Abstract
Objective
With the advent of highly active anti-retroviral therapy, HIV disease has become a chronic condition, but with a number of metabolic complications including insulin resistance and diabetes mellitus, dyslipidemia and hypertension and an increased incidence of atherosclerosis. The aim of the current study was to test the safety and efficacy of chromium picolinate for HIV- associated insulin resistance.
Materials/Methods
The study was a randomized, double-blind, placebo-controlled trial with subjects receiving 500μg of chromium picolinate or placebo twice daily for two months. HIV- infected subjects were selected based on a fasting concentration of plasma glucose greater than 5.5mmol/L or a plasma glucose concentration of greater than 7.7mmol/L (but less than 11mmol/L) 2h after oral ingestion of 75g of glucose. Insulin sensitivity was assessed with a hyper-insulinemic-euglycemic clamp and glucose tolerance was assessed with the oral glucose tolerance test. Subjects were monitored closely for alterations in viral load, CD4+ cells, hemoglobin and hematocrit, kidney and liver function, and fasting lipid profiles.
Results
Forty-three subjects were enrolled and 39 completed the protocol (20 in the chromium-supplemented and 19 in the placebo arm). Following chromium-supplementation, there were no significant changes in either insulin sensitivity or glucose tolerance. There was a significant improvement in serum HDL cholesterol concentration in the group supplemented with chromium.
Conclusions
Chromium picolinate supplementation at this level was well-tolerated, but overall was not an effective therapy for insulin resistance in these HIV-infected subjects.
Keywords: Chromium picolinate, Glucose intolerance, Prediabetes
Introduction
Multi-drug regimens called highly active antiretroviral therapy (HAART) have changed HIV disease from a life-threatening, terminal illness to a chronic disease. Unfortunately, prolonged survival is accompanied by metabolic abnormalities in carbohydrate metabolism, including an increased incidence of insulin resistance, dyslipidemia, hypertension, and increased waist-to-hip ratio [1,2], a compilation of abnormalities now known as the metabolic syndrome [3] and overt diabetes mellitus. The prevalence of insulin resistance among HIV-infected individuals is high, with estimates of up to 46% reported by Behrens et al. with 13% classified as diabetic based on an oral glucose tolerance test [4]. In the Multicenter AIDS Cohort Study, Brown et al found the prevalence of overt diabetes mellitus to be 14% representing a fourfold elevation in HIV-infected men compared to sero-negative men [5]. Since insulin resistance and diabetes are independent risk factors for cardiovascular disease [6-8], HAART results in a decline in HIV-associated deaths, but increased mortality and morbidity due to heart disease [9-11] with an increased relative risk of acute myocardial infarction of 1.75-fold in subjects with HIV-disease compared to subjects without HIV disease [11].
While there are medications which can delay the progression of insulin resistance to overt diabetes, co-morbidities in the HIV-infected population may make the use of such medications problematic; co-morbidities including HIV medications, history of drug abuse, alcoholism, hepatitis, and sexually transmitted diseases [12,13]. In addition, some of the available insulin sensitizers are also problematic in this population, as evidenced by the elevated risk of cardiovascular disease associated with rosiglitazone use and the very rare potential for metabolic acidosis associated with metformin administration [14-16]. Thus both the increased risk of diabetes and the potential for adverse side-effects with current medications in individuals with HIV-infection contribute to the need for new treatments for this population.
Chromium is a nutrient that potentiates the action of insulin and may be an essential element for glucose metabolism [17]. Improved insulin sensitivity in response to chromium supplementation in subjects with insulin resistance associated with diabetes, ageing, and other conditions has been reported by numerous investigators (e.g. [18-29]). Moreover, chromium supplementation appears to be safe. An estimated 10 million Americans use chromium daily [30], with only a few scattered case reports of serious side effects [31,32]. The ability of chromium to improve insulin sensitivity with apparently few serious side effects suggested a possible role for chromium supplementation in subjects with insulin resistance associated with HIV disease. A preliminary, open-label study of 6 subjects given 1000μg chromium picolinate for 8 weeks demonstrated an improvement of 25% in insulin sensitivity [33]. Based on these encouraging preliminary results, the current study was designed as a randomized, double- blind, placebo-controlled, 2-month trial of daily oral 1000μg of chromium picolinate in subjects with HIV disease and impaired glucose tolerance. The primary outcome was to assess quantitative improvements in insulin-mediated glucose disposal using the hyerinsulinemic-euglycemic clamp and the secondary outcome was to assess changes in AKT (a measure of insulin signaling) after chromium.
Methods
Subjects
Subjects for this study were recruited from the patient population at Stony Brook University Medical Center and surrounding areas. Subjects had clinically stable HIV disease (CD4+ cells above 300/ml and viral burden less than 35,000 copies/ml), included both genders over the age of 18 years, and had been on stable antiretroviral regimens for at least 3 months prior to study. All subjects were on highly active anti-retroviral therapy (HAART). Subjects were screened with an oral glucose tolerance test and were deemed eligible if their fasting glucose was between 5.56 and 7mmol/L and/or their two hour post-glucose load was between 7.78 and 11.11mmol/L. Subjects with overt diabetes were excluded. Because of the potential for chromium picolinate to cause oxidative damage, subjects were also monitored for plasma concentration of selenium and zinc (fluorometric analysis and flame atomic absorption, respectively by Associated Regional and University Pathologists, Inc. Salt Lake City, UT) and were excluded if deficient (i.e., less than 85 μg selenium /ml or less than 0.35 μg zinc/ml), thus ensuring that enrolled subjects would not have a deficit in anti-oxidant capacity. Over 100 subjects were screened and forty-three subjects were randomized.
Study design
The study was a single-center, randomized, double-blind, placebo-controlled trial. Subjects with glucose intolerance based on the oral glucose tolerance test were randomized with a permuted block design stratified by gender and age (above and below 45y) to receive either 500 μg of chromium picolinate (Nutrition 21 Company, Purchase, NY with independent analysis confirming 541 μg chromium per tablet) twice daily or matching placebo (dicalcium phosphate with independent analysis confirming no detectable chromium) for a period of 8 weeks. All study personnel and participants were blinded to treatment assignment until data collection and laboratory analyses were complete. Measurements of insulin sensitivity by oral glucose tolerance and hyperinsulinemiceuglycemic clamp were performed in the fasting from 22:00 h of the previous day) state. Samples of adipose tissue for in vitro analysis of the insulin signaling pathway through AKT (protein kinase B) at baseline and following 8 weeks of chromium supplementation were taken under local anesthesia from the lateral thigh.
Subjects returned for monitoring of safety parameters at 2 weeks, 1 m and 2 m. Safety monitoring included assessment of serum creatinine, liver function (assessed by total bilirubin, direct bilirubin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, albumin and total protein), CD4+ cells and viral burden. This study was approved by the Committee on Research Involving Human Subjects, the Stony Brook University IRB. All subjects gave their informed written consent and this trial was registered at ClinicalTrials.gov reference number NCT00109746.
Measurements and assays
Insulin sensitivity
Sensitivity to insulin was assessed from fasting glucose and insulin values; i.e. (fasting glucose in mmol/L × fasting insulin in μU/ml)/22 based on the Homeostasis Model Assessment (HOMA) described by Matthews et al. [34] and from an oral glucose tolerance test (OGTT) where plasma glucose concentrations were measured at 30 minute intervals up to 180 minutes following ingestion of 75g of glucose (Glucola, Ames Co., Elkhart, IN) after an overnight fast. Insulin sensitivity from the OGTT was assessed as the area under the plasma glucose concentration × time curve. Insulin sensitivity was also assessed as glucose disposal (Rd) during an hyperinsulinemic euglycemic clamp, determined from the rate of glucose infusion necessary to maintain plasma glucose at 5 mmol/L during intravenous infusion of 1.2 mU insulin (Humulin, Eli Lily, Indianapolis, IN) /kg/ min as previously described [35-39]. Glucose disposal is expressed per kg lean body mass to correct for differences in body composition among the subjects.
Insulin signaling
The ability of chromium supplementation to affect insulin signaling through the AKT or protein kinase B pathway in adipose tissue was assessed in biopsy specimens (50-75mg) incubated in 10nM insulin in Hanks buffered saline solution for 30 min at 37°C, blotted and frozen in liquid nitrogen until analysis. Determination of total and phosphor AKT was made following homogenization in RIPA (radio-immunoprecipitation assay) buffer with protease and phosphatase inhibitors and centrifugation. The lysate was assessed for total and phospho AKT with PathScan TotalAKT1 and PathScan PhosphoAKT1 (Ser473) assay kits from Cell Signaling Technology (Danvers, MA). Data were normalized to protein content with a bicinchoninic acid (BCA) kit also from Cell Signaling Technology.
Chromium status and compliance
Chromium status at study entry and compliance with the regimen of chromium supplementation was assessed from 24 hour collections of urine analyzed for chromium by inductively coupled plasma mass spectrometry (reference interval 0.5-5.0 μg/liter (Associated Regional and University Pathologists, Inc. Salt Lake City, UT) and corrected for completeness of collection by expression as the chromium to creatinine ratio. The ability of 24-hour urinary chromium excretion to indicate recent chromium intake is supported by the study of Anderson et al. [20]. Compliance was determined from urinary chromium excretion and from the number of pills returned at 2 weeks, 1 month and 2 months. Subjects were taken to be compliant if they returned less than 20% of their pills.
Lipodystrophy score
A lipodystrophy score (scale 0-18) was based on clinical assessment of loss of fat from the face, limbs, and buttock, the presence of, prominent superficial veins, increased fat on back of neck (buffalo hump), lipomas, increased abdominal fat, and breast hypertrophy
Body composition
Lean body mass and distribution of body fat were determined by Dual Energy X-ray absorptiometry (DEXA) with a whole-body scanner (Hologic Inc., Bedford, MA).
Viral load
Plasma samples for the quantification of HIV RNA were frozen and sent to the Department of Pathology at Stony Brook for analysis (NY State approved) RT-PCR. The assay has a lower limit of detection of 50 copies/ml.
CD4+ cells
Measurements of CD4+ cells in HIV-infected individuals were made by the Flow Cytometry Core Facility, Stony Brook Medical Center. The laboratory is an AIDS Clinical Trials Group certified and monitored laboratory.
Plasma proteins and metabolites
Glucose was measured by a glucose oxidase method with a Beckman Glucose Analyzer 2 (Fullerton, CA). Insulin was measured by radioimmunoassay (Diagnostic Products Corporation, Los Angeles, CA). C-reactive protein (CRP) and hemoglobin A1C were measured in the clinical laboratory of the University Hospital Medical Center by nephelometry and HPLC respectively.
Oxidative stress (8-hydroxydeoxyguanosine and total alkenals)
Early morning urine samples were assessed for 8-hydroxydeoxguanosine, a measure of oxidative damage of DNA [40] and total alkenals, a measure of the potential for lipid peroxidation [41,42]. 8-hydroxydeoxyguanosine was determined with an ELISA assay and total alkenals by spectrophotometric assay (Genox, Baltimore, MD); both values were normalized to urinary creatinine.
Statistical analyses
Subject demographic characteristics between the chromium-supplemented and placebo groups were compared with the Chi Square test of association for categorical variables and the independent samples t-test for continuous variables. Within the groups, a paired samples T-test was used to assess the change in mean values for the clinical measures from baseline to week 8, with one exception; HOMAIR is presented as the median value at the two time points and change over time (within groups) was evaluated with the non-parametric Wilcoxon test. Because the baseline measures of all clinical values for the treatment and placebo groups were statistically similar (p > 0.05), the independent samples T-test was used to compare the absolute change (post supplement – baseline measures) except for the change in HOMA-IR which was evaluated with the Mann Whitney U test. Data were analyzed as 2-sided tests the SPSS statistical package (version 19.0, SPSS® Inc., Chicago, IL) and differences were considered significance if P < 0.05.
Results
Forty-three subjects with HIV disease were randomized and 39 completed the study; 19 in the placebo arm and 20 in the chromium-supplemented group. Two subjects were withdrawn for safety concerns and three subjects were not able to complete their study visits.
Subject characteristics at baseline
The demographics of the two subject groups are shown in Table 1. The mean age for the study was 47 years and the subjects were predominantly African American and male. There were no significant differences between the placebo and chromium-supplemented groups. The subjects were mostly smokers (35/39) and had HIV disease for an average of 16 years but were stable with CD4+ cells of >600/ml and viral burden of about 200 copies/mL No subject had a change in antiretroviral regimen in the three months prior to study. At baseline, the plasma glucose concentration was 5.7mmol/L ± 0.7 in the placebo group and 5.8 ± 0.1 in the chromium-supplemented group with hemoglobin A1C of 5.6% ± 0.7 (placebo group) and 5.5 ± 0.5 (chromium-supplemented group). In the chromium-supplemented group, 20% of the patients had baseline glucose of ≥ 6.1 mmol/L, compared to 16% in the placebo group. There were no differences in insulin sensitivity at baseline between the groups assigned to the placebo group or the chromium-supplemented group. At baseline the mean HOMA values were 1.55 ± 0.46 in the placebo group and 1.24 ± 0.29 in the group supplemented with chromium. The mean area under the curve for glucose concentration (mmoles/L) × time (min) for the interval 0-180min during the OGTT was 1490 in the placebo group and 1495 in the group receiving chromium. The mean Rd in the placebo group was 8.19 ± 0.58 mg glucose/kg/min and 7.44 ± 0.69 in the chromium-supplemented group. Fasting serum LDL-cholesterol levels were 2.3nmol/L ± 0.24 in the placebo group and 2.41nmol/L ± 0.18 in the chromium-supplemented group and HDL-cholesterol was 1.14nmol/L ± 0.11 in group receiving placebo and 1.01nmol/L ± 0.05 in the group receiving chromium. Serum triglycerides were also similar in the two groups; 1.57nmol/L ± 0.23 in the group receiving placebo and 1.68nmol/L ± 0.17 in the group receiving chromium. Subjects were in the overweight category with body mass index of 28kg/m2 ± 1 in the placebo group and 28 ± 1 in the chromium-supplemented group. Clinical assessment of body habitus indicated a similar degree of lipodystrophy, with a lipodystrophy rating. 2.2 ± 0.41 in the group receiving chromium and 2.10 ± 0.41 in the placebo group on a scale (0 to 18) based on assessment of fat loss from the face, arms, legs and buttocks, the presence of prominent superficial veins, a Buffalo hump, lipomas, and increased abdominal fat, and breast hypertrophy. DEXA assessment confirmed similar proportion of body fat in the limbs in both groups at baseline; placebo 48.9% ± 1.7 and chromium-supplemented group 45.8% ± 1.
Table 1.
Subject characteristics and medications.
| Chromium (n = 20) | Placebo (n = 19) | |
|---|---|---|
| Age (y) | 47.6 ± 1.7 | 47.3 ± 1.7 |
| Gender (male/female) | 13/7 | 13/6 |
| Race (Caucasian/African American) | 7/13 | 6/13 |
| BMI (kg/m2) | 28.2 ± 0.8 | 26.9 ± 1 |
| CD4 (count/mm3) | 676 ± 78 | 686 ± 61 |
| Duration of HIV Infection (y) | 16.8 ± 1.4 | 16.3 ± 1.0 |
| LipodystrophyScorea | 2.2 ± 0.41 | 2.1 ± 0.41 |
| Smokers (%) | 17/20 (85%) | 18/19 (94.7%) |
| Viral Load (copies/mL) | 188 ± 67 | 268 ± 210 |
| Subjects with fasting glucose ≥ 6.1 mmol/L (%) | 4/20 (20%) | 3/19 (15.8%) |
| Subjects with triglycerides ≥ 2 mmol/L (%) | 6/20 (30%) | 7/18 (38.9%) |
| Subjects with total cholesterol ≥ 5.5 mmol/L (%) | 2/20 (10%) | 1/18 (5.6%) |
| Subjects with HDL-cholesterol ≤ 0.9 mmol/L (%) | 7/20 (35%) | 6/18 (33.3%) |
| Medications | Chromium (n = 20) | Placebo (n = 19) |
|---|---|---|
| Lipid Lowering | 5/20 (25%) | 1/19 (5.3%) |
| Cardiovascular | 5/20 (25%) | 3/19 (15.8%) |
| Antidepressant | 5/20 (25%) | 6/19 (31.6%) |
| Nucleoside Reverse Transcriptase Inhibitors | 15/20 (75%) | 13/19 (68.4%) |
| Non- Nucleoside Reverse Transcriptase Inhibitors | 4/20 (20%) | 2/19 (10.5%) |
| Protease Inhibitors | 11/20 (55%) | 12/19 (63.2%) |
| Fusion Inhibitors | 1/20 (5%) | 0/19 (0%) |
| Atripla (NNRTI + NRTI + NRTI) | 4/20 (20%) | 3/19 (15.8%) |
Results are reported as Mean ± SEM or % of subjects. Chi-square and un-paired Student t-test were used to compare the two groups and there were no significant differences between the groups. NNRTI is non-nucleoside reverse transcriptase inhibitors; NRTI is nucleoside reverse transcriptase inhibitors.
Lipodystrophyscore based physician assessment (scale 0-18)
Subjects were on a variety of anti-retroviral regimens including nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors and protease inhibitors, but the spectrum of medications was not different between the groups (Table 1). Typical of this population, study subjects were also on medications for medical problems, but again these were not different between the groups.
Prior to the intervention, there was no difference in chromium excretion between subjects allocated to placebo (0.05μg Cr/g creatinine ± 0.01) or chromium-supplementation (0.03μg Cr/g creatinine ± 0.004).
Changes in metabolic parameters with intervention
Following 8 weeks of treatment with either placebo or 1000 μg chromium (as chromium picolinate) /d supplementation, urinary chromium excretion was unchanged in the placebo group but increased significantly in the group receiving chromium supplements (0.03μgCr/g creatinine ± 0.004 vs 0.52 ± 0.058, P<0.001 with paired t). However, there were no statistically significant differences in response of fasting glucose levels, HOMA-IR, area under the glucose concentration × time curve for 180min following ingestion of 75g of glucose, or glucose disposal measured during the hyperinsulinemic-euglycemic clamp between the group receiving placebo and the group receiving chromium supplementation (assessed by independent t test, Table 2). Since the action of chromium has been linked to enhanced intracellular signaling in response to insulin (e.g. [43-45]), an assessment was made of the phosphorylation of AKT (protein kinase B) in biopsy specimens incubated in high-dose insulin as an index of the capacity of insulin signaling to be altered by chromium supplementation. There were also no changes in the degree of phosphorylation of AKT in biopsy specimens of adipose tissue incubated with 10mM insulin (data not shown). The serum levels HDL cholesterol was significantly increased in the chromium treated patients compared to the placebo and serum levels of triglycerides and C- reactive protein were unchanged in both groups (Table 2).
Table 2.
Metabolic changes with chromium supplementation for 2 months compared to placebo.
| Chromium (N = 20) | Placebo (N = 19) | P-value changea | |||
|---|---|---|---|---|---|
| Variable | Baseline | Week 8 | Baseline | Week 8 | |
| HOMA – IRb (units) | 0.96 (1.35) | 1.25 (2.09) | 0.64 (3.24) | 0.64 (2.04) | NSc |
| Glucose AUC | 5.77 ± 0.11 | 6.07 ± 0.13 | 5.78 ± 0.07 | 5.87 ± 0.15 | NS |
| Triglycerides (mmol/L) | 1.68 ± 0.17 | 1.64 ± 0.17 | 1.65 ± 0.23 | 1.30 ± 0.21 | NS |
| Total Cholesterol (mmol/L) | 4.18 ± 0.20 | 4.21 ± 0.18 | 4.38 ± 0.21 | 4.04 ± 0.18 | 0.013 |
| LDL Cholesterol (mmol/L) | 2.41 ± 0.18 | 2.61 ± 0.28 | 2.43 ± 0.21 | 2.24 ± 0.20 | NS |
| HDL Cholesterol (mmol/L) | 1.01 ± 0.05 | 1.12 ± 0.06 | 1.20 ± 0.09 | 1.21 ± 0.09 | 0.043 |
| CD4 Count | 676 ± 78 | 762 ± 104 | 686 ± 60.67 | 674.5 ± 63.21 | NS |
| CRP (mg/dL) | 2.9 ± 0.3 | 5.5 ± 0.5 | 3.2 ± 0.2 | 3.1 ± 0.2 | NS |
| Systolic BP (mm/Hg) | 128 ± 2 | 130 ± 3 | 124.3 ± 3.29 | 124.3 ± 2.86 | NS |
| Diastolic BP (mm/Hg) | 77 ± 2 | 74 ± 3 | 77.3 ± 2.03 | 74 ± 1.94 | NS |
| BMI (kg/m2) | 28.2 ± 0.8 | 27.3 ± 0.7 | 26.8 ± 1.1 | 27.0 ± 0.83 | NS |
| Limb Fat % | 45.9 ± 1.8 | 45.6 ± 2.4 | 49.2 ± 1.81 | 48.5 ± 7.84 | NS |
| Trunk Fat % | 54.2 ± 1.8 | 52.7 ± 1.8 | 50.9 ± 1.81 | 50.7 ± 1.65 | NS |
from an independent t –test comparing the change in the chromium group and the change in the placebo group
HOMA – IR is represented as the median (range), this variable only
from a Mann Whitney U test for this variable only
Glucose AUC is the area under the curve of the glucose × time curve for 0-180min following oral ingestion of 75g of glucose; CRP is C-reactive protein; limb and trunk fat % are the proportion of fat present in limbs or trunk as a proportion of total body fat, expressed as a %. Data are expressed as mean ± SEM. NS is non-significant, i.e. P>0.05.
Body composition, measured with DEXA, including (head fat, left arm fat, right arm fat, trunk fat, left leg fat and right leg fat) did not change with chromium supplementation. The distribution of body fat in the periphery, i.e. body fat present in the limbs expressed as a proportion of total body fat (% limb fat), was not altered by supplementation with chromium. Similarly there was no change in % trunk fat or BMI. Systolic and diastolic blood pressure also remained unchanged in both groups during the intervention period.
Safety parameters
The subjects were followed very closely during this 8 week therapy trial. Two subjects from the chromium-supplemented group were withdrawn from the trial; one because of hives and one for elevated liver function tests. Both the hives and the liver function abnormalities resolved after discontinuation of the therapy. There were no significant changes in any other parameters, viral load, CD4 count, hemoglobin or hematocrit, electrolytes, or renal function. As a measure of oxidative DNA damage, urinary excretion of 8-hydroxydeoxyguanosine (8,OHdG) was assessed at baseline, and at 1 month and 2 months of study. To correct for incomplete urine collections, the data are expressed as ng per mg of urinary creatinine. There was a significant difference in baseline excretion of 8, OHdG between the individual allocated to receive placebo (10.9 ± 0.3 ng/mg creatinine) and those allocated to receive chromium (7.21 ± 0.29). In both groups, 8,OHdG excretion was higher at 1 month compared to excretion at baseline, but returned to baseline values by 2 months. The change within groups with time is significant (P<0.001, repeated measures test), but there was no significant time × drug interaction (P=0.95). Since the urinary excretion of 8, OHdG was higher in individuals receiving placebo as well as chromium, it is unlikely that this excretion was related to chromium supplementation per se. Urine at baseline and 2 months was collected in the controlled hospital setting which may have differed from home environments in important aspects such as smoking, which is known to affect 8,OHdG [46]. Urinary excretion of total alkenals adjusted for urinary creatinine excretion was not different between the groups at baseline and did not change over time.
Compliance was assessed by pill counts performed at 2 week, 1 month and 2 month follow up appointments via a questionnaire. Subjects were deemed compliant if they consumed 80% of their allotted pills. Based on this criterion, in the placebo group, 15 of the 19 subjects were compliant and in the group allocated to chromium supplementation, 18 of the 20 were compliant.
Discussion
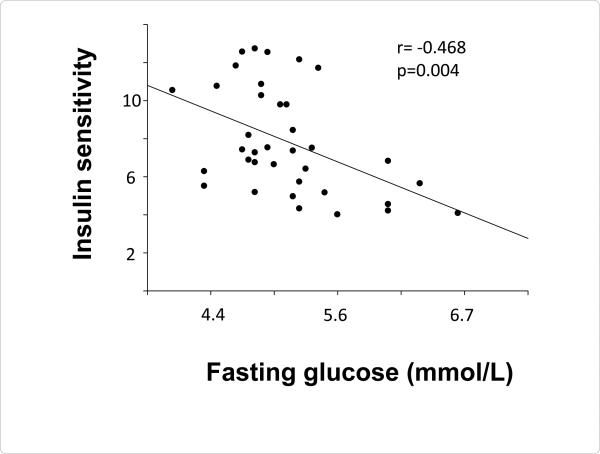
In this study to determine the effect of chromium supplementation on insulin sensitivity in subjects with HIV disease, multiple measurements of insulin sensitivity were employed. Although the hyperinsulinemiceuglycemic clamp has the greatest sensitivity for assessing changes in insulin sensitivity, it is not a practical measurement in a clinical setting. Therefore, more clinically relevant measures including fasting glucose, fasting glucose with a measurement of fasting insulin (HOMA-IR), and plasma glucose concentration after an oral glucose load, OGTT were also employed. Insulin sensitivity assessed with the hyperinsulinemiceuglycemic clamp (expressed as glucose disposal rate per kg lean body mass or Rd) was significantly related to the concentration of plasma glucose during fasting (r=-0.468, P=0.004, Figure 1 and HOMA-IR (r=0.474, P=0.007). Baseline assessment of insulin sensitivity by OGTT (i.e. the area under the curve of plasma glucose concentrations and time for 0-180min following ingestion of 75g of glucose) also correlated with fasting glucose concentration (r=0.33, P=0.04). In previously published work, we found a relationship between insulin sensitivity measured by the hyperinsulinemic-euglycemic clamp and the level of inflammation as determined by the type 2 soluble receptor for tumor necrosis factor α [39]. In the present study, inflammation was assessed by the more clinical measure of C-reactive protein (CRP) which did not correlate with insulin sensitivity (expressed as Rd, R-0.22, P=0.2).
Figure 1.
Correlation of fasting plasma glucose concentration with insulin sensitivity assessed with a hyperinsulinemic-euglycemic clamp.
The current study did not show any effect of chromium-supplementation on the any measures of insulin sensitivity. There were no changes in Rd, HOMA-IR or glucose AUC following ingestion of 75g glucose in either the chromium-supplement or placebo groups. This finding is very different from our pilot study which demonstrated a 25% increase in insulin sensitivity with the same time period and same treatment of 1000μg of chromium as chromium picolinate per day in subjects with HIV disease-associated insulin resistance [33]. The discrepancy between the initial study and the current one may arise from the greater number of subjects in the present study, but it is also possible that the result is due to the greater insulin resistance in the subjects of the pilot study (average Rd = 4.5) and the present study (average Rd=7.9).
Studies with chromium supplementation of subjects with type 2 diabetes have also found some inconsistency in response with some studies showing a clear benefit (e.g. review by Balk et al. [47]) while others have not (e.g. [48-50]. In subjects with type 2 diabetes, a clinical response to chromium supplementation may be greater in subjects with marginal or subclinical deficiency of chromium at baseline [51]. In our subject population, there was no demonstrable relationship between baseline chromium status assessed from urinary chromium excretion and the improvement in insulin sensitivity.
In studies examining the role of chromium supplementation in improving glucose metabolism in subjects with type 2 diabetes, it has been proposed that those with greater insulin resistance, i.e., higher proportion of hemoglobin as hemoglobin A1C, have a better response to chromium for improvement in glucose metabolism [21,52]. In a large study of 137 patients with type 2 diabetes, who were treated with 1000 micrograms of chromium picolinate daily for twenty-four weeks, the subjects who were more insulin resistant, with higher fasting plasma glucose concentrations and higher levels of hemoglobin A1C, had the greatest improvement in insulin sensitivity on chromium as assessed by the hyperinsulinemic-euglycemic clamp [21]. In earlier studies by others, baseline insulin sensitivity as measured by the clamp technique was the only variable which correlated significantly with the response to chromium, i.e., the lower the insulin sensitivity, the better the clinical response to chromium [52,53]. Therefore, differing sensitivity to insulin in the study populations may contribute to the divergent conclusions on the effect of chromium supplementation in subjects with HIV disease-associated insulin resistance between our pilot study and the current randomized, placebo- controlled trial.
In contrast, Aghdassi et al. reported that daily supplementation of HIV-infected individuals with 400mcg of chromium as chromium picolinate for 16 weeks with insulin resistance determined by HOMAIR > 2.5 showed a significant improvement in HOMA-IR, insulin levels, triglycerides, and total and trunk body fat in comparison to subjects receiving a placebo [54]. In that study there was no improvement in the plasma concentrations of fasting glucose, C-peptide, hemoglobin A1c or serum cholesterol, HDL and LDL [54]. While compliance is always an issue in human studies, the number of pills returned and from the urinary excretion of chromium at 8 weeks in the current study would suggest that most subjects were taking the chromium supplements. Although it is possible that an 8-week trial compared with the 16-week trial reported by Aghdassi et al. [54] was not sufficiently long to demonstrate improved insulin sensitivity. This too seems unlikely since our pilot study employed an 8-week supplementation period and demonstrated significant improvement in insulin sensitivity [33].
What seems more likely is that there are only a sub-set of subjects with insulin resistance who respond to chromium supplementation. In this group of 20 subjects taking supplemental chromium, 5 subjects responded with an increase in insulin sensitivity (in this group Rd was 6.84 ± 0.99 at baseline and 9.37 ± 1.7 after supplementation, P=0.03 by paired T test). Although there are good correlations of the multiple measures of insulin sensitivity employed in this study (i.e. fasting plasma glucose concentrations, HOMA-IR, OGTT-AUC), the only measure which identified a subgroup of responders was the hyperinsulinemiceuglycemic clamp, indicating greater sensitivity with the clamp technique compared to other measures as has been previously reported [55]. Distinction between responders and non-responders has also been reported in a study of diabetic subjects treated with chromium [52]. In that study, higher fasting plasma glucose concentrations and higher levels hemoglobin A1c at baseline were associated with better clinical outcome; with baseline insulin sensitivity accounting for about 40% of the variance in clinical response to chromium supplementation [52]. In the present study we did not see an overall relationship between baseline assessment of insulin sensitivity and response to chromium, but it is possible that the sample was too small or that there are other variables which contribute to a clinical response to chromium supplementation in individuals with HIV disease. Although it was not possible to identify these variables, the demonstrated safety of chromium supplementation suggests that chromium supplementation may be an appropriate treatment for some individuals.
The present study is limited by the small number of subjects [39] and the relatively short duration of the trial (8 weeks); although, in our pilot study, 8 weeks was sufficient to observe significant improvement in insulin sensitivity [33]. In human studies, compliance is always a concern, however the data on pill counts suggests that the most of the study subjects were at least 80% compliant in taking the study drug. In addition, the subjects in this study were mostly African American, so the findings may be limited to this racial group. Given these limitations, this randomized, double-blind, placebo-controlled trial assessing the safety and efficacy of chromium picolinate at 1000μg daily for 8 weeks in subjects with HIV disease did not demonstrate a significant improvement in insulin sensitivity measured by the hyperinulinemiceuglycemic clamp, HOMA-IR, or oral glucose tolerance test, though there were also no safety concerns. In addition, there were no changes in body composition or other metabolic parameters such as triglycerides or total cholesterol, but there was a significant increase in HDL cholesterol levels in the chromium-treated group. The results suggest that chromium treatment may be beneficial in only some subjects with HIV disease, possibly those who are more insulin resistant as demonstrated in our original pilot study and a subset of subjects in the current trial.
Acknowledgements
The authors thank Nutrition 21 for providing the chromium picolinate and placebo for this study. The authors also gratefully acknowledge the contributions of the clinical, nursing and core laboratory staff of the General Clinical Research Center and the study coordinators, Joyce Quick, Jean Kidd, Barbara Lubarda, Teresa Hunt-Goncalves and Jennifer Intravaia, without whose assistance this study could not have been performed.
Funding
This work was supported by NIH grant (MCG) number 1RO1AT002499 and the NCRR General Clinical Research Center grant MO1RR10710-02. Drs. Stein and Philips were supported by the Empire Clinical Research Investigator Program through the Department of Education in New York State.
Abbreviations
- AKT
Protein kinase B
- BMI
Body Mass Index
- AUC
Area Under The Curve
- Cr
Chromium
- CRP
C-reactive protein
- DEXA
Dual energy X-ray absorptiometry
- HAART
Highly Active Anti-Retroviral Therapy
- HOMA and HOMA-IR
Homeostasis Model Assessment and Homeostasis Model Assessment of insulin resistance, 8, OHdG, 8, hydroxydeoxyguansoine
- OGTT
Oral Glucose Tolerance Test
- Rd
The rate of glucose disposal during a hyperinsulinemiceuglycemic clamp
Footnotes
Citation: Stein SA, Mc Nurlan M, Phillips BT, Messina C, Mynarcik D, et al. (2013) Chromium Therapy for Insulin Resistance Associated with HIV-Disease. J AIDS Clin Res 4: 239. doi: 10.4172/2155-6113.1000239
Conflict of interest
None of the authors have any conflict-of-interest with the submitted studywhich was funded by the National Institutes of Health (ClinicalTrials.gov reference number NCT00109746). Nutrition 21 provided the chromium picolinate and matching placebo.
References
- 1.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 2.Guaraldi G, Stentarelli C, Zona S, Orlando G, Carli F, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208:222–227. doi: 10.1016/j.atherosclerosis.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 4.Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, et al. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–F70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 5.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.Després JP, Lamarche B, Mauriège P, Cantin B, Dagenais GR, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 7.Haffner SM. Epidemiological studies on the effects of hyperglycemia and improvement of glycemic control on macrovascular events in type 2 diabetes. Diabetes Care 22 Suppl. 1999;3:C54–56. [PubMed] [Google Scholar]
- 8.Haffner SM, Miettinen H. Insulin resistance implications for type II diabetes mellitus and coronary heart disease. Am J Med. 1997;103:152–162. doi: 10.1016/s0002-9343(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 9.Barbaro G, Di Lorenzo G, Cirelli A, Grisorio B, Lucchini A, et al. An open-label, prospective, observational study of the incidence of coronary artery disease in patients with HIV infection receiving highly active antiretroviral therapy. Clin Ther. 2003;25:2405–2418. doi: 10.1016/s0149-2918(03)80283-7. [DOI] [PubMed] [Google Scholar]
- 10.Friis-Møller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 11.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butt AA, Evans R, Skanderson M, Shakil AO. Comorbid medical and psychiatric conditions and substance abuse in HCV infected persons on dialysis. J Hepatol. 2006;44:864–868. doi: 10.1016/j.jhep.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Zenilman JM, Hook EW, 3rd, Shepherd M, Smith P, Rompalo AM, et al. Alcohol and other substance use in STD clinic patients: relationships with STDs and prevalent HIV infection. Sex Transm Dis. 1994;21:220–225. doi: 10.1097/00007435-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 15.Senior PA. Type 2 diabetes, metformin and lactic acidosis-defining the risk and promoting safe practice. Diabet Med. 2012;29:161–163. doi: 10.1111/j.1464-5491.2011.03469.x. [DOI] [PubMed] [Google Scholar]
- 16.Ziyadeh N, McAfee AT, Koro C, Landon J, Arnold Chan K. The thiazolidinediones rosiglitazone and pioglitazone and the risk of coronary heart disease: a retrospective cohort study using a US health insurance database. Clin Ther. 2009;31:2665–2677. doi: 10.1016/j.clinthera.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz K, Mertz W. A glucose tolerance factor and its differentiation from factor 3. Arch Biochem Biophys. 1957;72:515–518. doi: 10.1016/0003-9861(57)90228-x. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RA, Cheng N, Bryden NA, Polansky MM, Cheng N, et al. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RA, Polansky MM, Bryden NA, Canary JJ. Supplemental-chromium effects on glucose, insulin, glucagon, and urinary chromium losses in subjects consuming controlled low-chromium diets. Am J Clin Nutr. 1991;54:909–916. doi: 10.1093/ajcn/54.5.909. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RA, Polansky MM, Bryden NA, Roginski EE, Mertz W, et al. Chromium supplementation of human subjects: effects on glucose, insulin, and lipid variables. Metabolism. 1983;32:894–899. doi: 10.1016/0026-0495(83)90203-2. [DOI] [PubMed] [Google Scholar]
- 21.Cefalu WT, Rood J, Pinsonat P, Qin J, Sereda O, et al. Characterization of the metabolic and physiologic response to chromium supplementation in subjects with type 2 diabetes mellitus. Metabolism. 2010;59:755–762. doi: 10.1016/j.metabol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gürson CT, Saner G. Effect of chromium on glucose utilization in marasmic protein-calorie malnutrition. Am J Clin Nutr. 1971;24:1313–1319. doi: 10.1093/ajcn/24.11.1313. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins LL, Jr, Ransome-Kuti O, Majaj AS. Improvement of impaired carbohydrate metabolism by chromium 3 in manourished infants. Am J Clin Nutr. 1968;21:203–211. doi: 10.1093/ajcn/21.3.203. [DOI] [PubMed] [Google Scholar]
- 24.Levine RA, Streeten DH, Doisy RJ. Effects of oral chromium supplementation on the glucose tolerance of elderly human subjects. Metabolism. 1968;17:114–125. doi: 10.1016/0026-0495(68)90137-6. [DOI] [PubMed] [Google Scholar]
- 25.Morris BW, Kouta S, Robinson R, MacNeil S, Heller S. Chromium supplementation improves insulin resistance in patients with Type 2 diabetes mellitus. Diabet Med. 2000;17:684–685. doi: 10.1046/j.1464-5491.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 26.Offenbacher EG, Pi-Sunyer FX. Beneficial effect of chromium-rich yeast on glucose tolerance and blood lipids in elderly subjects. Diabetes. 1980;29:919–925. doi: 10.2337/diab.29.11.919. [DOI] [PubMed] [Google Scholar]
- 27.Potter JF, Levin P, Anderson RA, Freiberg JM, Andres R, et al. Glucose metabolism in glucose-intolerant older people during chromium supplementation. Metabolism. 1985;34:199–204. doi: 10.1016/0026-0495(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 28.Urberg M, Zemel MB. Evidence for synergism between chromium and nicotinic acid in the control of glucose tolerance in elderly humans. Metabolism. 1987;36:896–899. doi: 10.1016/0026-0495(87)90100-4. [DOI] [PubMed] [Google Scholar]
- 29.Wilson BE, Gondy A. Effects of chromium supplementation on fasting insulin levels and lipid parameters in healthy, non-obese young subjects. Diabetes Res Clin Pract. 1995;28:179–184. doi: 10.1016/0168-8227(95)01097-w. [DOI] [PubMed] [Google Scholar]
- 30.Neilsen F. Controversial chromium: does the superstar mineral of the mountebanks 500 receive appropriate attention from clinicians and nutritionists? Nutrition Today. 1996;31:226–233. [Google Scholar]
- 31.Cerulli J, Grabe DW, Gauthier I, Malone M, McGoldrick MD. Chromium picolinate toxicity. Ann Pharmacother. 1998;32:428–431. doi: 10.1345/aph.17327. [DOI] [PubMed] [Google Scholar]
- 32.Wasser WG, Feldman NS, D'Agati VD. Chronic renal failure after ingestion of over-the-counter chromium picolinate. Ann Intern Med. 1997;126:410. doi: 10.7326/0003-4819-126-5-199703010-00019. [DOI] [PubMed] [Google Scholar]
- 33.Feiner JJ, McNurlan MA, Ferris RE, Mynarcik DC, Gelato MC. Chromium picolinate for insulin resistance in subjects with HIV disease: a pilot study. Diabetes Obes Metab. 2008;10:151–158. doi: 10.1111/j.1463-1326.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Gavi S, Feiner JJ, Melendez MM, Mynarcik DC, Gelato MC, et al. Limb fat to trunk fat ratio in elderly persons is a strong determinant of insulin resistance and adiponectin levels. J Gerontol A Biol Sci Med Sci. 2007;62:997–1001. doi: 10.1093/gerona/62.9.997. [DOI] [PubMed] [Google Scholar]
- 36.Gavi S, Qurashi S, Stuart LM, Lau R, Melendez MM, et al. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity (Silver Spring) 2008;16:893–895. doi: 10.1038/oby.2007.138. [DOI] [PubMed] [Google Scholar]
- 37.Gelato MC, Mynarcik DC, Quick JL, Steigbigel RT, Fuhrer J, et al. Improved insulin sensitivity and body fat distribution in HIV-infected patients treated with rosiglitazone: a pilot study. J Acquir Immune Defic Syndr. 2002;31:163–170. doi: 10.1097/00126334-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 38.Lydic ML, McNurlan M, Bembo S, Mitchell L, Komaroff E, et al. Chromium picolinate improves insulin sensitivity in obese subjects with polycystic ovary syndrome. Fertil Steril. 2006;86:243–246. doi: 10.1016/j.fertnstert.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 39.Mynarcik DC, McNurlan MA, Steigbigel RT, Fuhrer J, Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–321. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 40.Shigenaga MK, Ames BN. Assays for 8-hydroxy-2'-deoxyguanosine: a biomarker of in vivo oxidative DNA damage. Free Radic Biol Med. 1991;10:211–216. doi: 10.1016/0891-5849(91)90078-h. [DOI] [PubMed] [Google Scholar]
- 41.Ollinger K, Brunmark A. Effect of different oxygen pressures and N,N'-diphenyl-p-phenylenediamine on Adriamycin toxicity to cultured neonatal rat heart myocytes. Biochem Pharmacol. 1994;48:1707–1715. doi: 10.1016/0006-2952(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 43.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132:1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 44.Davis CM, Vincent JB. Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry. 1997;36:4382–4385. doi: 10.1021/bi963154t. [DOI] [PubMed] [Google Scholar]
- 45.Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr. 2006;136:415–420. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- 46.Tamae K, Kawai K, Yamasaki S, Kawanami K, Ikeda M, et al. Effect of age, smoking and other lifestyle factors on urinary 7-methylguanine and 8-hydroxydeoxyguanosine. Cancer Sci. 2009;100:715–721. doi: 10.1111/j.1349-7006.2009.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized controlled trials. Diabetes Care. 2007;30:2154–2163. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 48.Gunton JE. Chromium supplementation does not improve glucose tolerance, 544 insulin sensitivity, or lipid profile: a randomized, placebo-controlled, double-blind trial of 545 supplementation in subjects with impaired glucose tolerance. Diabetes Care. 2005;28:712–3. 547. doi: 10.2337/diacare.28.3.712. [DOI] [PubMed] [Google Scholar]
- 49.Iqbal N, Cardillo S, Volger S, Bloedon LT, Anderson RA, et al. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metab Syndr Relat Disord. 2009;7:143–150. doi: 10.1089/met.2008.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleefstra N, Houweling ST, Jansman FG, Groenier KH, Gans RO, et al. Chromium treatment has no effect in patients with poorly controlled, insulin-treated type 2 diabetes in an obese Western population: a randomized, double-blind, placebo-controlled trial. Diabetes Care. 2006;29:521–525. doi: 10.2337/diacare.29.03.06.dc05-1453. [DOI] [PubMed] [Google Scholar]
- 51.Anderson RA. Chromium, glucose intolerance and diabetes. J Am Coll Nutr. 1998;17:548–555. doi: 10.1080/07315724.1998.10718802. [DOI] [PubMed] [Google Scholar]
- 52.Wang ZQ, Qin J, Martin J, Zhang XH, Sereda O, et al. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism. 2007;56:1652–1655. doi: 10.1016/j.metabol.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albarracin CA, Fuqua BC, Evans JL, Goldfine ID. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes Metab Res Rev. 2008;24:41–51. doi: 10.1002/dmrr.755. [DOI] [PubMed] [Google Scholar]
- 54.Aghdassi E, Arendt BM, Salit IE, Mohammed SS, Jalali P, et al. In patients with HIV-infection, chromium supplementation improves insulin resistance and other metabolic abnormalities: a randomized, double-blind, placebo controlled trial. Curr HIV Res. 2010;8:113–120. doi: 10.2174/157016210790442687. [DOI] [PubMed] [Google Scholar]
- 55.Mather KJ, Hunt AE, Steinberg HO, Paradisi G, Hook G, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab. 2001;86:5457–5464. doi: 10.1210/jcem.86.11.7880. [DOI] [PubMed] [Google Scholar]