Abstract
AIM
To explore the efficacy of preoperative intravitreal bevacizumab (IVB) injection combined with Ahmed glaucoma valve (AGV) implantation in the treatment of neovascular glaucoma (NVG).
METHODS
This retrospective study included 35 eyes from 35 patients who underwent preoperative IVB and AGV implantation for treatment of NVG. Findings such as intraocular pressure (IOP) number of anti-glaucoma medications, visual acuity (VA), surgical success rates, and complications were recorded.
RESULTS
After AGV implantation, IOP was 18.2±4.0 mm Hg, 15.5±3.3 mm Hg and 9.8±2.6 mm Hg at 6, 12 and 36mo, significantly decreased compared with pre-IOP (P<0.01). The number of anti-glaucoma medications was 0.9±0.5, 0.8±0.9 and 0.8±0.6 at 6, 12 and 36mo, significantly decreased compared to pre-treatment (P<0.01). At last visit, there were 19 eyes with stable VA, 4 with VA improvement, 12 with diminished VA and 3 with complete loss light perception. There were 7 cases that failed during 3-year fellow up period. Cumulative probabilities of valve survival by Kaplan-Meier analysis were 82.9%, 74.1% and 71.0% at 12, 24 and 36mo, respectively. Cox stepwise regression analysis found that the survival time was significant associated with the pre-visual acuity <2/400 (P<0.05). Post-operative complications occurred in 8 eyes, of which hyphema presented in 2 eyes, choroidal effusion in 2 eyes.
CONCLUSION
The procedure of preoperative IVB and AGV implantation should be one of treatments for NVG because of its safety and effectiveness.
Keywords: Ahmed glaucoma valve, bevacizumab, intravitreal injection, neovascular glaucoma
INTRODUCTION
Neovascular glaucoma (NVG), one of the refractory glaucoma, is closely related to retinal ischemic diseases, such as diabetic retinopathy and retinal vein occlusion. Ischemia triggers the release of various angiogenic factors, including vascular endothelial growth factor (VEGF), which penetrate into the anterior chamber to cause the neovascularization of iris and angle[1],[2]. Although panretinal photocoagulation (PRP) is considered the most effective treatment for retinal ischemia, it may be difficult to perform in patients with NVG secondary to media opacities, including corneal edema, cataract, anterior chamber or vitreous hemorrhage, and poor papillary dilation.
Bevacizumab, a recombinant antibody against VEGF, can be used as a novel therapeutic method for management of NVG. Previous studies have found that this procedure has been used successfully in the treatment of proliferative diabetic retinopathy[3], iris neovascularization[4], neovascular age-related macular degeneration[5], also very effective for complications retinal arterial macroaneurysms[6] and more recently, it has been used in NVG[7]–[10]. In addition, drainage valves are widely used for all kinds of refractory glaucoma, including NVG, but their success rate is low when the drainage valve is implanted alone, compared with the concurrent use of anti-angiogenesis therapies[1],[11],[12].
This retrospective study aimed to evaluate the efficacy of the preoperative use of intravitreal bevacizumab injection (IVB) combined with Ahmed glaucoma valve (AGV) implantation for treatment of NVG patients in terms of surgical success rate and postoperative complications.
SUBJECTS AND METHODS
This retrospective and consecutive case series study was conducted in NVG patients referred to the department of ophthalmology in the first affiliated hospital of Xinxiang Medical College, between January 2008 and February 2009. Thirty-four cases (97.1%) of 35 subjects included in this study have received PRP before the research conducted. And then all patients that were included in this study had undergone IVB combined with AGV implantation for increased intraocular pressure (IOP) that was not responsive to pharmacotherapy (more than 3 kinds of eyedrops including cholinergic drugs, adrenergic agonists, beta adrenergic antagonists or prostaglandin analogs were taken, and also combined with acetazolamide 250 mg tablets, 2-4 times daily, orally.) and /or laser treatment. In patients with bilateral implantation, chart analysis was analyzed from the first eye that had received surgery. NVG, diagnosed by a glaucoma sub specialist (Zhang HT), was defined as neovascularization of the iris and/or anterior chamber angle with elevated IOP ≥22 mm Hg. Glaucomatous optic nerve cupping was not required for diagnosis. The study was approved by the Institutional Review Board and conducted in accordance with the Declaration of Helsinki, all patients were informed of the procedure and informed consent was obtained.
Exclusion criteria included patient age <30y, previous cyclodestructive treatment, and earlier glaucoma drainage device implantation. Patients with less than 6mo follow-up were excluded from the analysis to allow evaluation of the results after the immediate postoperative period.
Preoperative information (before IVB) included gender, age, history of laser and surgical treatments, glaucoma diagnosis, lens status, glaucoma medications, IOP measured by Goldmann applanation tonometry, and visual acuity. Postoperative data (after AGV implantation) regarding IOP, number of glaucoma medicines, visual acuity, and complications were obtained at 1d, 1wk, 1, 2, 3, 6, 12mo, and every 6mo thereafter for 3y.
Criteria for success were defined before reviewing the data. Surgical success was defined as IOP of 6 mm Hg or greater and 21 mm Hg or less, with or without the use of additional glaucoma medicines, without further glaucoma surgery including cyclophotocoagulation or major complications that required removal of the implant, and without loss of light perception. The definition of the hypotony was an IOP≤5 mm Hg and hypertensive was defined by an IOP>21 mm Hg on 2 consecutive visits.
For IVB, after topical anesthesia and disinfection with iodine in the operating room, commercially available bevacizumab (Avastin 100 mg/4 mL intravenously; Genentech, USA) at a dose of 1.25 mg/0.05 mL was injected using a tuberculin syringe with a 27-G needle into the vitreous cavity through the pars plana at a position 3.5 mm posterior to the corneal limbus. After the injection, a topical antibiotic was added to the glaucoma medication regimen for (3d).
AGV implantation was carried out 7-14d after IVB according to the degree of iris neovascularization atrophy and anterior segment inflammation. The technique of Ahmed valve (Model FP7, New World, USA) implantation was as follows: a fornix-based conjunctival flab was created to expose 2 recti muscles; a pocket was performed between the episclera and tenon's capsule by blunt dissection. The tube of the valve was then irrigated with balanced saline solution to open the valve mechanism. Then, the valve plate was secured to the sclera by 9/0 nylon sutures. The valve plate was inserted with its anterior edge 8-9 mm from the limbus. The tube was then trimmed and beveled so that the bevel faced the corneal endothelial surface and extend 3 mm from the limbus inside the anterior chamber. A limbal-based 2/3 thickness scleral flap was then dissected and a 23-G needle was used to enter the anterior chamber under the scleral flap, then the tube was inserted into the anterior chamber and the flap was sutured over the tube using 9/0 nylon sutures. The conjunctiva was then closed with 10/0 Vicryl. Topical steroid and antibiotic was used at the end of the operation.
Statistical Analysis
All statistical analysis was performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Comparisons of preoperative and each follow-up point data such as IOP level, the number of anti-glaucoma medications used were performed using one-way ANOVA. Comparisons of preoperative and final IOP, the number of anti-glaucoma medications used were performed using paired t-test. The cumulative probability of success was analyzed by the Kaplan-Meier life-table method. Cox proportional hazard regression models were performed stepwise to assess the relationship between survival outcomes and multiple predictors, including age, gender, diabetes mellitus, hypertension, preoperative IOP, glaucoma medications, preoperative number of surgeries, and preoperative visual acuity. A P-value less than 0.05 was considered significant.
RESULTS
In total, 35 patients (35 eyes) with NVG were enrolled in the study, including 20 (57.1%) males and 15 (42.9%) females, and the mean age was 53.2±12.2y. The mean follow up period was 24.6±14.2mo (range 6-32mo). NVG was mainly caused by proliferative retinopathy (22/35, 62.9%) and retinal vein occlusion (9/35, 25.7%). The mean baseline IOP was 44.9±4.8 mm Hg and the mean number of glaucoma medications used preoperatively was 3.3±0.5. The characteristics of subjects are presented in Table 1.
Table 1. Characteristics of subjects with NVG.
| Characteristics | Subjects (n=35) |
| Gender, n (%) | |
| M | 20 (57.1) |
| F | 15 (42.9) |
| Age (a) | 53.2±12.2 |
| Diabetes, n (%) | 26 (74.3) |
| Hypertension, n (%) | 13 (37.1) |
| Lens status, n (%) | |
| Phakic | 22 (62.9) |
| Aphakic | 2 (5.7) |
| Pseudophakic | 11 (31.4) |
| Previous surgery history, n (%) | 13 (37.1) |
| Previous laser history, n (%) | 34 (97.1) |
| Pre-IOP, mm Hg | 44.9±4.8 |
| Pre-medications NO. | 3.3±0.5 |
| Pre-vision, n (%) | |
| ≥20/80 | 1 (2.9) |
| <20/80-20/200 | 3 (8.6) |
| <20/200-20/400 | 6 (17.1) |
| <20/400-counting fingers | 8 (22.9) |
| ≤Hand movement | 17 (48.6) |
| Etiology, n (%) | |
| Proliferative diabetic retinopathy | 22 (62.9) |
| Retinal vein occlusion | 9 (25.7) |
| Retinal artery occluson | 3 (8.6) |
| Ocular ischemia | 1 (2.9) |
The examination demonstrated that iris neovascularization (INV) was diminished after IVB. INV was absent in 22 (62.9%) patients, presented in one quadrant area in 9 (25.7%) patients, presented in 2 quadrants for 4 cases (16%) and without complications caused by IVB.
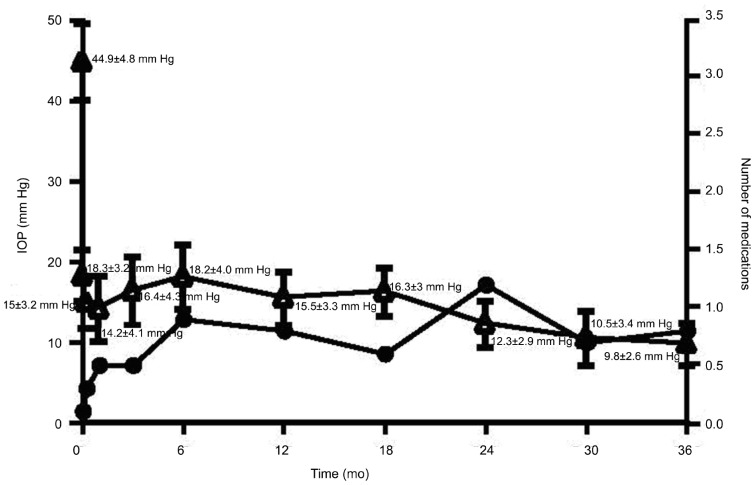
The mean IOP values in the 1st, 3rd, 6th, 12th, 24th and 36th months after AGV implantation were as follows: 14.2±4.1 mm Hg, 16.4±4.3 mm Hg, 18.2±4.0 mm Hg, 15.5±3.3 mm Hg, 12.3±2.9 mm Hg and 9.8±2.6 mm Hg, respectively (Figure 1). The difference between the mean baseline IOP and the IOP at each follow-up point was statistically significant (F=222.938, P<0.01).
Figure 1. IOP changes in eyes with NVG after surgery and number of anti-glaucoma medications in eyes with NVG after surgery.
The mean number of anti-glaucoma medications which included Timolol 0.5%, 1-2 times/d, Acetazolamide 250 mg tablets, 2-4 times daily, orally, Pilocarpine 0.5% 4 times/d and Adrenaline 1.0% 2-4 times/d used in the 1st, 3rd, 6th, 12th and 36th months after AGV implantation were as follows: 0.5±0.6, 0.5±0.6, 0.9±0.5, 0.8±0.9, 1.2±0.7 and 0.8±0.6, respectively (Figure 1). The difference between the before surgery and each follow-up point after surgery was statistically significant (F=69.312, P<0.01).
At the final visit, the IOP decreased from 40.5±5.0 mm Hg (baseline) to 15.5±7.0 mm Hg, and the difference was statistically significant (t=15.958, P<0.01). The number of glaucoma medications used decreased from 3.3±0.5 (baseline) to 0.9±0.8, and the difference was statistically significant (t=15.305, P<0.01). Compared with the preoperative examination outcomes, the postoperative visual acuity maintained stability in 19 eyes (54.3%), improved (Snellen visual acuity increased more than 1 line) in 4 eyes (11.4%), decreased (Snellen visual acuity decreased more than 1 line) for in 12 eyes (34.3%) and lost light perception in 3 eyes (8.6%).
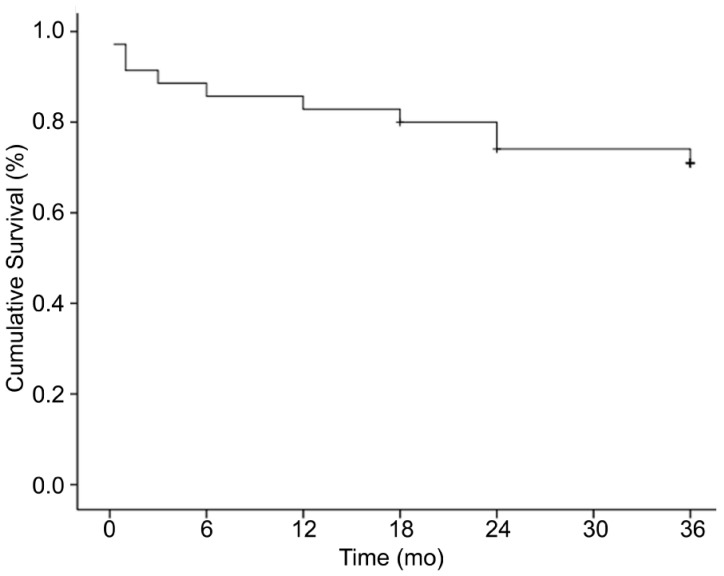
According to the definition of surgical success, 7 eyes with NVG were considered surgical failures (Table 2). The majority of eyes failed owing to increased IOP (2 eyes, 5.7%) and hypotony (2 eyes, 5.7%). Three eyes (8.6%) developed loss of light perception. The majority of eyes failed owing to increased IOP (2 eyes, 5.7%) and hypotony (2 eyes, 5.7%). Three eyes (8.6%) developed loss of light perception. One eye (2.9%) received the cyclocryotherapy after the intravitreal bevacizumab and ahmed glaucoma valve implantation. Although the IOP was under control (6-21 mm Hg), we still considered it was the failed case. The majority of control eyes failed owing to increased IOP (2 eyes, 5.7%) and hypotony (2 eyes, 5.7%). Three eyes (8.6%) developed loss of light perception, with 1 (2.9%) of these meeting no other criteria for surgical failure. The Kaplan-Meier survival analysis for success is shown in Figure 2. The cumulative probability of success was 91.4%, 88.6%, 85.7%, 82.9%, 80%, 74.1% and 71.0% at 1, 3, 6, 12, 18, 24 and 36mo, respectively. The mean survival time was 29.3±2.2mo (Figure 2). A Cox proportional hazard model was used to evaluate risk factors for surgical failure. Factors analyzed included age, gender, diabetes mellitus, hypertension, preoperative IOP, glaucoma medications, preoperative number of surgeries, and preoperative visual acuity. Preoperative visual acuity was detected as a risk factor for surgical failure (Wald=8.031, RR=3.571, P=0.033<0.05), whereas other factors were not statistically significant risk factors for failure.
Table 2. Reasons for failure cases after surgery.
| Reasons | Failure cases (n=7)1 |
| IOP>21 mm Hg | 2 (1 lost light perception)1 |
| IOP<6 mm Hg | 2 (1 lost light perception) |
| Further glaucoma surgery | 1 (1 lost light perception)1 |
| Loss of light perception | 23 |
1Cases in reasons for failure add up to more than 7 because some eyes had more than one criterion for failure; 1One of 2 cases with IOP>21 mm Hg received cyclocryosurgery;2One of 3 eyes lost light perception with IOP≥6 mm Hg and ≤21 mm Hg.
Figure 2. Survival curve of subjects with NVG after surgery.
As shown in Table 2, complications occurred in 8 (22.0%) of 35 eyes with NVG after AGV surgery. Anterior chamber hemorrhage occurred in 2 eyes (5.7%), choroidal effusion in 2 eyes (5.7%), anterior chamber was not present in 1 eye (2.9%) and obstruction of drainage tube by fibrous exudation in 1 case (2.9%). Excluding drug therapy, additional surgeries were performed successfully on 4 of 8 eyes with complications, and there were no cases requiring the removal the AGV owing to complications.
DISCUSSION
NVG has been recognized as one of the refractory ocular diseases and the failure rate of the simple glaucoma filtering operation almost achieved 80%[12]. To date, the ciliary body destructive surgery, drainage valve implantation and trabeculectomy with mitomycin C are considered to be effective for NVG treatment when drug therapies failed. However, the ciliary body destructive surgery has the risk of eyeball atrophy and visual loss[13]. Thus, it is suggested that the drainage valve implantation can be selected for NVG of patients who have a certain level of vision[1]. The success of drainage valve implantation requires the preoperative removal of existing neovascularization in the iris and anterior chamber angle. The trabeculectomy augmented with mitomycin C had the risk of hyphema[14]. Recently, the intravitreal injection of anti-VEGF drugs such as bevacizumab has shown promising results in regression of neovascularization. However, some studies found that the drainage valve implantation combined with IVB at the same time didn't improve the therapeutic effect on NVG[15]. But more and more publication suggested that the IVB is a useful preparatory step to safely and effectively implant[16],[17]. In the present study, we adopted the method of preoperative use of IVB for treating neovascularization, and then performed AGV implantation to reduce the high IOP to evaluate the efficacy of this therapy in NVG patients.
In present study, we found obvious iris atrophy occurred in all subjects after IVB, without any complication associated with the injection, which was in accordance with previous studies[7],[10]. Our results confirmed the effectiveness of IVB for the treatment of INV. After AGV implantation, the postoperative IOP levels as well as the number of antiglaucoma medications used were significantly reduced compared with the preoperative, and the results of cumulative survival rates at 1, 2 and 3y were 82.9%, 74.1% and 71.0%, respectively. WuDunn et al[18] investigated the therapeutic efficacy of refractory glaucoma by adapting the method of Baerveldt 250-mm2 glaucoma valve implantation and showed that the postoperative IOP and anti-glaucoma medications used were significantly reduced, with higher cumulative survival rates of 88% and 79% at 1 and 2y, respectively. In another study, Yalvac et al[19] reported that the AGV was successful for early and intermediate-term of IOP control but in long term this implant was failed to achieve control of IOP in patients with NVG. Netland et al[20] compared the results of AGV surgery in patients with and without NVG and results showed that NVG patients have greater risk of surgical failure after AGV surgery compared with controls, with success at 1y=73.1%, 2y=61.9%, and 5y=20.6% respectively. More recently, two studies[21],[22] compared the outcomes of adjunctive with and without IVB before AGV implantation in the treatment of NVG, and both of them found that the success rate in the IVB group was significantly higher than control group. In this study, our results showed that the success rate of combination of AVG implantation with preoperative IVB achieved a higher cumulative survival rate (71.0%) at 3y postoperatively when compared to Netland's study. However, it should be pointed out that 34 cases (97.1%) of 35 subjects included in this study have received PRP before the research conducted, so our results didn't indicate that the IBV could replace the role of PRP treatment. On the contrary, our results confirmed that IBV, as the adjunctive treatment for PRP, combining with AVG implantation will improve clinical effect on NVG treatment. In a recent study, Eid et al[17] found that a 90% success rate of aqueous shunting tube surgery when IVB was followed by PRP compared with 80% when bevacizumab was not followed by photocoagulation and 70% when photocoagulation was done without bevacizumab injection before the shunt surgery.
Seven failures (20%) in the current study included: high IOP in 2 cases (5.7%), low IOP in 2 cases and lost light perception in 3 cases (8.6%). Uncontrolled IOP was the main reason for the failure of surgery. Moreover, although AGV has a flow limitation function, we should pay attention to the low IOP after surgery due to the excessive drainage of aqueous humor. The reasons for visual acuity decline and even the development to non light perception may be associated with factors, such as poorly controlled IOP and progression of underlying disease. Netland et al[20] found that more than 50 percent of patients of 23.7% cases with postoperative IOP above 5 mm Hg and less than 22 mm Hg and they suggested that progression of underlying disease is an important cause of vision loss in NVG. In present study, only one of 3 (8.6%) cases lost light perception with normal IOP, and we considered that it may be related to re-injection of bevacizumab postoperatively, which to some extent prevented the progression of visual loss.
Hyphema was the most common complication after glaucoma valve implantation. However, no hyphema cases occurred during the AGV implantation surgery and only 2 cases (5.7%) had hyphema after surgery. Similar, there was a low occurrence rate of choroid effusion, which may be related to postoperative ocular hypotension. Almobarak and Khan[23] reported that the postoperative tube incorrect positioning that required surgical revision was common, but there was no case with incorrect tube in our study. This may be partly related to our extensive experience with this procedure. Overall, there were few intro-operative and postoperative complications that occurred in the present study.
Impact factors for effectiveness of glaucoma valve implantation are still unclear. In this study, we found that preoperative visual acuity <20/400 was correlated with postoperative survival time (RR=3.571) determined by Cox regression analysis, while other preoperative factors have no correlation with postoperative survival time. Yalvac et al[19] found that preoperative visual acuity <2/200 (P=0.003), diagnosis of diabetes mellitus (P=0.050), and preoperative IOP>or=35 mm Hg (P=0.038) were poor prognostic factors for post-surgical success. However, because of the different research subjects and treatment methods (subjects in this study had received IVB before surgery and only AGV was implanted), no correlation of diabetes mellitus and preoperative IOP with surgical therapeutic effects was found.
Considering the different follow-up time for patients after surgery and the influence of missing data on results in this study, survival analysis is more reasonable and accurate than other statistical methods to analyze the retrospective data. However, due to the lack of any other treatment as control, for example, trabeculectomy with intraoperative mitomycin C after an adjunctive treatment with IVB and PRP, it is still not possible to determine which treatment is more effective, and further comparative studies are needed[24].
In conclusion, preoperative IVB combined with AGV implantation is a good treatment modality in the management of eyes with NVG. Using this method, the success rate was still maintained at more than 70% at 3y postoperatively and few complications occurred. We hope that further prospective studies with a control group and larger sample size will confirm the efficacy and safety of this method.
Acknowledgments
Conflicts of Interest: Zhang HT, None; Yang YX, None; Xu YY, None; Yang RM, None; Wang BJ, None; Hu JX, None.
REFERENCES
- 1.Sivak-Callcott JA, O'Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108(10):1767–1776. quiz1777–1800. doi: 10.1016/s0161-6420(01)00775-8. [DOI] [PubMed] [Google Scholar]
- 2.Horsley MB, Kahook MY. Anti-VEGF therapy for glaucoma. Curr Opin Ophthalmol. 2010;21(2):112–117. doi: 10.1097/ICU.0b013e3283360aad. [DOI] [PubMed] [Google Scholar]
- 3.Sawada O, Kawamura H, Kakinoki M, Sawada T, Ohji M. Vascular endothelial growth factor in aqueous humor before and after intravitreal injection of bevacizumab in eyes with diabetic retinopathy. Arch Ophthalmol. 2007;125(10):1363–1366. doi: 10.1001/archopht.125.10.1363. [DOI] [PubMed] [Google Scholar]
- 4.Ishibashi S, Tawara A, Sohma R, Kubota T, Toh N. Angiographic changes in iris and iridocorneal angle neovascularization after intravitreal bevacizumab injection. Arch Ophthalmol. 2010;128(12):1539–1545. doi: 10.1001/archophthalmol.2010.282. [DOI] [PubMed] [Google Scholar]
- 5.Arevalo JF, Fromow-Guerra J, Sanchez JG, Maia M, Berrocal MH, Wu L, Saravia MJ, Costa RA. Primary intravitreal bevacizumab for subfoveal choroidal neovascularization in age-related macular degeneration: results of the Pan-American Collaborative Retina Study Group at 12 months follow-up. Retina. 2008;28(10):1387–1394. doi: 10.1097/IAE.0b013e3181884ff4. [DOI] [PubMed] [Google Scholar]
- 6.Pichi F, Morara M, Torrazza C, Manzi G, Alkabes M, Balducci N, Vitale L, Lembo A, Ciardella AP, Nucci P. Intravitreal bevacizumab for macular complications from retinal arterial macroaneurysms. Am J Ophthalmol. 2013;155(2):287–294.e1. doi: 10.1016/j.ajo.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Canut MI, Alvarez A, Nadal J, Abreu R, Abreu JA, Pulido JS. Anterior segment changes following intravitreal bevacizumab injection for treatment of neovascular glaucoma. Clin Ophthalmol. 2011;5:715–719. doi: 10.2147/OPTH.S17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf S, Sarra GM. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142(6):1054–1056. doi: 10.1016/j.ajo.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 9.Mahdy RA, Nada WM, Fawzy KM, Alnashar HY, Almosalamy SM. Efficacy of intravitreal bevacizumab with panretinal photocoagulation followed by Ahmed valve implantation in neovascular glaucoma. J Glaucoma. 2013;22(9):768–772. doi: 10.1097/IJG.0b013e318259aec4. [DOI] [PubMed] [Google Scholar]
- 10.Kotecha A, Spratt A, Ogunbowale L, dell'Omo R, Kulkarni A, Bunce C, Franks WA. Intravitreal bevacizumab in refractory neovascular glaucoma: a prospective, observational case series. Arch Ophthalmol. 2011;129(2):145–150. doi: 10.1001/archophthalmol.2010.350. [DOI] [PubMed] [Google Scholar]
- 11.SooHoo JR, Seibold LK, Kahook MY. Recent advances in the management of neovascular glaucoma. Semin Ophthalmol. 2013;28(3):165–172. doi: 10.3109/08820538.2012.730103. [DOI] [PubMed] [Google Scholar]
- 12.Mietz H, Raschka B, Krieglstein GK. Risk factors for failures of trabeculectomies performed without antimetabolites. Br J Ophthalmol. 1999;83(7):814–821. doi: 10.1136/bjo.83.7.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pokroy R, Greenwald Y, Pollack A, Bukelman A, Zalish M. Visual loss after transscleral diode laser cyclophotocoagulation for primary open-angle and neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2008;39(1):22–29. doi: 10.3928/15428877-20080101-09. [DOI] [PubMed] [Google Scholar]
- 14.Kojima S, Inatani M, Shobayashi K, Haga A, Inoue T, Tanihara H. Risk factors for hyphema after trabeculectomy with mitomycin C. J Glaucoma. 2014;23(5):307–311. doi: 10.1097/IJG.0b013e3182741c85. [DOI] [PubMed] [Google Scholar]
- 15.Ma KT, Yan JY, Kim JH, Kim NR, Hong S, Lee ES, Seong GJ, Kim CY. Surgical results of Ahmed valve implantation with intraoperative bevacizumab injection in patients with neovascular glaucoma. J Glaucoma. 2012;21(5):331–336. doi: 10.1097/IJG.0b013e31820e2fd0. [DOI] [PubMed] [Google Scholar]
- 16.Pichi F, Morara M, Lembo A, Ciardella AP, Meduri A, Nucci P. Neovascular glaucoma induced by peripheral retinal ischemia in neurofibromatosis type 1: management and imaging features. Case Rep Ophthalmol. 2013;4(1):69–73. doi: 10.1159/000350956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eid TM, Radwan A, el-Manawy W, el-Hawary I. Intravitreal bevacizumab and aqueous shunting surgery for neovascular glaucoma: safety and efficacy. Can J Ophthalmol. 2009;44(4):451–456. doi: 10.3129/i09-108. [DOI] [PubMed] [Google Scholar]
- 18.WuDunn D, Phan AD, Cantor LB, Lind JT, Cortes A, Wu B. Clinical experience with the Baerveldt 250-mm2 Glaucoma Implant. Ophthalmology. 2006;113(5):766–772. doi: 10.1016/j.ophtha.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 19.Yalvac IS, Eksioglu U, Satana B, Duman S. Long-term results of Ahmed glaucoma valve and Molteno implant in neovascular glaucoma. Eye (Lond) 2007;21(1):65–70. doi: 10.1038/sj.eye.6702125. [DOI] [PubMed] [Google Scholar]
- 20.Netland PA, Ishida K, Boyle JW. The Ahmed glaucoma valve in patients with and without neovascular glaucoma. J Glaucoma. 2010;19(9):581–586. doi: 10.1097/IJG.0b013e3181ca7f7f. [DOI] [PubMed] [Google Scholar]
- 21.Zhou MW, Wang W, Huang WB, Chen SD, Li XY, Gao XB, Zhang XL. Adjunctive with versus without intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in the treatment of neovascular glaucoma. Chin Med J (Engl) 2013;126(8):1412–1417. [PubMed] [Google Scholar]
- 22.Sevim MS, Buttanri IB, Kugu S, Serin D, Sevim S. Effect of intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in neovascular glaucoma. Ophthalmologica. 2013;229(2):94–100. doi: 10.1159/000345490. [DOI] [PubMed] [Google Scholar]
- 23.Almobarak F, Khan AO. Complications and 2-year valve survival following Ahmed valve implantation during the first 2 years of life. Br J Ophthalmol. 2009;93(6):795–798. doi: 10.1136/bjo.2008.150037. [DOI] [PubMed] [Google Scholar]
- 24.Alkawas AA, Shahien EA, Hussein AM. Management of neovascular glaucoma with panretinal photocoagulation, intravitreal bevacizumab, and subsequent trabeculectomy with mitomycin C. J Glaucoma. 2010;19(9):622–626. doi: 10.1097/IJG.0b013e3181ccb794. [DOI] [PubMed] [Google Scholar]