Abstract
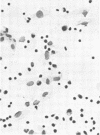
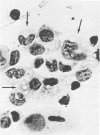
Intraperitoneal (i.p.) infection of BALB/c mice with 1,000 50% mouse lethal doses of the Karp strain of Rickettsia tsutsugamushi was inevitably lethal, and associated pathological alterations were confined to the peritoneal cavity. These included: (i) continuous proliferation of rickettsial organisms in peritoneal macrophages until death; (ii) hepatic granulomas appearing 6 days after infection and increasing in size and number until death; (iii) splenomegaly, resulting principally from proliferation of lymphoid tissue, and (iv) terminal peritonitis. Under two circumstances, i.p. infections with R. tsutsugamushi were not lethal: (i) infection with 100 50% mouse infectious doses of the Gilliam strain, which, in fact, resulted in immune protection against otherwise lethal Karp challenge; and (ii) Karp infection of animals immunized with the Gilliam strain. In both cases, the associated pathological abnormalities were, as with primary Karp infection, restricted to the peritoneal cavity. Also similar was the striking splenomegaly due to lymphoid proliferation, which was particularly prominent in immunized animals. In contrast to primary and lethal Karp infection, however, these infections were characterized by: (i) minimal and transient proliferation of rickettsial organisms in peritoneal macrophages; (ii) disappearance of hepatic granulomas; and (iii) absence of peritonitis. It was concluded that the survival of an animal bearing an i.p. infection of scrub typhus depended on its ability to concentrate a sufficiently vigorous immune response in the peritoneal cavity, resulting in the evolution of rickettsiacidal macrophages capable of suppressing the infection.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boak J. L., Christie G. H., Ford W. L., Howard J. G. Pathways in the development of liver macrophages: alternative precursors contained in populations of lymphocytes and bone-marrow cells. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):307–327. doi: 10.1098/rspb.1968.0013. [DOI] [PubMed] [Google Scholar]
- Fedorko M. E., Hirsch J. G. Structure of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):109–124. [PubMed] [Google Scholar]
- JACKSON E. B., SMADEL J. E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951 May;53(3):326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- KUNDIN W. D., LIU C., HARMON P., RODINA P. PATHOGENESIS OF SCRUB TYPHUS INFECTION (RICKETTSIA TSUTSUGAMUSHI) AS STUDIED BY IMMUNOFLUORESCENCE. J Immunol. 1964 Nov;93:772–781. [PubMed] [Google Scholar]
- KUWATA T. Analysis of immunity in experimental Tsutsugamushi disease. J Immunol. 1952 Feb;68(2):115–120. [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- SCHOENBERG M. D., MUMAW V. R., MOORE R. D., WEISBERGER A. S. CYTOPLASMIC INTERACTION BETWEEN MACROPHAGES AND LYMPHOCYTIC CELLS IN ANTIBODY SYNTHESIS. Science. 1964 Feb 28;143(3609):964–965. doi: 10.1126/science.143.3609.964. [DOI] [PubMed] [Google Scholar]