;149(5):762–767. doi: 10.1016/j.ajo.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Bozkurt E, Bayraktar S, Yazgan S, Cakir M, Cekic O, Erdogan H, Yilmaz OF. Comparison of conventional and torsional mode (OZil) phacoemulsification; randomized prospective clinical study. Eur J Ophthalmol. 2009;19(6):984–989. doi: 10.1177/112067210901900614. [DOI] [PubMed] [Google Scholar]
- 14.Fiskind W, Bakewell B, Donnenfeld ED, Rose AD, Watkins LA, Olson RJ. Comparative clinical trial of ultrasound phacoemulsification with and without the WhiteStar system. J Cataract Refract Surg. 2006;32(1):45–49. doi: 10.1016/j.jcrs.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, Wee WR, Lee JH, Kim MK. The comparison between torsional and conventional mode phacoemulsification in moderate and hard cataracts. Korean J Ophthalmol. 2010;24(6):336–340. doi: 10.3341/kjo.2010.24.6.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ventura AS, Walti R, Böhnke M. Corneal thickness and endothelial density before and after cataract surgery. Br J Ophthalmol. 2001;85(1):18–20. doi: 10.1136/bjo.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swartz T, Marten L, Wang M. Measuring the cornea: the latest developments in corneal topography. Curr Opin Ophthalmol. 2007;18(4):325–333. doi: 10.1097/ICU.0b013e3281ca7121. [DOI] [PubMed] [Google Scholar]
- 18.Mencucci R, Ponchietti C, Virgili G, Giansanti F, Menchini U. Corneal endothelial damage after intraocular surgery; microinsicion versus standard tecnique. J Cataract Refract Surg. 2006;32(8):1351–1354. doi: 10.1016/j.jcrs.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 19.Wilczynski M, Drobniewski I, Synder A, Omulecki W. Evaluation of early corneal endothelial cell loss in bimanual microinsicion cataract surgery (MICS) in comparison with Standard phacoemulsification. Eur J Ophthalmol. 2006;16(6):798–803. doi: 10.1177/112067210601600603. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K, Yoshida M, Manabe S, Hirata A. Cataract surgery in eyes with low corneal endothelial cell density. J Cataract Refract Surg. 2011;37(8):1419–1425. doi: 10.1016/j.jcrs.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Mahdy MA, Eid MZ, Mohammed MA, Hafez A, Bhatia J. Relationship between endothelial cell loss microcoaxial phacoemulsification parameters in noncomplicated cataract surgery. Clin Ophthalmol. 2012;6:503–510. doi: 10.2147/OPTH.S29865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assaf A, Roshdy MM. Comparative analysis of corneal morphological changes after transversal and torsional phacoemulsification through 2.2 mm corneal incision. Clin Ophthalmol. 2013;7:55–61. doi: 10.2147/OPTH.S39019. [DOI] [PMC free article] [PubMed] [Google Scholar]